Insulin Production and Resistance in Different Models of Diet-Induced Obesity and Metabolic Syndrome
Abstract
:1. Introduction
2. Results
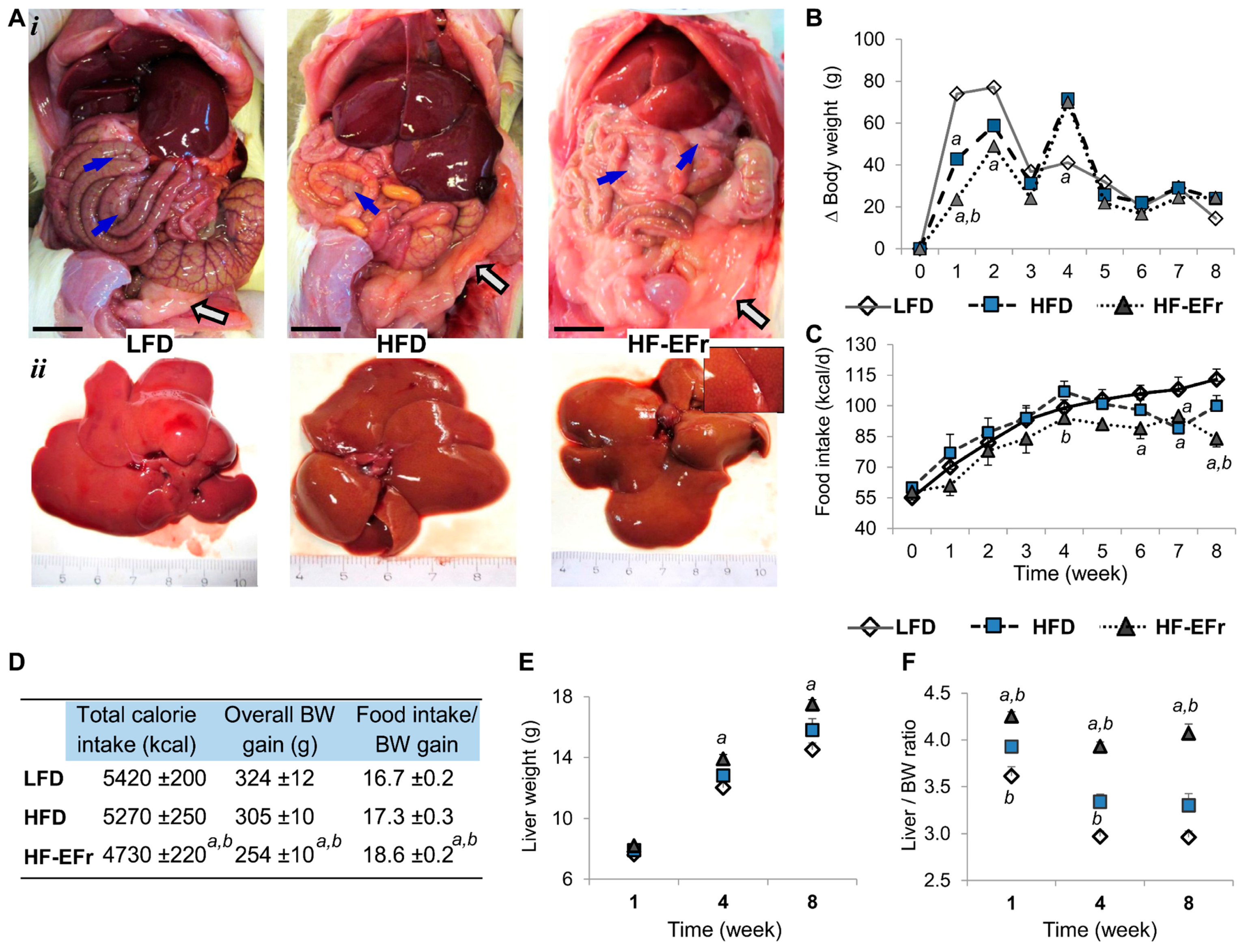
2.1. Chronic Consumption of Ethanol-Plus-Fructose-Enriched-HFD (HF-EFr) Induced Less Weight Gain Than LFD, But Increased Liver Weight and Visceral Fat
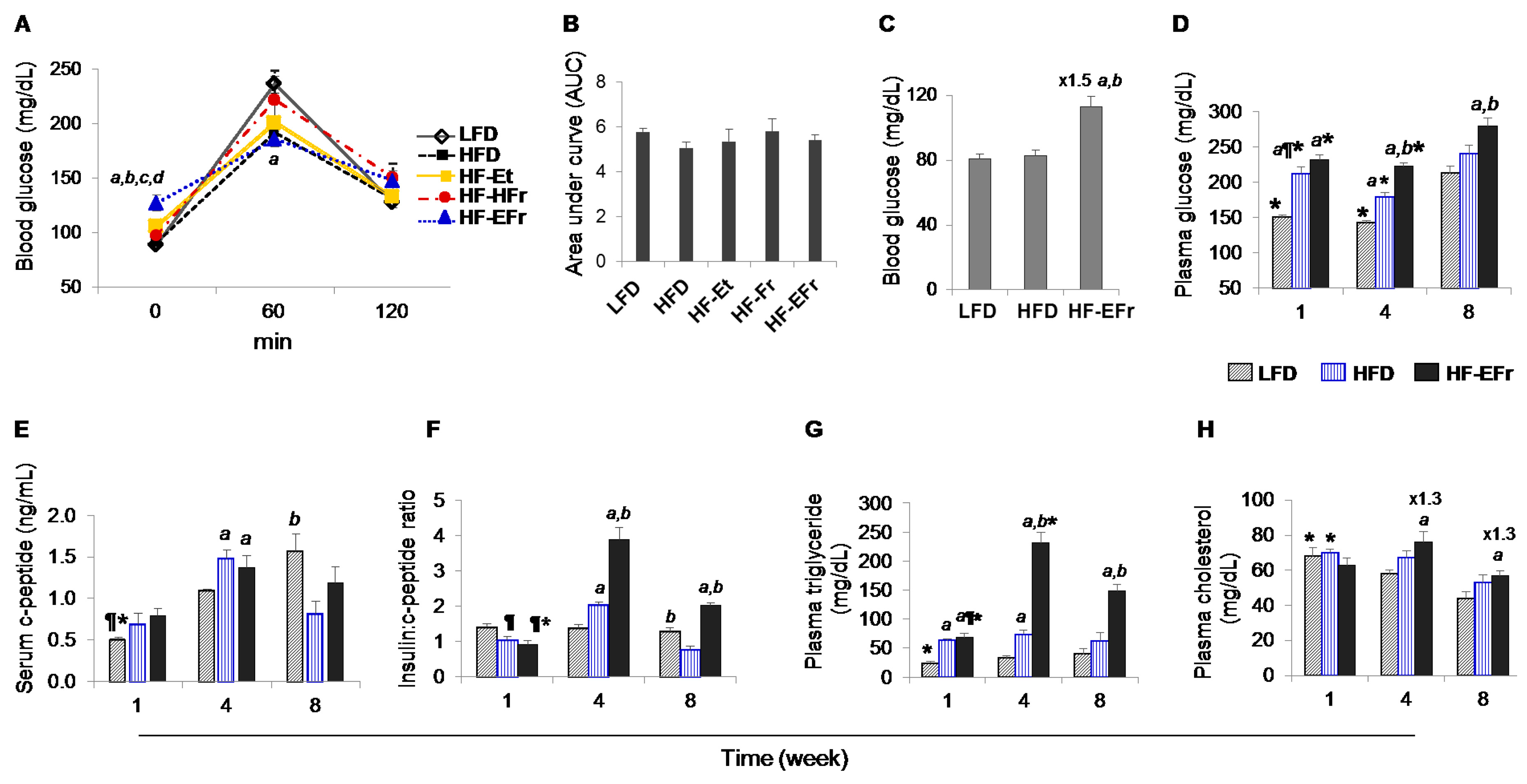
2.2. Effects of Short- and Long-Term Diet Consumption on the Levels of Glucose Homeostasis
2.3. Changes in Metabolic Parameters and Liver Enzymes
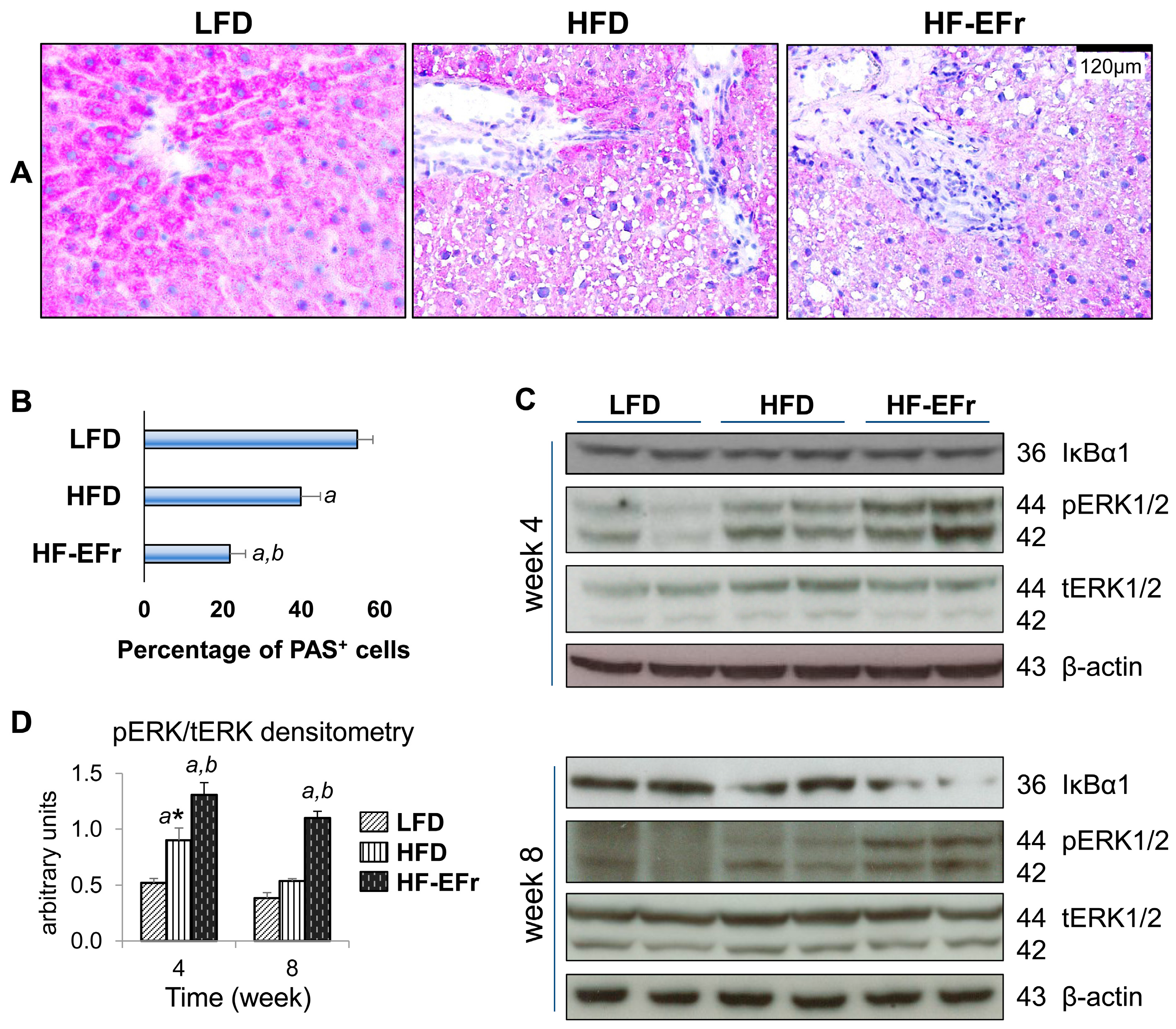
2.4. Histology of the Liver and the Onset of Hepatic Steatosis
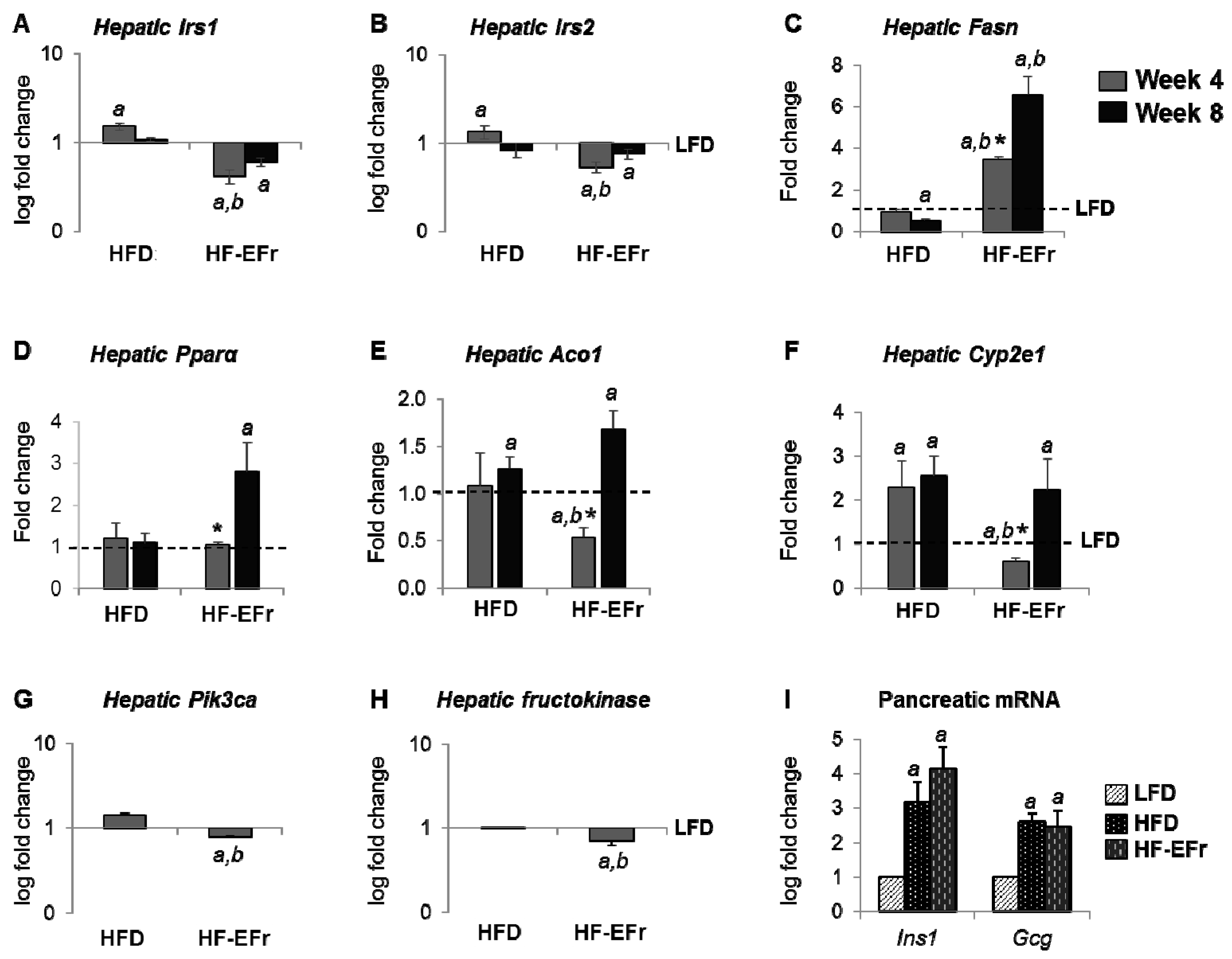
2.5. Alterations of mRNA Expression in the Liver and Pancreas
2.6. Consumption of Alcohol-Plus-Fructose-Containing HFD Progressively Induces an Activation of Extracellular Signal-Regulated Protein Kinases-1 and -2 (ERK1/2)
3. Discussion
3.1. Obesity, Calorie Consumption and Liver Weight
3.2. Consumption of Ethanol-Plus-Fructose and Liver Injury
3.3. MetS, IR and Insulin Clearance
4. Materials and Methods
4.1. Animals
4.2. Induction of Fatty Liver and Animal Sacrifice
4.3. Glucose Tolerance Test and Baseline Fasting Glucose
4.4. Biochemical Analyses
4.5. Radioimmunoassay (RIA)
4.6. Histopathological Studies
4.6.1. H&E Staining
4.6.2. Oil Red O Staining and Determination of Hepatic Lipid Content
4.6.3. Periodic Acid Schiff (PAS)
4.7. Hepatic and Pancreatic RNA Isolation Real-Time Reverse Transcription-PCR
4.8. Western Immunoblots for Hepatic Extracts
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Masarone, M.; Federico, A.; Abenavoli, L.; Loguercio, C.; Persico, M. Non alcoholic fatty liver: epidemiology and natural history. Rev. Recent Clin. Trials 2014, 9, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.A.; Waters, O.R.; Knuiman, M.W.; Elliott, R.R.; Olynyk, J.K. NAFLD as a risk factor for the development of diabetes and the metabolic syndrome: An eleven-year follow-up study. Am. J. Gastroenterol. 2009, 104, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.L.; Leung, J.C.-F.; Loong, T.C.-W.; Wong, G.L.-H.; Yeung, D.K.-W.; Chan, R.S.-M.; Chan, H.L.-Y.; Chim, A.M.-L.; Woo, J.; Chu, W.C.-W.; et al. Prevalence and Severity of Nonalcoholic Fatty Liver Disease in Non-Obese Patients: A Population Study Using Proton-Magnetic Resonance Spectroscopy. Am. J. Gastroenterol. 2015, 110, 1306–1314. [Google Scholar] [CrossRef] [PubMed]
- Leite, N.C.; Salles, G.F.; Araujo, A.L.E.; Villela-Nogueira, C.A.; Cardoso, C.R.L. Prevalence and associated factors of non-alcoholic fatty liver disease in patients with type-2 diabetes mellitus. Liver Int. 2009, 29, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Newton, K.P.; Hou, J.; Crimmins, N.A.; Lavine, J.E.; Barlow, S.E.; Xanthakos, S.A.; Africa, J.; Behling, C.; Donithan, M.; Clark, J.M.; et al. Prevalence of Prediabetes and Type 2 Diabetes in Children With Nonalcoholic Fatty Liver Disease. JAMA Pediatr. 2016, 170, e161971. [Google Scholar] [CrossRef] [PubMed]
