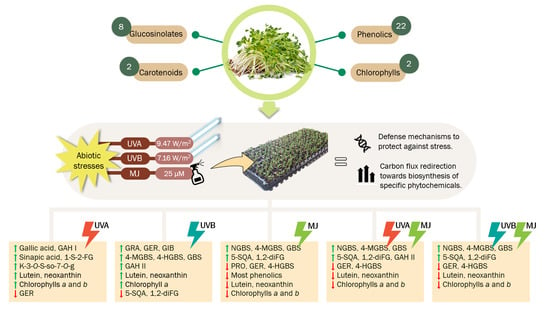
UVA, UVB Light, and Methyl Jasmonate, Alone or Combined, Redirect the Biosynthesis of Glucosinolates, Phenolics, Carotenoids, and Chlorophylls in Broccoli Sprouts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of UVA or UVB Light and Methyl Jasmonate on the Accumulation of Glucosinolates
2.2. Effect of UVA or UVB Light and Methyl Jasmonate on the Accumulation of Phenolic Compounds
2.3. Effect of UVA or UVB Light and Methyl Jasmonate on the Accumulation of Carotenoids and Chlorophylls
3. Materials and Methods
3.1. Chemical and Plant Material
3.2. Sprouting Method
3.3. UV and MJ Treatments
3.4. Phytochemical Analyses
3.4.1. Extraction of Phytochemicals
3.4.2. Analysis of Glucosinolates
Desulfation of Glucosinolates
Identification and Quantification of Desulfoglucosinolates by HPLC-DAD and HPLC-ESI-MSn
3.4.3. Analysis of Phenolic Compounds
Identification and Quantification of Phenolic Compounds by HPLC-DAD and HPLC-ESI-MSn
3.4.4. Analysis of Carotenoids and Chlorophylls
Identification and Quantification of Carotenoids and Chlorophylls by HPLC-DAD
3.5. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vale, A.P.; Santos, J.; Brito, N.V.; Fernandes, D.; Rosa, E.; Beatriz, M.; Oliveira, P.P. Evaluating the impact of sprouting conditions on the glucosinolate content of Brassica oleracea sprouts. Phytochemistry 2015, 115, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, E.H.; Araya, M. Physiological effects of broccoli consumption. Phytochem. Rev. 2009, 8, 283–298. [Google Scholar] [CrossRef]
- Paja̧k, P.; Socha, R.; Gałkowska, D.; Rożnowski, J.; Fortuna, T. Phenolic profile and antioxidant activity in selected seeds and sprouts. Food Chem. 2014, 143, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.W.; Wehage, S.L.; Holtzclaw, W.D.; Kensler, T.W.; Egner, P.A.; Shapiro, T.A.; Talalay, P. Protection of humans by plant glucosinolates: Efficiency of conversion of glucosinolates to isothiocyanates by the gastrointestinal microflora. Cancer Prev. Res. 2012, 5, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Kostov, R.V. Glucosinolates and isothiocyanates in health and disease. Trends Mol. Med. 2012, 18, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Herr, I.; Büchler, M.W. Dietary constituents of broccoli and other cruciferous vegetables: Implications for prevention and therapy of cancer. Cancer Treat. Rev. 2010, 36, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Higdon, J.; Delage, B.; Williams, D.; Dashwood, R. Cruciferous vegetables and human cancer risk: Epidemiologic evidence and mechanistic basis. Pharmacol. Res. 2007, 55, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Cartea, M.E.; Francisco, M.; Soengas, P.; Velasco, P. Phenolic Compounds in Brassica Vegetables. Molecules 2010, 16, 251–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres-Contreras, A.M.; Nair, V.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Stability of bioactive compounds in broccoli as affected by cutting styles and storage time. Molecules 2017, 22, 636. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, F.; Tomás-Barberán, F.; García-Viguera, C. Health-promoting compounds in broccoli as influenced by refrigerated transport and retail sale period. J. Agric. Food Chem. 2003, 51, 3029–3034. [Google Scholar] [CrossRef] [PubMed]
