Metabolic Response of Human Osteoarthritic Cartilage to Biochemically Characterized Collagen Hydrolysates
Abstract
:1. Introduction
2. Results
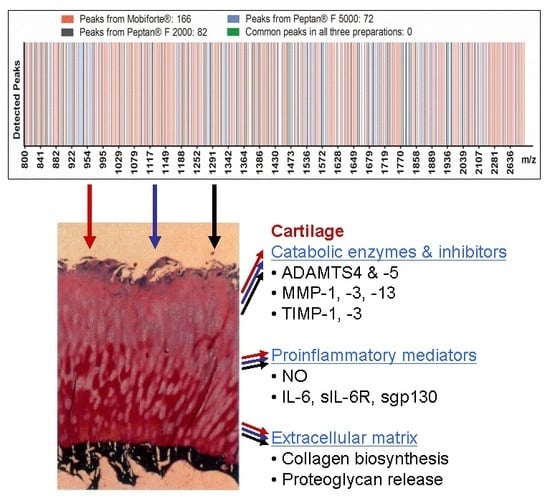
2.1. MALDI-TOF-MS Analysis of CHs
2.2. NMR Analysis of CHs
2.3. Amyloid Fibrillization and Atomic Force Microscopy of CHs
2.4. Collagen Synthesis of Human Cartilage Treated with CHs
2.5. Proteoglycan Loss from CH-Treated Cartilage Explants
2.6. In Vitro Effects of CHs on Aggrecanase Activity
2.7. Levels of Catabolic MMPs and TIMPs
2.8. NO Production in Human Cartilage Explants
2.9. Levels of IL-6, sIL-6R and sgp130 in Cultured Cartilage Explants
2.10. Viability of Chondrocytes in Explants after CH Treatment
3. Discussion
3.1. Biochemical Characterization of CHs
3.2. Collagen Biosynthesis of Human Cartilage as Modulated by CHs
3.3. Effects of CHs on Catabolic Enzymes and Proinflammatory Mediators
4. Materials and Methods
4.1. MALDI-TOF Mass Spectrometric Analysis
4.2. Nuclear Magnetic Resonance Spectroscopy
4.3. Amyloid Fibrillization and Atomic Force Microscopy of CHs
4.4. Specimen Selection
4.5. Articular Cartilage Explant Culture
4.6. Analysis of Collagen Synthesis
4.7. Analysis of Proteoglycan Loss
4.8. Aggrecanase Activity
4.9. Levels of Catabolic MMPs and TIMPs
4.10. NO Production
4.11. Analysis of IL-6, sIL-6R and sgp130
4.12. Chondrocyte Viability
4.13. Statistical Analyses
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| OA | Osteoarthritis |
| CH | Collagen Hydrolysate |
| MS | Mass Spectrometry |
| MMP | Matrix Metalloproteinase |
| ADAMTS | A Disintegrin and Metalloproteinase with a Thrombospondin Motif |
| TIMP | Tissue Inhibitor of Metalloproteinases |
| IL | Interleukin |
| sIL-6R | Soluble Interleukin-6 Receptor |
| sgp 130 | Soluble Glycoprotein 130 |
| MALDI-TOF | Matrix-Assisted Laser Desorption/Ionization-Time of Flight |
| TFA | Trifluoroacetic Acid |
| TOCSY | Total Correlation Spectroscopy |
| NMR | Nuclear Magnetic Resonance |
| ThT | Thioflavin T |
| BMI | Body Mass Index |
| DOSY | Diffusion Ordered Spectroscopy |
References
- Steinmeyer, J.; Konttinen, Y.T. Oral treatment options for degenerative joint disease-presence and future. Adv. Drug Deliv. Rev. 2006, 58, 168–211. [Google Scholar] [CrossRef] [PubMed]
- Schadow, S.; Siebert, H.-C.; Lochnit, G.; Kordelle, J.; Rickert, M.; Steinmeyer, J. Collagen metabolism of human osteoarthritic articular cartilage as modulated by bovine collagen hydrolysates. PLoS ONE 2013, 8, e53955. [Google Scholar] [CrossRef] [PubMed]
- Henrotin, Y.; Lambert, C.; Couchourel, D.; Ripoll, C.; Chiotelli, E. Nutraceuticals: Do they represent a new era in the management of osteoarthritis? A narrative review from the lessons taken with five products. Osteoarthr. Cartil. 2011, 19, 1–21. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, Nutrition and Allergies. Scientific Opinion on the substantiation of a health claim related to collagen hydrolysate and maintenance of joints pursuant to Article 13(5) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2291. [Google Scholar]
- Rayman, M.P.; Pattison, D.J. Dietary manipulation in musculoskeletal conditions. Best Pract. Res. Clin. Rheumatol. 2008, 22, 535–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Vijven, J.P.J.; Luijsterburg, P.A.J.; Verhagen, A.P.; van Osch, G.J.V.M.; Kloppenburg, M.; Bierma-Zeinstra, S.M.A. Symptomatic and chondroprotective treatment with collagen derivatives in osteoarthritis: A systematic review. Osteoarthr. Cartil. 2012, 20, 809–821. [Google Scholar] [CrossRef] [PubMed]