- Liangpunsakul, S.; Chalasani, N. What should we recommend to our patients with NAFLD regarding alcohol use? Am. J. Gastroenterol. 2012, 107, 976–978. [Google Scholar] [CrossRef]
- Arslanian, S.A.; Saad, R.; Lewy, V.; Danadian, K.; Janosky, J. Hyperinsulinemia in african-american children: decreased insulin clearance and increased insulin secretion and its relationship to insulin sensitivity. Diabetes 2002, 51, 3014–3019. [Google Scholar] [CrossRef] [PubMed]
- Svedberg, J.; Björntorp, P.; Smith, U.; Lönnroth, P. Free-fatty acid inhibition of insulin binding, degradation, and action in isolated rat hepatocytes. Diabetes 1990, 39, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Kotronen, A.; Juurinen, L.; Tiikkainen, M.; Vehkavaara, S.; Yki-Järvinen, H. Increased Liver Fat, Impaired Insulin Clearance, and Hepatic and Adipose Tissue Insulin Resistance in Type 2 Diabetes. Gastroenterology 2008, 135, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.N.; Pei, D.; Staris, P.; Polonsky, K.S.; Chen, Y.D.; Reaven, G.M. Alterations in the glucose-stimulated insulin secretory dose-response curve and in insulin clearance in nondiabetic insulin-resistant individuals. J. Clin. Endocrinol. Metab. 1997, 82, 1834–1838. [Google Scholar] [CrossRef] [PubMed]
- Duckworth, W.C.; Bennett, R.G.; Hamel, F.G.; Affairs, V. Insulin Degradation: Progress and Potential 1. Endocr. Rev. 1998, 19, 608–624. [Google Scholar]
- Miyaaki, H.; Ichikawa, T.; Taura, N.; Miuma, S.; Honda, T.; Shibata, H.; Toriyama, K.; Nakao, K. Significance of Hepatic Insulin Clearance in Patients with Chronic Hepatitis C and Non-alcoholic Fatty Liver Disease. Intern. Med. 2016, 55, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Camporez, J.P.G.; Kanda, S.; Petersen, M.C.; Jornayvaz, F.R.; Samuel, V.T.; Bhanot, S.; Petersen, K.F.; Jurczak, M.J.; Shulman, G.I. ApoA5 knockdown improves whole-body insulin sensitivity in high-fat-fed mice by reducing ectopic lipid content. J. Lipid Res. 2015, 56, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Kamon, J.; Minokoshi, Y.; Ito, Y.; Waki, H.; Uchida, S.; Yamashita, S.; Noda, M.; Kita, S.; Ueki, K.; et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 2002, 8, 1288–1295. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Hamel, F.G.; Siford, G.; Duckworth, W.C. Alteration of rat hepatic insulin metabolism by glyburide and glipizide. J. Pharmacol. Exp. Ther. 1993, 264, 1293–1298. [Google Scholar] [PubMed]