- Villarreal-García, D.; Nair, V.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Plants as biofactories: Postharvest stress-induced accumulation of phenolic compounds and glucosinolates in broccoli subjected to wounding stress and exogenous phytohormones. Front. Plant Sci. 2016, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- Jacobo-Velázquez, D.A.; Cisneros-Zevallos, L. Correlations of antioxidant activity against phenolic content revisited: A new approach in data analysis for food and medicinal plants. J. Food Sci. 2009, 74, R107–R113. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.L.; Ramos-Romero, S.; Pérez-Jiménez, J. Key Aspects of polyphenols and health. In Advances in Technologies for Producing Food-Relevant Polyphenols; Valenzuela, J.C., Vergara-Salinas, J.R., Perez-Correa, J.R., Eds.; Taylor & Francis Group: Boca Raton, FL, USA, 2016; pp. 33–62. [Google Scholar]
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Chlorogenic acid: Recent advances on its dual role as a food additive and a nutraceutical against metabolic syndrome. Molecules 2017, 22, 358. [Google Scholar] [CrossRef] [PubMed]
- Clotault, J.; Peltier, D.; Berruyer, R.; Thomas, M.; Briard, M.; Geoffriau, E. Expression of carotenoid biosynthesis genes during carrot root development. J. Exp. Bot. 2008, 59, 3563–3573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dos Reis, L.C.R.; de Oliveira, V.R.; Hagen, M.E.K.; Jablonski, A.; Flôres, S.H.; de Oliveira Rios, A. Carotenoids, flavonoids, chlorophylls, phenolic compounds and antioxidant activity in fresh and cooked broccoli (Brassica oleracea var. Avenger) and cauliflower (Brassica oleracea var. Alphina F1). LWT-Food Sci. Technol. 2015, 63, 177–183. [Google Scholar]
- Villarreal-García, D.; Alanís-Garza, P.A.; Cuéllar-Villarreal, R.; Redondo-Gil, M.; Mora-Nieves, J.L.; Jacobo-Velázquez, D.A. Effect of different defrosting methods on the stability of bioactive compounds and consumer acceptability of frozen broccoli. CyTA-J. Food 2015, 13, 312–320. [Google Scholar] [CrossRef]
- Bernal, J.; Mendiola, J.A.; Ibáñez, E.; Cifuentes, A. Advanced analysis of nutraceuticals. J. Pharm. Biomed. Anal. 2011, 55, 758–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niyogi, K.K.; Wolosiuk, R.A.; Malkin, R. Photosynthesis. In Biochemistry and Molecular Biology of Plants; Buchanan, B.B., Gruissem, W., Jones, R.L., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 568–588. [Google Scholar]
- Ferruzzi, M.G.; Blakeslee, J. Digestion, absorption, and cancer preventative activity of dietary chlorophyll derivatives. Nutr. Res. 2007, 27, 1–12. [Google Scholar] [CrossRef]
- Cisneros-Zevallos, L. The use of controlled postharvest abiotic stresses as a tool for enhancing the nutraceutical content and adding-value. J. Food Sci. 2003, 68, 1560–1565. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A.; Cisneros-Zevallos, L. An alternative use of horticultural crops: Stressed plants as biofactories of bioactive phenolic compounds. Agriculture 2012, 2, 259–271. [Google Scholar] [CrossRef]
- Jenkins, G.I.; Brown, B.A. UV-B Perception and signal transduction. In Light and Plant Development; Blackwell Publishing Ltd.: Oxford, UK, 2007; pp. 155–182. [Google Scholar]
