The Promoting Effect of the Extracellular Matrix Peptide TNIIIA2 Derived from Tenascin-C in Colon Cancer Cell Infiltration
Abstract
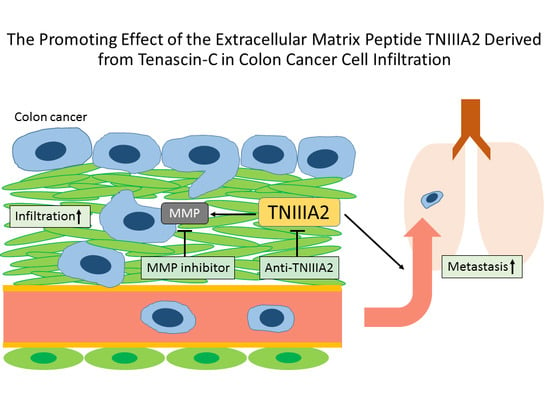
:1. Introduction
2. Results
2.1. Functional Analysis of Tenascin C (TNC)/TNIIIA2 in the Invasion of Colon Cancer Cells
2.2. The Effect of TNIIIA2 on a Mouse Model of Metastasis
3. Discussion
4. Materials and Methods
4.1. Cells and Animals
4.2. Reagents and Instruments
4.3. Invasion Assay
4.4. RT-PCR
4.5. Animal Model of Metastasis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Hynes, R.O. Integrins: Versatility, modulation, and signaling in cell adhesion. Cell 1992, 69, 11–25. [Google Scholar] [CrossRef]
- Calderwood, D.A.; Shattil, S.J.; Ginsberg, M.H. Integrins and actin filaments: Reciprocal regulation of cell adhesion and signaling. J. Biol. Chem. 2000, 275, 22607–22610. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O. Integrins: Bidirectional, allosteric signaling mashines. Cell 2002, 110, 673–687. [Google Scholar] [CrossRef]
- Howe, A.K.; Aplin, A.E.; Juliano, R.L. Anchorage-dependent ERK signaling-mechanisms and consequences. Curr. Opin. Genet. Dev. 2002, 12, 30–35. [Google Scholar] [CrossRef]
- Chiquet-Ehrismann, R. What distinguishes tenascin from fibronectin? FASEB J. 1990, 4, 2598–2604. [Google Scholar] [PubMed]
- Chiquet-Ehrismann, R.; Kalla, P.; Pearson, C.A.; Beck, K.; Chiquet, M. Tenascin interferes with fibronectin action. Cell 1988, 53, 383–390. [Google Scholar] [CrossRef]
- Bourdon, M.A.; Ruoslahti, E. Tenascin mediates cell attachment through an RGD-dependent receptor. J. Cell Biol. 1989, 108, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Joshi, P.; Chung, C.Y.; Aukhil, I.; Erickson, H.P. Endothelial cells adhere to the RGD domain and the fibrinogen-like terminal knob of tenascin. J. Cell Sci. 1993, 106, 389–400. [Google Scholar] [PubMed]
- Klein, G.; Beck, S.; Müller, C.A. Tenascin is a cytoadhesive extracellular matrixcomponent of the human hematopoietic microenvironment. J. Cell Biol. 1993, 123, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Hemesath, T.J.; Stefansson, K. Expression of tenascin in thymus and thymic nonlymphoid cells. J. Immunol. 1994, 152, 422–428. [Google Scholar] [PubMed]
- Hindermann, W.; Berndt, A.; Borsi, L.; Luo, X.; Hyckel, P.; Katenkamp, D.; Kosmehl, H. Synthesis and protein distribution of the unspliced large tenascin-C isoform in oral squamous cell carcinoma. J. Pathol. 1999, 189, 475–480. [Google Scholar] [CrossRef]
- Ghert, M.A.; Jung, S.T.; Qi, W.; Harrelson, J.M.; Erickson, H.P.; Block, J.A.; Scully, S.P. The clinical significance of tenascin-C splice variant expression in chondrosarcoma. Oncology 2001, 61, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Tsunoda, T.; Inada, H.; Kalembeyi, I.; Imanaka-Yoshida, K.; Sakakibara, M.; Okada, R.; Katsuta, K.; Sakakura, T.; Majima, Y.; Yoshida, T. Involvement of large tenascin-C splice variants in breast cancer progression. Am. J. Pathol. 2003, 162, 1857–1867. [Google Scholar] [CrossRef]