- Tiikkainen, M.; Häkkinen, A.-M.; Korsheninnikova, E.; Nyman, T.; Mäkimattila, S.; Yki-Järvinen, H. Effects of rosiglitazone and metformin on liver fat content, hepatic insulin resistance, insulin clearance, and gene expression in adipose tissue in patients with type 2 diabetes. Diabetes 2004, 53, 2169–2176. [Google Scholar] [CrossRef] [PubMed]
- García-Monzón, C.; Lo Iacono, O.; Mayoral, R.; González-Rodríguez, A.; Miquilena-Colina, M.E.; Lozano-Rodríguez, T.; García-Pozo, L.; Vargas-Castrillón, J.; Casado, M.; Boscá, L.; et al. Hepatic insulin resistance is associated with increased apoptosis and fibrogenesis in nonalcoholic steatohepatitis and chronic hepatitis C. J. Hepatol. 2011, 54, 142–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basu, S.; Yoffe, P.; Hills, N.; Lustig, R.H. The relationship of sugar to population-level diabetes prevalence: An econometric analysis of repeated cross-sectional data. PLoS ONE 2013, 8, e57873. [Google Scholar] [CrossRef] [PubMed]
- Lozano, I.; Van der Werf, R.; Bietiger, W.; Seyfritz, E.; Peronet, C.; Pinget, M.; Jeandidier, N.; Maillard, E.; Marchioni, E.; Sigrist, S.; et al. High-fructose and high-fat diet-induced disorders in rats: Impact on diabetes risk, hepatic and vascular complications. Nutr. Metab. 2016, 13, 15. [Google Scholar] [CrossRef] [PubMed]
- Lê, K.A.; Ith, M.; Kreis, R.; Faeh, D.; Bortolotti, M.; Tran, C.; Boesch, C.; Tappy, L. Fructose overconsumption causes dyslipidemia and ectopic lipid deposition in healthy subjects with and without a family history of type 2 diabetes. Am. J. Clin. Nutr. 2009, 89, 1760–1765. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Giovannucci, E.; Pischon, T.; Hankinson, S.E.; Ma, J.; Rifai, N.; Rimm, E.B. Fructose, glycemic load, and quantity and quality of carbohydrate in relation to plasma C-peptide concentrations in US women. Am. J. Clin. Nutr. 2004, 80, 1043–1049. [Google Scholar] [PubMed]
- Landmann, M.; Wagnerberger, S.; Kanuri, G.; Ziegenhardt, D.; Bergheim, I. Beer Is Less Harmful for the Liver than Plain Ethanol: Studies in Male Mice Using a Binge-Drinking Model. Alcohol Alcohol. 2015, 50, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Godos, J.; Federico, A.; Dallio, M.; Scazzina, F. Mediterranean diet and nonalcoholic fatty liver disease: Molecular mechanisms of protection. Int. J. Food Sci. Nutr. 2016. [Google Scholar] [CrossRef] [PubMed]
- Boesch, C.; Elsing, C.; Wegmüller, H.; Felblinger, J.; Vock, P.; Reichen, J. Effect of ethanol and fructose on liver metabolism a dynamic 31Phosphorus magnetic resonance spectroscopy study in normal volunteers. Magn. Reson. Imaging 1997, 15, 1067–1077. [Google Scholar] [CrossRef]