- Schreiner, M.; Mewis, I.; Huyskens-Keil, S.; Jansen, M.A.K.; Zrenner, R.; Winkler, J.B.; O’Brien, N.; Krumbein, A. UV-B-induced secondary plant metabolites-potential benefits for plant and human health. CRC Crit. Rev. Plant. Sci. 2012, 31, 229–240. [Google Scholar] [CrossRef]
- Mewis, I.; Schreiner, M.; Nguyen, C.N.; Krumbein, A.; Ulrichs, C.; Lohse, M.; Zrenner, R. UV-B irradiation changes specifically the secondary metabolite profile in broccoli sprouts: Induced signaling overlaps with defense response to biotic stressors. Plant Cell Physiol. 2012, 53, 1546–1560. [Google Scholar] [CrossRef] [PubMed]
- Moreira-Rodríguez, M.; Nair, V.; Benavides, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. UVA, UVB light doses and harvesting time differentially tailor glucosinolate and phenolic profiles in broccoli sprouts. Molecules 2017, 22, 1065. [Google Scholar] [CrossRef] [PubMed]
- De Geyter, N.; Gholami, A.; Goormachtig, S.; Goossens, A. Transcriptional machineries in jasmonate-elicited plant secondary metabolism. Trends Plant Sci., 2012, 17, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Balibrea, S.; Moreno, D.A.; García-Viguera, C. Improving the phytochemical composition of broccoli sprouts by elicitation. Food Chem. 2011, 129, 35–44. [Google Scholar] [CrossRef]
- Barrientos Carvacho, H.; Pérez, C.; Zúñiga, G.; Mahn, A. Effect of methyl jasmonate, sodium selenate and chitosan as exogenous elicitors on the phenolic compounds profile of broccoli sprouts. J. Sci. Food Agric. 2014, 94, 2555–2561. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, M.D.; Larsen-Petersen, B.; Glawischnig, E.; Bøgh-Jensen, A.; Andreasson, E.; Halkier, B.A. Modulation of CYP79 genes and glucosinolate profiles in Arabidopsis by defense signaling pathways. Plant Physiol. 2003, 131, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Ku, K.M.; Juvik, J.A. Environmental stress and methyl jasmonate-mediated changes in flavonoid concentrations and antioxidant activity in broccoli florets and kale leaf tissues. Hortic. Sci. 2013, 48, 996–1002. [Google Scholar]
- Wiesner, M.; Hanschen, F.S.; Schreiner, M.; Glatt, H.; Zrenner, R. Induced Production of 1-Methoxy-Indol-3-Ylmethyl Glucosinolate by Jasmonic Acid and Methyl Jasmonate in Sprouts and Leaves of Pak Choi (Brassica Rapa ssp. Chinensis). Int. J. Mol. Sci. 2013, 14, 14996–15016. [Google Scholar] [CrossRef] [PubMed]
- Skirycz, A.; Reichelt, M.; Burow, M.; Birkemeyer, C.; Rolcik, J.; Kopka, J.; Zanor, M.I.; Gershenzon, J.; Strnad, M.; Szopa, J.; et al. DOF transcription factor AtDof1.1 (OBP2) is part of a regulatory network controlling glucosinolate biosynthesis in Arabidopsis. Plant J. 2006, 47, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Pfalz, M.; Mikkelsen, M.D.; Bednarek, P.; Olsen, C.E.; Halkier, B.A.; Kroymann, J. Metabolic engineering in Nicotiana benthamiana reveals key enzyme functions in Arabidopsis indole glucosinolate modification. Plant Cell 2011, 23, 716–729. [Google Scholar] [CrossRef] [PubMed]
- Pfalz, M.; Mukhaimar, M.; Perreau, F.; Kirk, J.; Hansen, C.I.C.; Olsen, C.E.; Agerbirk, N.; Kroymann, J. Methyl transfer reactions in glucosinolate biosynthesis mediated by Indole glucosinolate O-methyltransferase 5. Plant Physiol. 2016, 172, 2190–2203. [Google Scholar] [CrossRef] [PubMed]