- Jeevithan, E.; Qingbo, Z.; Bao, B.; Wu, W. Biomedical and pharmaceutical application of fish collagen and gelatin: A review. J. Nutr. Ther. 2013, 2, 218–227. [Google Scholar]
- Asserin, J.; Lati, E.; Shioya, T.; Prawitt, J. The effect of oral collagen peptide supplementation on skin moisture and the dermal collagen network: Evidence from an ex vivo model and randomized, placebo-controlled clinical trials. J. Cosmet. Dermatol. 2015, 14, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Mortarino, P.A.; Goy, D.P.; Abramson, D.B.; Cabello, J.; Bumaguin, G.E.; Vitelli, E.J.; Toledo, J.; Sarrio, L.; Pezzotto, S.M.; Mardegan Issa, J.P.; et al. Emerging therapy in arthritis: Modulation of markers of the inflammatory process. Microsc. Res. Technol. 2016, 79, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Comblain, F.; Sanchez, C.; Lesponne, I.; Balligand, M.; Serisier, S.; Henrotin, Y. Curcuminoids extract, hydrolyzed collagen and green tea extract synergically inhibit inflammatory and catabolic mediator´s synthesis by normal bovine and osteoarthritic human chondrocytes in monolayer. PLoS ONE 2015, 10, e0121654. [Google Scholar] [CrossRef] [PubMed]
- Oesser, S.; Seifert, J. Stimulation of type II collagen biosynthesis and secretion in bovine chondrocytes cultured with degraded collagen. Cell Tissue Res. 2003, 311, 393–399. [Google Scholar] [PubMed]
- Raabe, O.; Reich, C.; Wenisch, S.; Hild, A.; Burg-Roderfeld, M.; Siebert, H.C.; Arnhold, S. Hydrolyzed fish collagen induced chondrogenic differentiation of equine adipose tissue-derived stromal cells. Histochem. Cell Biol. 2010, 134, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Sun, J. Potential application of hydrolyzed fish collagen for inducing the multidirectional differentiation of rat bone marrow mesenchymal stem cells. Biomacromolecules 2014, 15, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Kuzan, A.; Smulczynska-Demel, A.; Chwilkowska, A.; Saczko, J.; Frydrychowsky, A.; Dominiak, M. An estimation of the biological properties of fish collagen in an experimental in vitro study. Adv. Clin. Exp. Med. 2015, 24, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Siebert, H.-C.; Burg-Roderfeld, M.; Eckert, T.; Stötzel, S.; Kirch, U.; Diercks, T.; Humphries, M.J.; Frank, M.; Wechselberger, R.; Tajkhorshid, E.; et al. Interaction of the α2A domain of integrin with small collagen fragments. Protein Cell 2010, 1, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Stötzel, S.; Schurink, M.; Wienk, H.; Siebler, U.; Burg-Roderfeld, M.; Eckert, T.; Kulik, B.; Wechselberger, R.; Sewing, J.; Steinmeyer, J.; et al. Molecular organization of different collagen hydrolysates and collagen fragments as revealed by a combination of Atomic Force Microscopy (AFM) and Diffusion Ordered NMR Spectroscopy (DOSY). ChemPhysChem 2012, 13, 3117–3125. [Google Scholar] [CrossRef] [PubMed]
- Jeevithan, E.; Jingyi, Z.; Wang, N.; He, L.; Bao, B.; Wu, W. Physico-chemical, antioxidant and intestinal adsorption properties of whale shark type-II collagen based on its solubility with acid and pepsin. Process Biochem. 2015, 50, 463–472. [Google Scholar] [CrossRef]
- Oesser, S.; Adam, M.; Babel, W.; Seifert, J. Oral administration of 14C-labeled gelatin hydrolysate leads to an accumulation of radioactivity in cartilage of mice (C57/BL). J. Nutr. 1999, 129, 1891–1895. [Google Scholar] [PubMed]
- Bondeson, J.; Wainwright, S.; Hughes, C.; Caterson, B. The regulation of the ADAMTS4 and ADAMTS5 aggrecanases in osteoarthritis: A review. Clin. Exp. Rheumatol. 2008, 26, 139–145. [Google Scholar] [PubMed]