- Uzuegbu, U.E.; Onyesom, I. Fructose-induced increase in ethanol metabolism and the risk of Syndrome X in man. C. R. Biol. 2009, 332, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Chen, T.; Prough, R.A.; Cave, M.C.; McClain, C.J. Chronic Alcohol Consumption Causes Liver Injury in High-Fructose-Fed Male Mice Through Enhanced Hepatic Inflammatory Response. Alcohol. Clin. Exp. Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Shulga, N.; Pastorino, J.G. Mitoneet mediates TNFα-induced necroptosis promoted by exposure to fructose and ethanol. J. Cell Sci. 2014, 127, 896–907. [Google Scholar] [CrossRef] [PubMed]
- Alwahsh, S.M.; Xu, M.; Schultze, F.C.; Wilting, J.; Mihm, S.; Raddatz, D.; Ramadori, G. Combination of alcohol and fructose exacerbates metabolic imbalance in terms of hepatic damage, dyslipidemia, and insulin resistance in rats. PLoS ONE 2014, 9, e104220. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Luben, R.; Khaw, K.-T.; Bingham, S.; Wareham, N.J.; Forouhi, N.G. Dietary energy density predicts the risk of incident type 2 diabetes: the European Prospective Investigation of Cancer (EPIC)-Norfolk Study. Diabetes Care 2008, 31, 2120–2125. [Google Scholar] [CrossRef] [PubMed]
- Sideri, A.; Stavrakis, D.; Bowe, C.; Shih, D.Q.; Fleshner, P.; Arsenescu, V.; Arsenescu, R.; Turner, J.R.; Pothoulakis, C.; Karagiannides, I. Effects of obesity on severity of colitis and cytokine expression in mouse mesenteric fat. Potential role of adiponectin receptor 1. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, G591–G604. [Google Scholar] [CrossRef] [PubMed]
- Stanhope, K.L.; Schwarz, J.M.; Keim, N.L.; Griffen, S.C.; Bremer, A.A.; Graham, J.L.; Hatcher, B.; Cox, C.L.; Dyachenko, A.; Zhang, W.; et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J. Clin. Investig. 2009, 119, 1322–1334. [Google Scholar] [CrossRef] [PubMed]
- Fabbrini, E.; Sullivan, S.; Klein, S. Obesity and nonalcoholic fatty liver disease: Biochemical, metabolic, and clinical implications. Hepatology 2010, 51, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Lieber, C.S.; Leo, M.A.; Mak, K.M.; Decarli, L.M.; Sato, S. Choline fails to prevent liver fibrosis in ethanol-fed baboons but causes toxicity. Hepatology 1985, 5, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Miyakawa, H.; Iida, S.; Leo, M.; Greenstein, R.; Zimmon, D.; Lieber, C. Citation Pathogenesis of precirrhotic portal hypertension in alcohol-fed baboons. Gastroenterology 1985, 88, 143–150. [Google Scholar] [CrossRef]
- Lieber, C.S.; Decarli, L.M.; Sorrell, M.F. Experimental methods of ethanol administration. Hepatology 1989, 10, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Lieber, C.S.; DeCarli, L.M. Liquid diet technique of ethanol administration: 1989 update. Alcohol Alcohol. 1989, 24, 197–211. [Google Scholar] [PubMed]