- Baskar, V.; Gururani, M.A.; Yu, J.W.; Park, S.W. Engineering Glucosinolates in Plants: Current Knowledge and Potential Uses. Appl. Biochem. Biotechnol. 2012, 168, 1694–1717. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, W.J.; Yan, X.F.; Wang, Y. Glucosinolate content and related gene expression in response to enhanced UV-B radiation in Arabidopsis. Afr. J. Biotechnol. 2011, 10, 6481–6491. [Google Scholar]
- Demukra, P.V.; Abdala, G.; Baldwin, I.T.; Ballaré, C.L. Jasmonate-dependent and independent pathways mediate specific effects of solar ultraviolet B radiation on leaf phenolics and antiherbivore defense. Plant Physiol. 2010, 152, 1084–1095. [Google Scholar] [CrossRef] [PubMed]
- Heredia, J.B.; Cisneros-Zevallos, L. The effect of exogenous ethylene and methyl jasmonate on pal activity, phenolic profiles and antioxidant capacity of carrots (Daucus carota) under different wounding intensities. Postharvest Biol. Technol. 2009, 51, 242–249. [Google Scholar] [CrossRef]
- Park, W.T.; Kim, Y.B.; Seo, J.M.; Kim, S.J.; Chung, E.; Lee, J.H.; Park, S.U. Accumulation of anthocyanin and associated gene expression in radish sprouts exposed to light and methyl jasmonate. J. Agric. Food Chem. 2013, 61, 4127–4132. [Google Scholar] [CrossRef] [PubMed]
- Verdaguer, D.; Jansen, M.A.K.; Llorens, L.; Morales, L.O.; Neugart, S. UV-A radiation effects on higher plants: Exploring the known unknown. Plant Sci. 2017, 255, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Dolan, W.L.; Anderson, N.A.; Chapple, C. Indole glucosinolate biosynthesis limits phenylpropanoid accumulation in Arabidopsis thaliana. Plant Cell 2015, 27, 1529–1546. [Google Scholar] [CrossRef] [PubMed]
- Jacobo-Velázquez, D.A.; González-Agüero, M.; Cisneros-Zevallos, L. Cross-talk between signaling pathways: The link between plant secondary metabolite production and wounding stress response. Sci. Rep. 2015, 5, 8608. [Google Scholar] [CrossRef] [PubMed]
- Nićiforović, N.; Abramovič, H. Sinapic acid and its derivatives: Natural sources and bioactivity. Compr. Rev. Food Sci. Food Saf. 2014, 13, 34–51. [Google Scholar] [CrossRef]
- Kopsell, D.A.; Sams, C.E. Increases in shoot tissue pigments, glucosinolates, and mineral elements in sprouting broccoli after exposure to short-duration blue light from light emitting diodes. J. Am. Soc. Hortic. Sci. 2013, 138, 31–37. [Google Scholar]
- Taylor, K.L.; Brackenridge, A.E.; Vivier, M.A.; Oberholster, A. High-performance liquid chromatography profiling of the major carotenoids in Arabidopsis thaliana leaf tissue. J. Chromatogr. A 2006, 1121, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Castle, W.S.; Coates, G.A. High-performance liquid chromatography for the characterization of carotenoids in the new sweet orange (Earlygold) grown in Florida, USA. J. Chromatogr. A 2001, 913, 371–377. [Google Scholar] [CrossRef]
- Becerra-Moreno, A.; Alanís-Garza, P.A.; Mora-Nieves, J.L.; Mora-Mora, J.P.; Jacobo-Velázquez, D.A. Kale: An excellent source of vitamin C, pro-vitamin A, lutein and glucosinolates. CyTA-J. Food 2014, 12, 298–303. [Google Scholar] [CrossRef]