- Naito, S.; Shiomi, T.; Okada, A.; Kimura, T.; Chijiiwa, M.; Fujita, Y.; Yatabe, T.; Komiya, K.; Enomoto, H.; Fujikawa, K.; et al. Expression of ADAMTS4 (aggreacanse-1) in human osteoarthritic cartilage. Pathol. Int. 2007, 57, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Mosyak, L.; Georgiadis, K.; Shane, T.; Svenson, K.; Hebert, T.; McDonagh, T.; Mackie, S.; Olland, S.; Lin, L.; Zhong, X.; et al. Crystal structures of the two major aggrecan degrading enzymes, ADAMTS4 and ADAMTS5. Protein Sci. 2008, 17, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Shieh, H.S.; Tomasselli, A.G.; Mathis, K.J.; Schnute, M.E.; Woodard, S.S.; Caspers, N.; Williams, J.M.; Kiefer, J.R.; Munie, G.; Wittwer, A.; et al. Structure analysis reveals the flexibility of the ADAMTS5 active site. Protein Sci. 2011, 20, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Bertini, I.; Calderone, V.; Cosenza, M.; Fragai, M.; Lee, Y.-M.; Luchinat, C.; Mangani, S.; Terni, B.; Turano, P. Conformational variability of matrix metalloproteinases: Beyond a single 3D structure. Proc. Natl. Acad. Sci. USA 2005, 102, 5334–5339. [Google Scholar] [CrossRef] [PubMed]
- Alcaraz, L.A.; Banci, L.; Bertini, I.; Cantini, F.; Donaire, A.; Gonnelli, L. Matrix metallo-proteinase-inhibitor interaction: The solution structure of the catalytic domain of human matrix metalloproteinase-3 with different inhibitors. J. Biol. Inorg. Chem. 2007, 12, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheumatol. 2012, 64, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Malemud, C.J. Biologic basis of osteoarthritis: State of the evidence. Curr. Opin. Rheumatol. 2015, 27, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Troeberg, L.; Fushimi, K.; Scilabra, S.D.; Nakamura, H.; Dive, V.; Thogersen, I.B.; Enghild, J.J.; Nagase, H. The C-terminal domains of ADAMTS-4 and ADAMTS-5 promote association with N-TIMP-3. Matrix Biol. 2009, 28, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Jennings, L.; Wu, L.; King, K.B.; Hämmerle, H.; Cs-Szabo, G.; Mollenhauer, J. The effect of collagen fragments on the extracellular matrix metabolism of bovine and human chondrocytes. Connect Tissue Res. 2001, 42, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Fichter, M.; Körner, U.; Schömburg, J.; Jennings, L.; Cole, A.A.; Mollenhauer, J. Collagen degradation products modulate matrix metalloproteinase expression in cultured articular chondrocytes. J. Orthop. Res. 2006, 24, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Attur, M.G.; Dave, M.; Akamatsu, M.; Katoh, M.; Amin, A.R. Osteoarthritis or osteoarthrosis: The definition of inflammation becomes a semantic issue in the genomic era of molecular medicine. Osteoarthr. Cartil. 2002, 10, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Stannus, O.P.; Jones, G.; Blizzard, L.; Cicuttini, F.M.; Ding, C. Associations between serum levels of inflammatory markers and change in knee pain over 5 years in older adults: A prospective cohort study. Ann. Rheum. Dis. 2013, 72, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Shimura, Y.; Kursawa, H.; Sugawara, Y.; Tsuchiya, M.; Sawa, M.; Kaneko, H. The factors associated with pain severity in patients with knee osteoarthritis vary according to the radiographic disease severity: A cross-sectional study. Osteoarthr. Cartil. 2013, 21, 1179–1184. [Google Scholar] [CrossRef] [PubMed]