- Leeman, R.F.; Heilig, M.; Cunningham, C.L.; Stephens, D.N.; Duka, T.; O’Malley, S.S. Ethanol Consumption: How Should We Measure It? Achieving Consilience between Human and Animal Phenotypes. Addict. Biol. 2010, 15, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Sellmann, C.; Priebs, J.; Landmann, M.; Degen, C.; Engstler, A.J.; Jin, C.J.; Gärttner, S.; Spruss, A.; Huber, O.; Bergheim, I. Diets rich in fructose, fat or fructose and fat alter intestinal barrier function and lead to the development of nonalcoholic fatty liver disease over time. J. Nutr. Biochem. 2015, 26, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Louvet, A.; Mathurin, P. Alcoholic liver disease: mechanisms of injury and targeted treatment. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Nakamuta, M.; Kohjima, M.; Morizono, S.; Kotoh, K.; Yoshimoto, T.; Miyagi, I.; Enjoji, M. Evaluation of fatty acid metabolism-related gene expression in nonalcoholic fatty liver disease. Int. J. Mol. Med. 2005, 16, 631–635. [Google Scholar] [PubMed]
- McCullough, A.J. Pathophysiology of nonalcoholic steatohepatitis. J. Clin. Gastroenterol. 2006, 40, S17–S29. [Google Scholar] [PubMed]
- Alwahsh, S.M.; Gebhardt, R. Dietary fructose as a risk factor for non-alcoholic fatty liver disease (NAFLD). Arch. Toxicol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Ishimoto, T.; Lanaspa, M.A.; Rivard, C.J.; Roncal-Jimenez, C.A.; Orlicky, D.J.; Cicerchi, C.; Mcmahan, R.H.; Abdelmalek, M.F.; Rosen, H.R.; Jackman, M.R.; et al. High-fat and high-sucrose (western) diet induces steatohepatitis that is dependent on fructokinase. Hepatology 2013, 58, 1632–1643. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Pan, B.; Liu, L.; Huang, X.; Liu, Z.; Tian, J. Alcohol exposure increases the expression of cardiac transcription factors through ERK1/2-mediated histone3 hyperacetylation in H9C2 cells. Biochem. Biophys. Res. Commun. 2015, 466, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Jiao, P.; Feng, B.; Li, Y.; He, Q.; Xu, H. Hepatic ERK activity plays a role in energy metabolism. Mol. Cell. Endocrinol. 2013, 375, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Weng, L.P.; Smith, W.M.; Brown, J.L.; Eng, C. PTEN inhibits insulin-stimulated MEK/MAPK activation and cell growth by blocking IRS-1 phosphorylation and IRS-1/Grb-2/Sos complex formation in a breast cancer model. Hum. Mol. Genet. 