- Hanamura, N.; Yoshida, T.; Matsumoto, E.; Kawarada, Y.; Sakakura, T. Expression of fibronectin and tenascin-C mRNA by myofibroblasts, vascular cells and epithelial cells in human colon adenomas and carcinomas. Int. J. Cancer 1997, 73, 10–15. [Google Scholar] [CrossRef]
- Kamiya, S.; Kato, R.; Wakabayashi, M.; Tohyama, T.; Enami, I.; Ueki, M.; Yajima, H.; Ishii, T.; Nakamura, H.; Katayama, T.; et al. Fibronectin peptides derived from two distinct regions stimulate adipocyte differentiation by preventing fibronectin matrix assembly. Biochemistry 2002, 41, 3270–3277. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Imazeki, H.; Miura, S.; Yoshimura, T.; Okutsu, H.; Harada, Y.; Ohwaki, T.; Nagao, O.; Kamiya, S.; Hayashi, R.; et al. A peptide derived from tenascin-C induces beta1 integrin activation thorough syndecan-4. J. Biol. Chem. 2007, 282, 34929–34937. [Google Scholar] [CrossRef] [PubMed]
- Frezza, E.E.; Wachtel, M.S.; Chiriva-Internati, M. Influence of obesity on the risk of developing colon cancer. Gut 2006, 55, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, M.; Bradburn, D.M. Overview of screening and management of familial adenomatous polyposis. Gut 1992, 33, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Half, E.; Bercovich, D.; Rozen, P. Familial adenomatous polyposis. Orphanet J. Rare Dis. 2009, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Riedl, S.; Tandara, A.; Reinshagen, M.; Hinz, U.; Faissner, A.; Bodenmuller, H.; Buhr, H.J.; Herfarth, C.; Moller, P. Serum tenascin-C is an indicator of inflammatory bowel disease activity. Int. J. Colorectal Dis. 2001, 16, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Kawakatsu, H.; Hirota, N.; Yokoyama, T.; Sakakura, T.; Saito, M. Specific expression of tenascin in human colonic neoplasms. Br. J. Cancer 1993, 67, 1058–1064. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Chiquet-Ehrismann, R.; Moyano, J.V.; Garcia-Pardo, A.; Orend, G. Interference of tenascin-C with syndecan-4 binding to fibronectin blocks cell adhesion and stimulates tumor cell proliferation. Cancer Res. 2001, 61, 8586–8594. [Google Scholar] [PubMed]
- Liotta, L.A.; Rao, C.N.; Wewer, U.M. Biochemicalinteractions of tumor cells with the basement membrane. Annu. Rev. Biochem. 1986, 55, 1037–1057. [Google Scholar] [CrossRef] [PubMed]
- Ueda, Y.; Imai, K.; Tsuchiya, H.; Fujimoto, N.; Nakanishi, I.; Katsuda, S.; Seiki, M.; Okada, Y. Matrix metalloproteinase 9 (gelatinase B) is expressed in multinucleated giant cells of human giant cell tumor of bone and is associated with vascular invasion. Am. J. Pathol. 1996, 148, 611–622. [Google Scholar] [PubMed]
- Tews, D.S.; Nissen, A. Expression of adhesion factors and degrading proteins in primary and secondary glioblastomas and their precursor tumors. Invasion Metastasis 1998–1999, 18, 271–284. [Google Scholar] [CrossRef]