- Stannus, O.; Jones, G.; Cicuttine, F.; Parameswaran, V.; Quinn, S.; Burgess, J.; Ding, C. Circulating levels of IL-6 and TNF-α are associated with knee radiographic osteoarthritis and knee cartilage loss in older adults. Osteoarthr. Cartil. 2010, 18, 1441–1447. [Google Scholar] [CrossRef] [PubMed]
- Livshits, G.; Zhai, G.; Hart, D.J.; Kato, B.S.; Wang, H.; Williams, F.M.; Spector, T.D. Interleukin-6 is a significant predictor of radiographic knee osteoarthritis: The Chingford Study. Arthritis Rheumatol. 2009, 60, 2037–2045. [Google Scholar] [CrossRef] [PubMed]
- Rowan, A.D.; Koshy, P.J.; Shingleton, W.D.; Degnan, B.A.; Heath, J.K.; Vernallis, A.B.; Spaull, J.R.; Life, P.F.; Hudson, K.; Cawston, T.E. Synergistic effects of glycoprotein 130 binding cytokines in combination with interleukin-1 on cartilage collagen breakdown. Arthritis Rheumatol. 2001, 44, 1620–1632. [Google Scholar] [CrossRef]
- Flannery, C.R.; Little, C.B.; Hughes, C.E.; Curtis, C.L.; Caterson, B.; Jones, S.A. IL-6 and its soluble receptor augment aggrecanase-mediated proteoglycan catabolism in articular cartilage. Matrix Biol. 2000, 19, 549–553. [Google Scholar] [CrossRef]
- Sui, Y.; Lee, J.H.; DiMicco, M.A.; Vanderploeg, E.J.; Blake, S.M.; Hung, H.H.; Plaas, A.H.; James, E.E.; Song, X.Y.; Lark, M.W.; et al. Mechanical injury potentiate proteoglycan catabolism induced by interleukin-6 with soluble interleukin-6 receptor and tumor necrosis factor α in immature bovine and adult human articular cartilage. Arthritis Rheumatol. 2009, 60, 2985–2996. [Google Scholar] [CrossRef] [PubMed]
- Rose-John, S. The soluble interleukin-6 receptor and related proteins. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Jostock, T.; Müllberg, J.; Ozbek, S.; Atreva, R.; Blinn, G.; Voltz, N.; Fischer, M.; Neurath, M.F.; Rose-John, S. Soluble gp130 is the natural inhibitor of soluble interlukin-6 receptor transsignaling responses. Eur. J. Biochem. 2001, 268, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Wang, W. Protein aggregation and its inhibition in biopharmaceutics. Int. J. Pharm. 2005, 289, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Bau, B.; Yang, H.; Soeder, S.; Aigner, T. Freshly isolated osteoarthritic chondrocytes are catabolically more active than normal chondrocytes, but less responsive to catabolic stimulation with interleukin-1beta. Arthritis Rheumatol. 2005, 52, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Aigner, T.; Haag, J.; Martin, J.; Buckwalter, J. Osteoarthritis: Aging of matrix and cells-going for a remedy. Curr. Drug Targets 2007, 8, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Moktar, N.M.; Yusof, H.M.; Yahaya, N.H.; Muhamad, R.; Das, S. The transcript level of interleukin-6 in the cartilage of idiopathic ostoarthritis of knees. Clin. Ther. 2010, 161, 25–28. [Google Scholar]
- Bayliss, M.T.; Ali, S.Y. Age-related changes in the composition and structure of human articular cartilage proteoglycans. Biochem. J. 1979, 176, 683–693. [Google Scholar] [CrossRef]
- Shigemura, Y.; Kubomura, D.; Sato, Y.; Sato, K. Dose-dependent changes in the levels of free and peptide forms of hydroxyproline in human plasma after collagen hydrolysate ingestion. Food Chem. 2014, 159, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wan, Q.; Qian, J.; Liang, Q.; Wang, Z.; Xu, J.; He, S.; Ma, H. Bioavailability and bioavailable forms of collagen after oral administration to rats. J. Agric. Food Chem. 2015, 63, 3752–3756. [Google Scholar] [CrossRef] [PubMed]