- Strati, I.F.; Sinanoglou, V.J.; Kora, L.; Miniadis-Meimaroglou, S.; Oreopoulou, V. Carotenoids from foods of plant, animal and marine origin: An efficient HPLC-DAD separation method. Foods 2012, 1, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, C.; Baranda, A.B.; De Marañón, I.M. The effect of high pressure and high temperature processing on carotenoids and chlorophylls content in some vegetables. Food Chem. 2014, 163, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Koltermann, D.; Schreiner, M.; Krumbein, A.; Mewis, I.; Ulrichs, C.; Huyskens-Keil, S. UV-B radiation mediated changes of bioactive compounds Brassica juncea L. In Proceedings of the 2007 Eco Summit (ES’07), Beijing, China, 22–27 May 2007; p. 161. [Google Scholar]
- Wierstra, I.; Kloppstech, K. Differential effects of methyl jasmonate on the expression of the early light-inducible proteins and other light-regulated genes in barley. Plant Physiol. 2000, 124, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Pérez, A.G.; Sanz, C.; Richardson, D.G.; Olías, J.M. Methyl jasmonate vapor promotes β-carotene synthesis and chlorophyll degradation in Golden Delicious apple peel. J. Plant Growth Regul. 1993, 12, 163–167. [Google Scholar] [CrossRef]
- Jung, S. Effect of chlorophyll reduction in Arabidopsis thaliana by methyl jasmonate or norflurazon on antioxidant systems. Plant Physiol. Biochem. 2004, 42, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Villaluenga, C.; Peñas, E.; Ciska, E.; Piskula, M.K.; Kozlowska, H.; Vidal-Valverde, C.; Frias, J. Time dependence of bioactive compounds and antioxidant capacity during germination of different cultivars of broccoli and radish seeds. Food Chem. 2010, 120, 710–716. [Google Scholar] [CrossRef]
- Maldini, M.; Baima, S.; Morelli, G.; Scaccini, C.; Natella, F.A. Liquid Chromatography-Mass Spectrometry approach to study “glucosinoloma” in broccoli sprouts. J. Mass Spectrom. 2012, 47, 1198–1206. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, G.; Pernice, R.; Maggio, A.; De Pascale, S.; Fogliano, V. Glucosinolates profile of Brassica Rapa L. subsp. Sylvestris L. janch. var. esculenta Hort. Food Chem. 2008, 107, 1687–1691. [Google Scholar]
- Bhandari, S.R.; Jo, J.S.; Lee, J.G. Comparison of glucosinolate profiles in different tissues of nine Brassica crops. Molecules 2015, 20, 15827–15841. [Google Scholar] [CrossRef] [PubMed]
- Torres-Contreras, A.M.; Nair, V.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Plants as biofactories: Stress-induced production of chlorogenic acid isomers in potato tubers as affected by wounding intensity and storage time. Ind. Crops Prod. 2014, 62, 61–66. [Google Scholar] [CrossRef]
- Jaiswal, A.K.; Abu-Ghannam, N.; Gupta, S.A. Comparative study on the polyphenolic content, antibacterial activity and antioxidant capacity of different solvent extracts of Brassica oleracea vegetables. Int. J. Food Sci. Technol. 2012, 47, 223–231. [Google Scholar] [CrossRef]
- Ferreres, F.; Sousa, C.; Pereira, D.M.; Valentão, P.; Taveira, M.; Martins, A.; Pereira, J.A.; Seabra, R.M.; Andrade, P.B. Screening of antioxidant phenolic compounds produced by in vitro shoots of Brassica oleracea L. var. costata DC. Comb. Chem. High Throughput Screen. 2009, 12, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Siger, A.; Czubinski, J.; Dwiecki, K.; Kachlicki, P.; Nogala-Kalucka, M. Identification and antioxidant activity of sinapic acid derivatives in Brassica napus L. seed meal extracts. Eur. J. Lipid Sci. Technol. 2013, 115, 1130–1138. [Google Scholar]