- Kalembeyi, I.; Inada, H.; Nishiura, R.; Imanaka-Yoshida, K.; Sakakura, T.; Yoshida, T. Tenascin-C upregulates matrix metalloproteinase-9 in breast cancer cells: Direct and synergistic effects with transforming growth factor beta1. Int. J. Cancer. 2003, 105, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Nuttall, R.K.; Liu, S.; Edwards, D.R.; Yong, V.W. Tenascin-C stimulates glioma cell invasion through matrix metalloproteinase-12. Cancer Res. 2006, 66, 11771–11780. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, S.; Rosenberg, D.W. The role of notch signaling in colon homeostasis and carcinogenesis. Cancer Sci. 2011, 102, 1938–1942. [Google Scholar] [CrossRef] [PubMed]
- Oskarsson, T.; Acharyya, S.; Zhang, X.H.; Vanharanta, S.; Tavazoie, S.F.; Morris, P.G.; Downey, R.J.; Manova-Todorova, K.; Brogi, E.; Massagué, J. Breast cancer cells produce tenascin C as a metastatic niche component to colonize the lungs. Nat. Med. 2011, 17, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Peddareddigari, V.G.; Wang, D.; Dubois, R.N. The tumor microenvironment in colorectal carcinogenesis. Cancer Microenviron. 2010, 3, 149–166. [Google Scholar] [CrossRef] [PubMed]
- Hawinkels, L.J.; Paauwe, M.; Verspaget, H.W.; Wiercinska, E.; van der Zon, J.M.; van der Ploeg, K.; Koelink, P.J.; Lindeman, J.H.; Mesker, W.; ten Dijke, P.; et al. Interaction with colon cancer cells hyperactivates TGF-b signaling in cancer-associated fibroblasts. Oncogene 2014, 33, 97–107. [Google Scholar] [CrossRef] [PubMed]
- De Wever, O.; Nguyen, Q.D.; van Hoorde, L.; Bracke, M.; Bruyneel, E.; Gespach, C.; Mareel, M. Tenascin-C and SF/HGF produced by myofibroblasts in vitro provide convergent proinvasive signals to human colon cancer cells through RhoA and Rac. FASEB J. 2004, 18, 1016–1018. [Google Scholar] [CrossRef] [PubMed]
- De Santis, R.; Albertoni, C.; Petronzelli, F.; Campo, S.; D’Alessio, V.; Rosi, A.; Anastasi, A.M.; Lindstedt, R.; Caroni, N.; Arseni, B.; et al. Low and High Tenascin-Expressing Tumors Are Efficiently Targeted by ST2146Monoclonal Antibody. Clin. Cancer Res. 2006, 12, 2191–2196. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Archer, G.E.; Wikstrand, C.J.; Morrison, S.L.; Zalutsky, M.R.; Bigner, D.D.; Batra, S.K. Generation and characterization of amouse/human chimeric antibody directed against extracellular matrix protein tenascin. J. Neuroimmunol. 1994, 52, 127–137. [Google Scholar] [CrossRef]
- Akabani, G.; Reardon, D.A.; Coleman, R.E.; Wong, T.Z.; Metzler, S.D.; Bowsher, J.E.; Barboriak, D.P.; Provenzale, J.M.; Greer, K.L.; DeLong, D.; et al. Dosimetry and radiographic analysis of 131I-labeled anti-tenascin 81C6 murine monoclonal antibody in newly diagnosed patients with malignant gliomas: A phase II study. J. Nucl. Med. 2005, 46, 1042–1051. [Google Scholar] [PubMed]