- Fukui, N.; Ikeda, Y.; Ohnuki, T.; Tanaka, N.; Hikita, A.; Mitomi, H.; Mori, T.; Juji, T.; Katsuragawa, Y.; Yamamoto, S.; et al. Regional differences in chondrocyte metabolism in osteoarthritis: A detailed analysis by laser capture microdissection. Arthritis Rheumatol. 2008, 58, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Patwari, P.; Gao, G.; Lee, J.H.; Grodzinsky, A.J.; Sandy, J.D. Analysis of ADAMTS4 and MT4-MMP indicates that both are involved in aggrecanolysis in interleukin-1-treated bovine cartilage. Osteoarthr. Cartil. 2005, 13, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Shlopov, B.V.; Lie, W.R.; Mainardi, C.L.; Cole, A.A.; Chubinskaya, S.; Hasty, K.A. Osteoarthritic lesions: Involvement of three different collagenases. Arthritis Rheumatol. 1997, 40, 2065–2074. [Google Scholar] [CrossRef]
- Hartog, A.; Cozijnsen, M.; de Vrij, G.; Garssen, J. Collagen hydrolysate inhibits zymosan-induced inflammation. Exp. Biol. Med. 2013, 238, 798–802. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kouguchi, T.; Shimizu, K.; Sato, M.; Takahata, Y.; Mormiatsu, F. Chicken collagen hydrolysate reduces proinflammatory cytokine production in C57BL/6. KOR-ApoEsh1 mice. J. Nutr. Sci. Vitaminol. 2010, 56, 208–210. [Google Scholar] [CrossRef] [PubMed]
- Oesser, S.; Proksch, E.; Schunck, M. Prophylactic treatment with a special collagen hydrolysate decreases cartilage tissue degeneration in the knee joints. Osteoarthr. Cartil. 2008, 16 (Suppl. 4), S45. [Google Scholar] [CrossRef]
- Collins, D.H. The Pathology of Articular and Spinal Diseases; Edward Arnold and Co.: London, UK, 1949; pp. 76–79. [Google Scholar]
- Steinmeyer, J.; Kordelle, J.; Stürz, H. In vitro inhibition of aggrecanase activity by tetracyclines and proteoglycan loss from osteoarthritic human articular cartilage. J. Orthop. Res. 2010, 28, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, J.L.; Farley, M.L.; Swaim, B.; Goldring, S.R.; Goldring, M.B.; Bierbaum, B.E.; Gray, M.L. Dual proline labeling protocol for individual “baseline” and “response” biosynthesis measurements in human articular cartilage. Osteoarthr. Cartil. 2008, 16, 1263–1266. [Google Scholar] [CrossRef] [PubMed]
- Farndale, R.W.; Buttle, D.J.; Barrett, A.J. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim. Biophys. Acta 1986, 83, 173–177. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Ben-Aderet, L.; Merquiol, E.; Fahham, D.; Kumar, A.; Reich, E.; Ben-Nun, Y.; Kandel, L.; Liebergall, M.; Kosinska, M.K.; Steinmeyer, J.; et al. Detecting cathepsin activity in human osteoarthritis via activity-based probes. Arthritis Res. Ther. 2015, 17, 69. [Google Scholar] [CrossRef] [PubMed]







© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schadow, S.; Simons, V.S.; Lochnit, G.; Kordelle, J.; Gazova, Z.; Siebert, H.-C.; Steinmeyer, J. Metabolic Response of Human Osteoarthritic Cartilage to Biochemically Characterized Collagen Hydrolysates. Int. J. Mol. Sci. 2017, 18, 207. https://doi.org/10.3390/ijms18010207
Schadow S, Simons VS, Lochnit G, Kordelle J, Gazova Z, Siebert H-C, Steinmeyer J. Metabolic Response of Human Osteoarthritic Cartilage to Biochemically Characterized Collagen Hydrolysates. International Journal of Molecular Sciences. 2017; 18(1):207. https://doi.org/10.3390/ijms18010207
Chicago/Turabian StyleSchadow, Saskia, Viktor S. Simons, Guenter Lochnit, Jens Kordelle, Zuzana Gazova, Hans-Christian Siebert, and Juergen Steinmeyer. 2017. "Metabolic Response of Human Osteoarthritic Cartilage to Biochemically Characterized Collagen Hydrolysates" International Journal of Molecular Sciences 18, no. 1: 207. https://doi.org/10.3390/ijms18010207