2001, 10, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, N.; Maurya, C.K.; Pandey, J.; Rai, A.K.; Tamrakar, A.K. Fructose-induced ROS generation impairs glucose utilization in L6 skeletal muscle cells. Free Radic. Res. 2015, 5762, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hirosumi, J.; Tuncman, G.; Chang, L.; Görgün, C.Z.; Uysal, K.T.; Maeda, K.; Karin, M.; Hotamisligil, G.S. A central role for JNK in obesity and insulin resistance. Nature 2002, 420, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Lanaspa, M.A.; Ishimoto, T.; Li, N.; Cicerchi, C.; Orlicky, D.J.; Ruzycki, P.; Ruzicky, P.; Rivard, C.; Inaba, S.; Roncal-Jimenez, C.A.; et al. Endogenous fructose production and metabolism in the liver contributes to the development of metabolic syndrome. Nat. Commun. 2013, 4, 2434. [Google Scholar] [PubMed]
- Commerford, S.R.; Bizeau, M.E.; McRae, H.; Jampolis, A.; Thresher, J.S.; Pagliassotti, M.J. Hyperglycemia compensates for diet-induced insulin resistance in liver and skeletal muscle of rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 281, R1380–R1389. [Google Scholar] [PubMed]
- Castro, M.C.; Francini, F.; Schinella, G.; Caldiz, C.I.; Zubiría, M.G.; Gagliardino, J.J.; Massa, M.L. Apocynin administration prevents the changes induced by a fructose-rich diet on rat liver metabolism and the antioxidant system. Clin. Sci. 2012, 123, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Spruss, A.; Henkel, J.; Kanuri, G.; Blank, D.; Püschel, G.P.; Bischoff, S.C.; Bergheim, I. Female mice are more susceptible to nonalcoholic fatty liver disease: sex-specific regulation of the hepatic AMP-activated protein kinase-plasminogen activator inhibitor 1 cascade, but not the hepatic endotoxin response. Mol. Med. 2012, 18, 1346–1355. [Google Scholar] [CrossRef] [PubMed]
- Jaspan, J.; Polonsky, K. Glucose Ingestion in Dogs Alters the Hepatic Extraction of Insulin. J. Clin. Investig. 1982, 69, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Flier, J.S.; Minaker, K.L.; Landsberg, L.; Young, J.B.; Pallotta, J.; Rowe, J.W. Impaired in vivo insulin clearance in patients with severe target-cell resistance to insulin. Diabetes 1982, 31, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Sookoian, S.; Pirola, C.J. How Safe Is Moderate Alcohol Consumption in Overweight and Obese Individuals? Gastroenterology 2016, 150, 1698–1703. [Google Scholar] [CrossRef]
- Joosten, M.M.; Beulens, J.W.J.; Kersten, S.; Hendriks, H.F.J. Moderate alcohol consumption increases insulin sensitivity and ADIPOQ expression in postmenopausal women: A randomised, crossover trial. Diabetologia 2008, 51, 1375–1381. [Google Scholar] [CrossRef] [PubMed]