- Gazzaniga, P.; Nofroni, I.; Gandini, O.; Silvestri, I.; Frati, L.; Aglianò, A.M.; Gradilone, A. Tenascin C and epidermal growth factor receptor as markers of circulating tumoral cells in bladder and colon cancer. Oncol. Rep. 2005, 14, 1199–1202. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Peng, F.; Li, G.; Fu, Y.; Huang, Y.; Chen, Z.; Chen, Y. Proteomic analysis of stromal proteins in different stages of colorectal cancer establishes Tenascin-C as a stromal biomarker for colorectal cancer metastasis. Oncotarget 2016, 7, 37226–37237. [Google Scholar] [CrossRef] [PubMed]
- Catalán, V.; Gómez-Ambrosi, J.; Rodríguez, A.; Ramírez, B.; Izaguirre, M.; Hernández-Lizoain, J.L.; Baixauli, J.; Martí, P.; Valentí, V.; Moncada, R.; et al. Increased Obesity-Associated Circulating Levels of the Extracellular Matrix Proteins Osteopontin, Chitinase-3 Like-1 and Tenascin C Are Associated with Colon Cancer. PLoS ONE 2016, 11, e0162189. [Google Scholar] [CrossRef] [PubMed]
- Saginati, M.; Siri, A.; Balza, E.; Ponassi, M.; Zardi, L. A simple procedure for tenascin purification. Eur. J. Biochem. 1992, 205, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, R.; Seki, Y.; Saito, Y.; Kamiya, S.; Fujita, M.; Okutsu, H.; Iyoda, T.; Takai, T.; Owaki, T.; Yajima, H.; et al. Tenascin-C-derived peptide TNIIIA2 highly enhances cell survival and platelet-derived growth factor (PDGF)-dependent cell proliferation through potentiated and sustained activation of integrin α5β1. J. Biol. Chem. 2014, 289, 17699–17708. [Google Scholar] [CrossRef] [PubMed]
- Welch, D.R. Technical considerations for studying cancer metastasis in vivo. Clin. Exp. Metastasis 1997, 15, 272–306. [Google Scholar] [CrossRef] [PubMed]





| GAPDH | Forward: 5′-TTCACCACCATGGAGAAGGC-3′ |
| Reverse: 5′-GGCATGGACTGTGGTCATGA-3′ | |
| MMP-2 | Forward: 5′-AGATCTTCTTCTTCAAGGACCGGTT-3′ |
| Reverse: 5′-GGCTGGTCAGTGGCTTGGGGTA-3′ | |
| MMP-9 | Forward: 5′-CACCACCACAACTGAACC-3′ |
| Reverse: 5′-GCCTAGACCCAACTTATCC-3′ |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, H.; Sasada, M.; Kamiya, S.; Ito, Y.; Watanabe, H.; Okada, Y.; Ishibashi, K.; Iyoda, T.; Yanaka, A.; Fukai, F. The Promoting Effect of the Extracellular Matrix Peptide TNIIIA2 Derived from Tenascin-C in Colon Cancer Cell Infiltration. Int. J. Mol. Sci. 2017, 18, 181. https://doi.org/10.3390/ijms18010181
Suzuki H, Sasada M, Kamiya S, Ito Y, Watanabe H, Okada Y, Ishibashi K, Iyoda T, Yanaka A, Fukai F. The Promoting Effect of the Extracellular Matrix Peptide TNIIIA2 Derived from Tenascin-C in Colon Cancer Cell Infiltration. International Journal of Molecular Sciences. 2017; 18(1):181. https://doi.org/10.3390/ijms18010181
Chicago/Turabian StyleSuzuki, Hideo, Manabu Sasada, Sadahiro Kamiya, Yuka Ito, Hikaru Watanabe, Yuko Okada, Kazuma Ishibashi, Takuya Iyoda, Akinori Yanaka, and Fumio Fukai. 2017. "The Promoting Effect of the Extracellular Matrix Peptide TNIIIA2 Derived from Tenascin-C in Colon Cancer Cell Infiltration" International Journal of Molecular Sciences 18, no. 1: 181. https://doi.org/10.3390/ijms18010181