- Sun, J.; Xiao, Z.; Lin, L.; Lester, G.E.; Wang, Q.; Harnly, J.M.; Chen, P. Profiling polyphenols in five Brassica species microgreens by UHPLC-PDA-ESI/HRMS. J. Agric. Food Chem. 2013, 61, 10960–10970. [Google Scholar] [CrossRef] [PubMed]

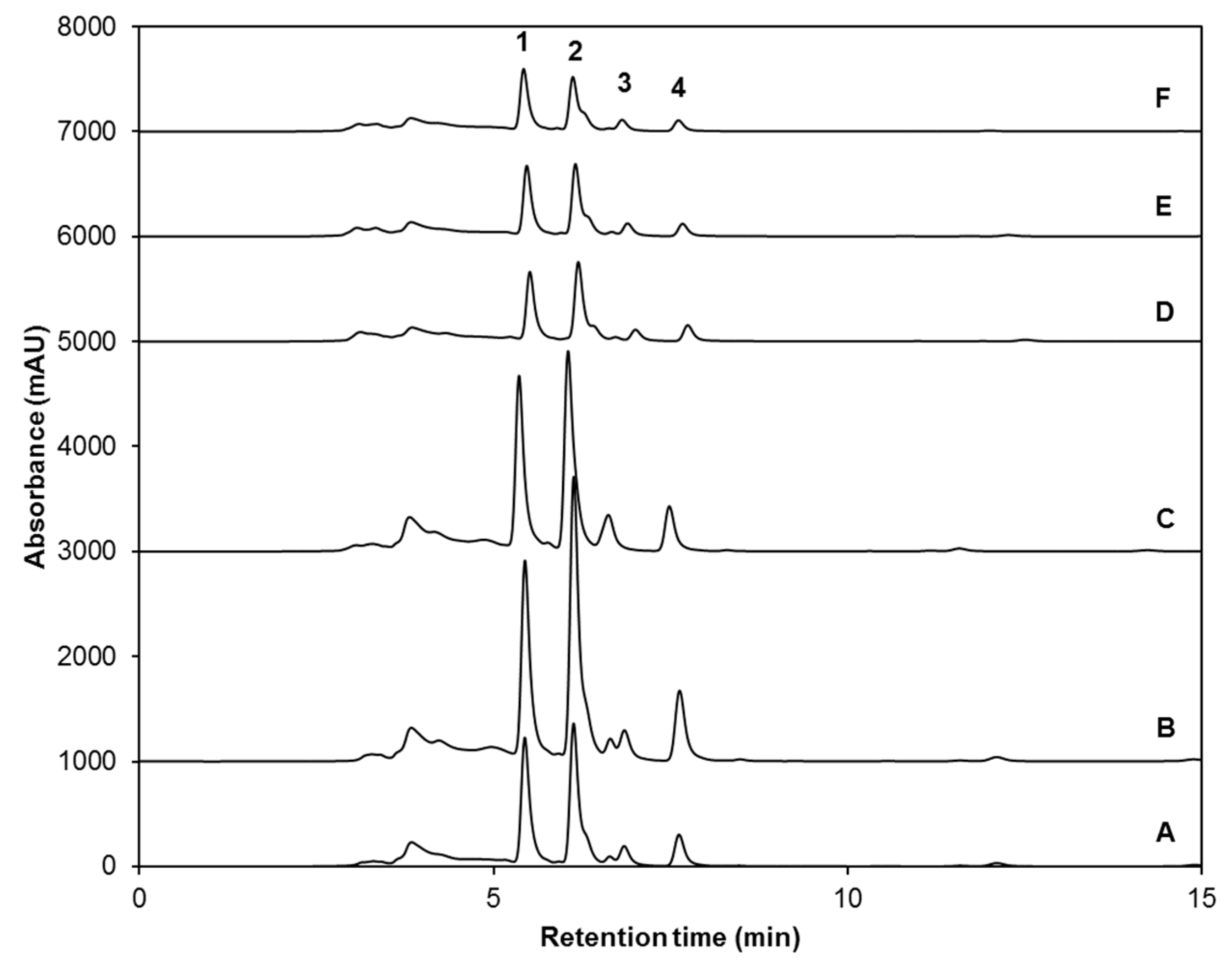

| Peak Number (Retention Time, min) | λmax (nm) | Identification | [M − H]− (m/z) | MS2 (m/z) a |
|---|---|---|---|---|
| 1 (5.3) | 222 | Glucoiberin-dsg | 342 | 179, 131 |
| 2 (5.8) | 224 | Progoitrin-dsg | 308 | 145, 129, 79 |
| 3 (6.6) | 222 | Glucoraphanin-dsg | 356 | 193 |
| 4 (13.6) | 221, 266 | 4-hydroxy-glucobrassicin-dsg | 383 | 221, 203, 153 |
| 5 (17.9) | 210 | Glucoerucin-dsg | 340 | 177, 160, 129, 113 |
| 6 (20.6) | 220, 280 | Glucobrassicin-dsg | 367 | 204, 187, 155, 129 |
| 7 (24.3) | 220, 268 | 4-methoxy-glucobrassicin-dsg | 397 | 234, 204, 154, 139 |
| 8 (30.3) | 222, 290 | Neoglucobrassicion-dsg | 397 | 234, 204, 154, 129 |
| Treatment 4 | Glucosinolate Concentration (mmol/kg DW) 1,2,3 | |||||||||
| GIB | PRO | GRA | 4-HGBS | GER | ||||||
| Control | 2.8 ± 0.4 | b | 0.7 ± 0.1 | a | 13.2 ± 1.4 | bc | 5.4 ± 0.3 | b | 4.1 ± 0.4 | b |
| UVA | 3.2 ± 0.3 | b | 0.6 ± 0.1 | a | 13.8 ± 0.9 | bc | 6.7 ± 1.1 | ab | 3.0 ± 0.3 | c |
| UVB | 5.1 ± 1.2 | a | 0.8 ± 0.1 | a | 23.6 ± 2.1 | a | 7.1 ± 0.4 | a | 6.6 ± 0.4 | a |
| MJ | 2.6 ± 0.2 | b | 0.4 ± 0.0 | b | 13.4 ± 0.6 | bc | 0.5 ± 0.1 | c | 0.3 ± 0.1 | d |
| UVA + MJ | 2.8 ± 0.1 | b | 0.6 ± 0.1 | ab | 11.7 ± 1.0 | c | 1.6 ± 0.2 | c | 0.8 ± 0.1 | d |
| UVB + MJ | 3.2 ± 0.3 | b | 0.8 ± 0.1 | a | 15.5 ± 0.8 | b | 1.3 ± 0.2 | c | 0.9 ± 0.2 | d |
| Treatment 4 | Glucosinolate Concentration (mmol/kg DW) 1,2,3 | |||||||||
| GBS | 4-MGBS | NGBS | TOTAL | |||||||
| Control | 10.3 ± 1.6 | d | 4.6 ± 0.3 | d | 15.1 ± 1.4 | c | 56 ± 5 | d | ||
| UVA | 11.5 ± 0.9 | cd | 3.9 ± 0.2 | d | 10.4 ± 1.5 | c | 53 ± 1 | d | ||
| UVB | 13.4 ± 0.9 | bc | 12.7 ± 0.5 | a | 12.3 ± 2.1 | c | 82 ± 5 | c | ||
| MJ | 15.1 ± 0.8 | ab | 6.4 ± 0.3 | c | 72.1 ± 3.5 | b | 111 ± 5 | b | ||
| UVA + MJ | 17.7 ± 0.3 | a | 10.6 ± 0.6 | b | 96.4 ± 1.5 | a | 142 ± 3 | a | ||
| UVB + MJ | 15.2 ± 0.5 | ab | 9.4 ± 0.6 | b | 92.8 ± 6.1 | a | 139 ± 6 | a | ||
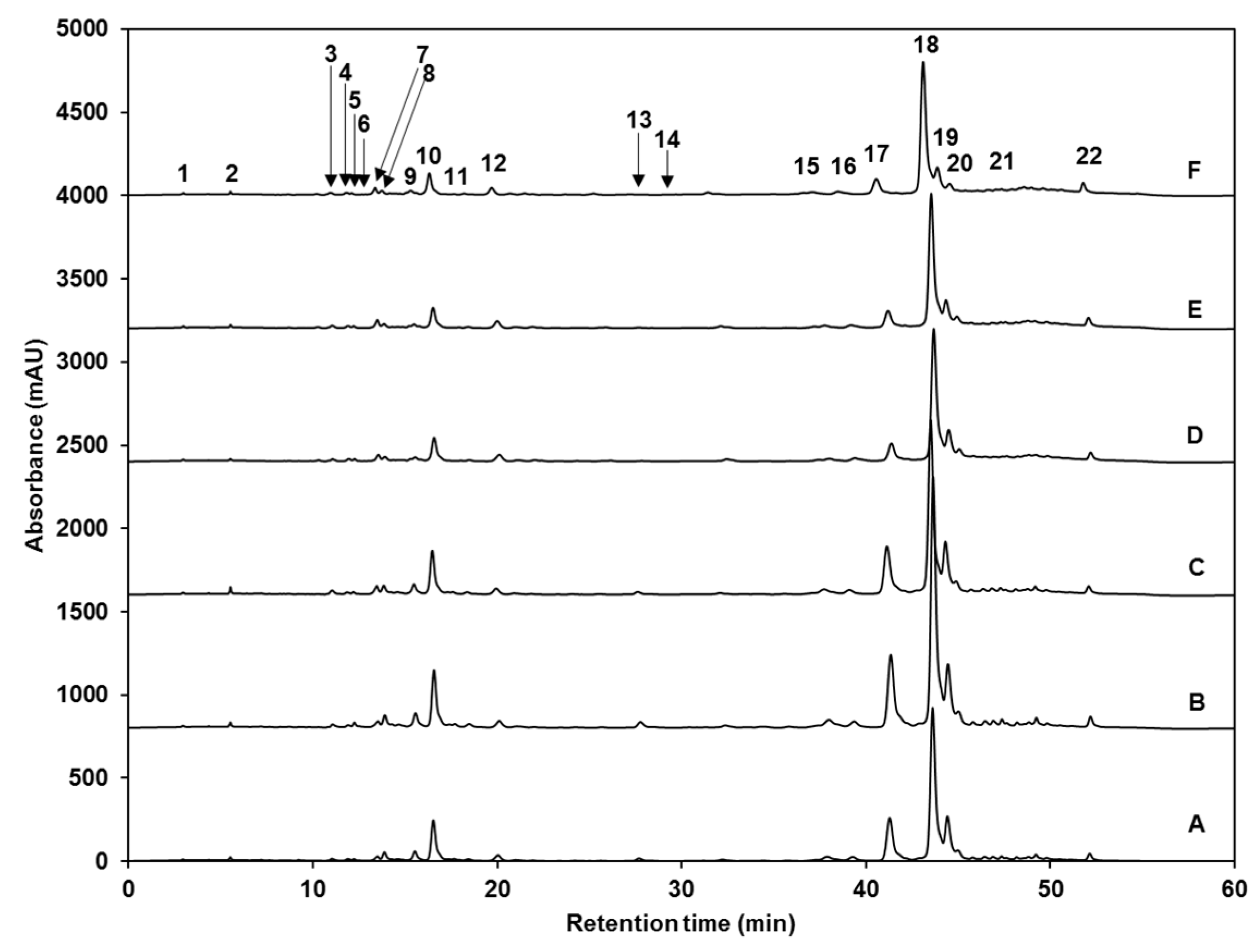
| Peak Number (Retention Time, min) | λmax (nm) | Identification | [M − H]− (m/z) | MS2 (m/z) a |
|---|---|---|---|---|
| 1 (4.2) | 262 | Gallic acid hexoside I | 331 | 162, 125 |
| 2 (6.9) | 210, 300 | Gallotannic acid | 1700 | 1530, 1378, 1225, 1091 |
| 3 (10.7) | 272 | p-hydroxybenzoic acid | 137 | 122, 111, 107 |
| 4 (11.8) | 218, 280 | Gallic acid hexoside II | 331 | 162, 125 |
| 5 (12.2) | 218sh, 326sh | 4-O-caffeoylquinic acid | 353 | 191, 179, 173 |
| 6 (12.7) | 220, 268 | digalloyl hexoside | 483 | 337, 169 |
| 7 (13.6) | 222, 265, 330 | 3-O-hexoside kaempferol | 447 | 285 |
| 8 (14.6) | 220, 268 | Gallic acid derivative | - | - |
| 9 (15.3) | 240sh, 328 | 1-O-sinapoyl-β-d-glucose | 385 | 223, 205, 173, 145 |
| 10 (16.2) | 240sh, 330 | Sinapoyl malate | 339 | 205.6, 173, 147, 132 |
| 11 (17.2) | 228, 330 | 1,2-diferuloylgentiobiose | 693 | 499, 175 |