- Kanuri, G.; Landmann, M.; Priebs, J.; Spruss, A.; Löscher, M.; Ziegenhardt, D.; Röhl, C.; Degen, C.; Bergheim, I. Moderate alcohol consumption diminishes the development of non-alcoholic fatty liver disease (NAFLD) in ob/ob mice. Eur. J. Nutr. 2016, 55, 1153–1164. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.H.; Choi, K.-M.; Liu, Q.; Kim, S.B.; Ji, H.-J.; Kim, M.; Shin, S.-K.; Do, S.-G.; Shin, E.; Jung, G.; et al. Anti-Obesity Effect of 6,8-Diprenylgenistein, an Isoflavonoid of Cudrania tricuspidata Fruits in High-Fat Diet-Induced Obese Mice. Nutrients 2015, 7, 10480–10490. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.M.; El Denshary, E.S.; Abdallah, D.M.; Popkin, B.; Malik, V.; Popkin, B.; Bray, G.; Despres, J.; Willett, W.; Grundy, S.; et al. Geraniol, Alone and in Combination with Pioglitazone, Ameliorates Fructose-Induced Metabolic Syndrome in Rats via the Modulation of Both Inflammatory and Oxidative Stress Status. PLoS ONE 2015, 10, e0117516. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Sebastian, B.M.; Tang, H.; McMullen, M.M.; Axhemi, A.; Jacobsen, D.W.; Nagy, L.E. Taurine supplementation prevents ethanol-induced decrease in serum adiponectin and reduces hepatic steatosis in rats. Hepatology 2009, 49, 1554–1562. [Google Scholar] [CrossRef] [PubMed]
- Papamiltiadous, E.S.; Roberts, S.K.; Nicoll, A.J.; Ryan, M.C.; Itsiopoulos, C.; Salim, A.; Tierney, A.C. A randomised controlled trial of a Mediterranean Dietary Intervention for Adults with Non Alcoholic Fatty Liver Disease (MEDINA): Study protocol. BMC Gastroenterol. 2016, 16, 14. [Google Scholar] [CrossRef] [PubMed]
- Cakir, M.; Akbulut, U.E.; Okten, A. Association between Adherence to the Mediterranean Diet and Presence of Nonalcoholic Fatty Liver Disease in Children. Child. Obes. 2016, 12, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Heikkinen, S.; Argmann, C.A.; Champy, M.-F.; Auwerx, J. Evaluation of glucose homeostasis. Curr. Protoc. Mol. Biol. 2007. [Google Scholar] [CrossRef]
- Xu, M.; Alwahsh, S.M.; Ramadori, G.; Kollmar, O.; Slotta, J.E. Upregulation of hepatic melanocortin 4 receptor during rat liver regeneration. J. Surg. Res. 2016, 203, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Alwahsh, S.M.; Xu, M.; Seyhan, H.A.; Ahmad, S.; Mihm, S.; Ramadori, G.; Schultze, F.C. Diet high in fructose leads to an overexpression of lipocalin-2 in rat fatty liver. World J. Gastroenterol. 2014, 20, 1807–1821. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Ulusoy, E.; Eren, B. Histological changes of liver glycogen storage in mice (Mus musculus) caused by high-protein diets. Histol. Histopathol. 2006, 21, 925–930. [Google Scholar] [PubMed]
- Gotoh, M.; Maki, T.; Kiyoizumi, T.; Satomi, S.; Monaco, A.P. An improved method for isolation of mouse pancreatic islets. Transplantation 1985, 40, 437–438. [Google Scholar] [CrossRef] [PubMed]


| Components | HFD | HF-EFr | ||
|---|---|---|---|---|
| Weight (g) | Energy (%) | Weight (g) | Energy (%) | |
| LDC | 132 | 637 | 83 | 400 |
| Maltose dextrin | 90 | 360 | 0 | |
| Ethanol | 0 | 42 | 299 | |