| 12 (22.5) | 220, 268 | 5-sinapoylquinic acid | 397 | 222, 191 |
| 13 (27. 1) | 235, 324 | Sinapic acid | 223 | 179, 163, 135, 119 |
| 14 (29.3) | 221, 290 | Gallic acid | 169 | 167, 141, 137, 125, 81 |
| 15 (36.2) | 238sh, 270, 330 | Kaempferol 3-O-sinapoyl-sophoroside 7-O-glucoside | 977 | 771, 609, 429, 285 |
| 16 (37.6) | 240sh, 268, 332 | 1,2-disinapoylgentiobiose | 753 | 529, 223 |
| 17 (39.9) | 240sh, 330 | 1-sinapoyl-2′-ferulolylgentiobiose | 723 | 449, 223 |
| 18 (42.4) | 240sh, 328 | 1,2,2′-trisinapoylgentiobiose b | 959 | 735, 223 |
| 19 (43.2) | 240sh, 331 | 1,2-disinapoyl-1′-ferulolylgentiobiose | 929 | 705, 223 |
| 20 (43.9) | 220, 238, 328 | 1,2-disinapoyl-2′-ferulolylgentiobiose | 929 | 705, 223 |
| 21 (46.6) | 242, 326 | 1-sinapoyl-2,2′-diferuloylgentiobiose | 899 | 705, 223 |
| 22 (51.2) | 238sh, 330 | 1,2,2′-trisinapoylgentiobiose b | 959 | 735, 223 |
| Treatment 5 | Phenolic Concentration (mg/kg DW) 1,2,3,4 | |||||||||||||||
| GAH I | GTA | p-HBA | GAH II | 4-O-CQA | diGH | 3-O-H-K | GAD | |||||||||
| Control | 522.6 ± 13.0 | b | 216.6 ± 6.9 | a | 321.9 ± 25.9 | b | 330.6 ± 10.4 | cd | 373.7 ± 58.3 | a | 310.5 ± 6.0 | a | 482.2 ± 26.9 | a | 158.9 ± 14.1 | ab |
| UVA | 577.3 ± 17.0 | a | 218.5 ± 6.5 | a | 302.3 ± 9.5 | bc | 386.9 ± 18.6 | bc | 394.0 ± 61.2 | a | 321.1 ± 25.8 | a | 442.3 ± 24.6 | a | 144.9 ± 7.0 | ab |
| UVB | 532.5 ± 23.6 | b | 224.0 ± 6.8 | a | 296.7 ± 19.3 | bc | 446.6 ± 19.6 | ab | 352.7 ± 43.1 | a | 283.7 ± 10.3 | a | 389.3 ± 5.9 | b | 132.3 ± 4.8 | b |
| MJ | 404.6 ± 9.6 | c | 168.7 ± 4.0 | b | 266.4 ± 4.7 | c | 310.8 ± 30.2 | d | 368.5 ± 15.4 | a | 214.6 ± 17.5 | bc | 216.2 ± 3.8 | c | 165.6 ± 14.0 | a |
| UVA + MJ | 388.0 ± 9.3 | c | 163.5 ± 2.0 | b | 368.1 ± 5.0 | a | 468,0 ± 11.3 | a | 356.5 ± 15.5 | a | 224.9 ± 14.9 | b | 206.6 ± 3.0 | c | 170.2 ± 11.3 | a |
| UVB + MJ | 391.5 ± 10.0 | c | 159.5 ± 3.2 | b | 301.6 ± 15.9 | bc | 414.1 ± 32.5 | ab | 389.6 ± 46.9 | a | 179.1 ± 5.5 | c | 211.2 ± 2.7 | c | 167.0 ± 11.0 | a |
| Treatment 5 | Phenolic Concentration (mg/kg DW) 1,2,3,4 | |||||||||||||||
| 1-O-S-β-d-g | Sinapoyl Malate | 1,2-diFG | 5-SQA | Sinapic Acid | Gallic Acid | K-3-O-S-so-7-O-g | 1,2-diSG | |||||||||
| Control | 559.9 ± 17.4 | b | 2420.7 ± 82.7 | a | 468.6 ± 9.0 | c | 295.6 ± 20.1 | c | 295.2 ± 34.1 | b | 201.9 ± 8.3 | c | 709.6 ± 10.0 | b | 431.8 ± 7.4 | a |
| UVA | 656.3 ± 33.2 | a | 2579.3 ± 169.6 | a | 461.9 ± 18.9 | cd | 212.9 ± 45.5 | cd | 363.4 ± 15.1 | a | 317.4 ± 20.4 | a | 890.0 ± 91.1 | a | 480.3 ± 43.1 | a |
| UVB | 565.8 ± 27.0 | b | 2605.7 ± 174.6 | a | 422.6 ± 13.1 | d | 128.2 ± 16.6 | d | 265.2 ± 24.6 | b | 205.7 ± 17.6 | c | 748.0 ± 25.4 | b | 445.5 ± 20.5 | a |
| MJ | 428.1 ± 10.2 | cd | 1091.8 ± 73.9 | b | 617.7 ± 20.6 | a | 1001.2 ± 65.7 | a | 154.2 ± 1.5 | c | 253.9 ± 15.1 | b | 301.2 ± 14.1 | c | 325.5 ± 4.8 | b |
| UVA + MJ | 454.6 ± 8.1 | c | 1092.8 ± 14.2 | b | 589.2 ± 3.8 | a | 988.5 ± 19.7 | a | 153.2 ± 1.0 | c | 237.0 ± 10.7 | bc | 356.8 ± 27.3 | c | 332.8 ± 9.4 | b |
| UVB + MJ | 383.0 ± 9.8 | d | 1182.3 ± 13.8 | b | 539.9 ± 8.8 | b | 707.8 ± 55.0 | b | 163.0 ± 2.4 | c | 228.3 ± 6.0 | bc | 283.2 ± 3.4 | c | 326.9 ± 2.8 | b |
| Treatment 5 | Phenolic Concentration (mg/kg DW) 1,2,3,4 | |||||||||||||||
| 1-S-2-FG | 1,2,2-triSG 6 | 1,2-diS-1-FG | 1,2-diS-2-FG | 1-S-2,2-diFG | 1,2,2-triSG 6 | TOTAL | ||||||||||
| Control | 3287.4 ± 55.6 | b | 9800.6 ± 207.3 | a | 2501.4 ± 149.7 | a | 457.1 ± 84.3 | a | 239.2 ± 7.5 | ab | 376.5 ± 13.8 | c | 24,762 ± 477 | ab | ||
| UVA | 4060.6 ± 424.5 | a | 11,078.0 ± 1145.9 | a | 2366.8 ± 199.7 | a | 286.8 ± 49.7 | b | 271.9 ± 21.2 | a | 447.8 ± 42.7 | a | 27,261 ± 2218 | a | ||
| UVB | 3440.1 ± 80.9 | b | 9646.5 ± 381.6 | a | 2276.4 ± 134.6 | a | 203.2 ± 7.5 | b | 250.5 ± 12.7 | ab | 388.6 ± 11.0 | bc | 24,250 ± 837 | b | ||
| MJ | 1269.3 ± 19.4 | c | 7815.6 ± 173.6 | b | 1615.4 ± 51.2 | b | 490.9 ± 20.5 | a | 186.8 ± 8.8 | c | 411.9 ± 14.3 | abc | 18,079 ± 272 | c | ||
| UVA + MJ | 1295.3 ± 26.9 | c | 8052.7 ± 144.9 | b | 1510.9 ± 41.1 | b | 474.6 ± 26.7 | a | 178.5 ± 9.9 | c | 410.9 ± 7.5 | abc | 18,474 ± 266 | c | ||
| UVB + MJ | 1210.5 ± 21.5 | c | 7791.3 ± 243.1 | b | 1535.8 ± 20.2 | b | 478.8 ± 16.9 | a | 217.3 ± 21.2 | bc | 444.6 ± 10.8 | ab | 17,706 ± 302 | c | ||
| Peak Number (Retention Time, min) | λmax (nm) | Tentative Identification | Method of Identification a |
|---|---|---|---|
| 1 (5.3) | 422sh, 445, 474 | Lutein | A, B, C |
| 2 (6.1) | 461 | Chlorophyll b | A, B, C |
| 3 (6.9) | 417sh, 441, 470 | Neoxanthin | A, B, C |
| 4 (7.6) | 335sh, 381sh, 413sh, 432 | Chlorophyll a | A, B, C |
| Treatment 4 | Carotenoid/Chlorophyll Concentration (mg/kg DW) 1,2,3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lutein | Chlorophyll b | Neoxanthin | Chlorophyll a | TOTAL | ||||||
| Control | 472.2 ± 22.3 | b | 6615.2 ± 453.1 | b | 116.7 ± 7.5 | b | 1326.6 ± 103.9 | b | 8531 ± 559 | b |
| UVA | 577.9 ± 39.4 | a | 8647.9 ± 803.1 | a | 155.8 ± 10.2 | a | 2216.1 ± 308.6 | a | 11,598 ± 1159 | a |
| UVB | 552.1 ± 28.7 | a | 7547.1 ± 386.2 | ab | 159.5 ± 6.8 | a | 1814.6 ± 157.6 | a | 10,073 ± 569 | ab |
| MJ | 228.8 ± 8.1 | c | 3174.8 ± 183.9 | c | 68.5 ± 5.1 | c | 744.1 ± 35.0 | c | 4216 ± 218 | c |
| UVA + MJ | 235.9 ± 6.5 | c | 2947.4 ± 83.8 | c | 69.2 ± 1.6 | c | 548.0 ± 7.3 | c | 3800 ± 93 | c |
| UVB + MJ | 210.3 ± 4.8 | c | 2259.6 ± 71.1 | c | 58.6 ± 0.6 | c | 457.8 ± 21.2 | c | 2986 ± 93 | c |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreira-Rodríguez, M.; Nair, V.; Benavides, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. UVA, UVB Light, and Methyl Jasmonate, Alone or Combined, Redirect the Biosynthesis of Glucosinolates, Phenolics, Carotenoids, and Chlorophylls in Broccoli Sprouts. Int. J. Mol. Sci. 2017, 18, 2330. https://doi.org/10.3390/ijms18112330
Moreira-Rodríguez M, Nair V, Benavides J, Cisneros-Zevallos L, Jacobo-Velázquez DA. UVA, UVB Light, and Methyl Jasmonate, Alone or Combined, Redirect the Biosynthesis of Glucosinolates, Phenolics, Carotenoids, and Chlorophylls in Broccoli Sprouts. International Journal of Molecular Sciences. 2017; 18(11):2330. https://doi.org/10.3390/ijms18112330
Chicago/Turabian StyleMoreira-Rodríguez, Melissa, Vimal Nair, Jorge Benavides, Luis Cisneros-Zevallos, and Daniel A. Jacobo-Velázquez. 2017. "UVA, UVB Light, and Methyl Jasmonate, Alone or Combined, Redirect the Biosynthesis of Glucosinolates, Phenolics, Carotenoids, and Chlorophylls in Broccoli Sprouts" International Journal of Molecular Sciences 18, no. 11: 2330. https://doi.org/10.3390/ijms18112330