| Fructose | 0 | 77 | 308 | |
| H2O | 790 | 798 | ||
| Total | 1012 | 997 | 1000 | 1007 |
| Parameters | Time Point | LFD | HFD | HF-EFr |
|---|---|---|---|---|
| Protein (g/dL) | Week 1 | 5.3 ± 0.1 ¶,* | 5.5 ± 0.1 | 5.4 ± 0.1 * |
| Week 4 | 5.9 ± 0.1 | 5.8 ± 0.1 | 5.9 ± 0.1 | |
| Week 8 | 5.7 ± 0.0 | 5.9 ± 0.2 | 5.7 ± 0.1 | |
| Albumin (g/dL) | Week 1 | 3.91 ± 0.01 | 3.92 ± 0.01 | 3.80 ± 0.02 *,a,b |
| Week 4 | 3.93 ± 0.01 | 3.83 ± 0.02 | 4.08 ± 0.08 * | |
| Week 8 | 3.90 ± 0.07 | 3.84 ± 0.06 | 3.72 ± 0.03 a,b | |
| ALT (U/L) | Week 1 | 44 ± 3 | 36 ± 1 | 32 ± 2 ¶,a |
| Week 4 | 50 ± 2 | 39 ± 1 a | 69 ± 3 a,b | |
| Week 8 | 43 ± 2 | 36 ± 3 | 57 ± 2 a,b | |
| AST (U/L) | Week 1 | 58 ± 1 * | 61 ± 2 | 56 ± 1 ¶,* |
| Week 4 | 73 ± 4 | 70 ± 2 | 87 ± 1 a,b | |
| Week 8 | 76 ± 2 | 63 ± 1 | 85 ± 2 b | |
| Creatinine (mg/dL) | Week 1 | 0.18 ± 0.02 ¶,* | 0.17 ± 0.00 ¶,* | 0.15 ± 0.01 ¶,* |
| Week 4 | 0.27 ± 0.01 * | 0.25 ± 0.00 * | 0.24 ± 0.02 | |
| Week 8 | 0.31 ± 0.02 | 0.29 ± 0.01 | 0.27 ± 0.02 | |
| HOMA-IR | Week 1 | 0.61 ± 0.02 * | 0.72 ± 0.10 ¶ | 0.88 ± 0.03 ¶,*,a |
| Week 4 | 0.71 ± 0.08 * | 2.85 ± 0.05 *,a | 6.31 ± 0.75 *,a,b | |
| Week 8 | 2.01 ± 0.13 | 0.78 ± 0.12 a | 3.70 ± 0.35 a,b | |
| QUICKI | Week 1 | 0.42 ± 0.01 * | 0.41 ± 0.01 ¶ | 0.39 ± 0.00 ¶,*,a |
| Week 4 | 0.41 ± 0.01 * | 0.33 ± 0.00 *a | 0.29 ± 0.01 *,a,b | |
| Week 8 | 0.34 ± 0.01 | 0.41 ± 0.01 a | 0.32 ± 0.00 a,b | |
| Pancreatic amylase | Week 1 | 1748 ± 10 * | 1955 ± 89 | 2003 ± 59 * |
| Week 4 | 1630 ± 49 | 1971 ± 80 a | 2125 ± 40 *,a | |
| Week 8 | 1482 ± 60 | 1695 ± 79 | 1774 ± 27 a | |
| Leptin (ng/mL) | Week 1 | 2.0 ± 0.0 * | 4.0 ± 0.7 a | 4.0 ± 0.7 *,a |
| Week 4 | 2.0 ± 0.2 * | 3.0 ± 0.1 a | 3.1 ± 0.3 *,a | |
| Week 8 | 3.6 ± 0.6 | 3.8 ± 0.6 | 9.1 ± 1.4 a,b |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alwahsh, S.M.; Dwyer, B.J.; Forbes, S.; Van Thiel, D.H.; Starkey Lewis, P.J.; Ramadori, G. Insulin Production and Resistance in Different Models of Diet-Induced Obesity and Metabolic Syndrome. Int. J. Mol. Sci. 2017, 18, 285. https://doi.org/10.3390/ijms18020285
Alwahsh SM, Dwyer BJ, Forbes S, Van Thiel DH, Starkey Lewis PJ, Ramadori G. Insulin Production and Resistance in Different Models of Diet-Induced Obesity and Metabolic Syndrome. International Journal of Molecular Sciences. 2017; 18(2):285. https://doi.org/10.3390/ijms18020285
Chicago/Turabian StyleAlwahsh, Salamah M., Benjamin J. Dwyer, Shareen Forbes, David H. Van Thiel, Philip J. Starkey Lewis, and Giuliano Ramadori. 2017. "Insulin Production and Resistance in Different Models of Diet-Induced Obesity and Metabolic Syndrome" International Journal of Molecular Sciences 18, no. 2: 285. https://doi.org/10.3390/ijms18020285





