Decreased Expression of Vitamin D Receptor Affects an Immune Response in Primary Biliary Cholangitis via the VDR-miRNA155-SOCS1 Pathway
Abstract
:1. Introduction
2. Results
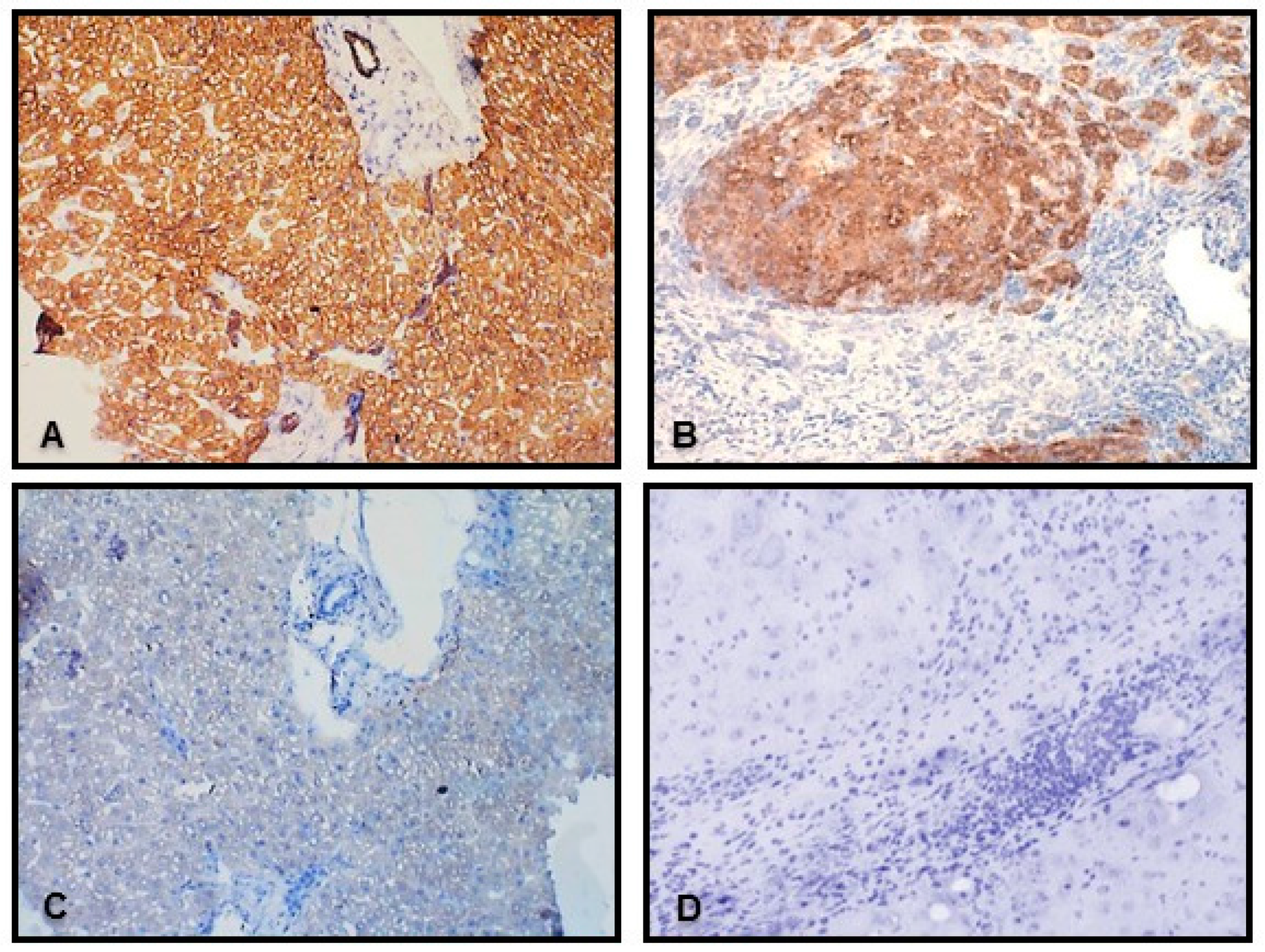
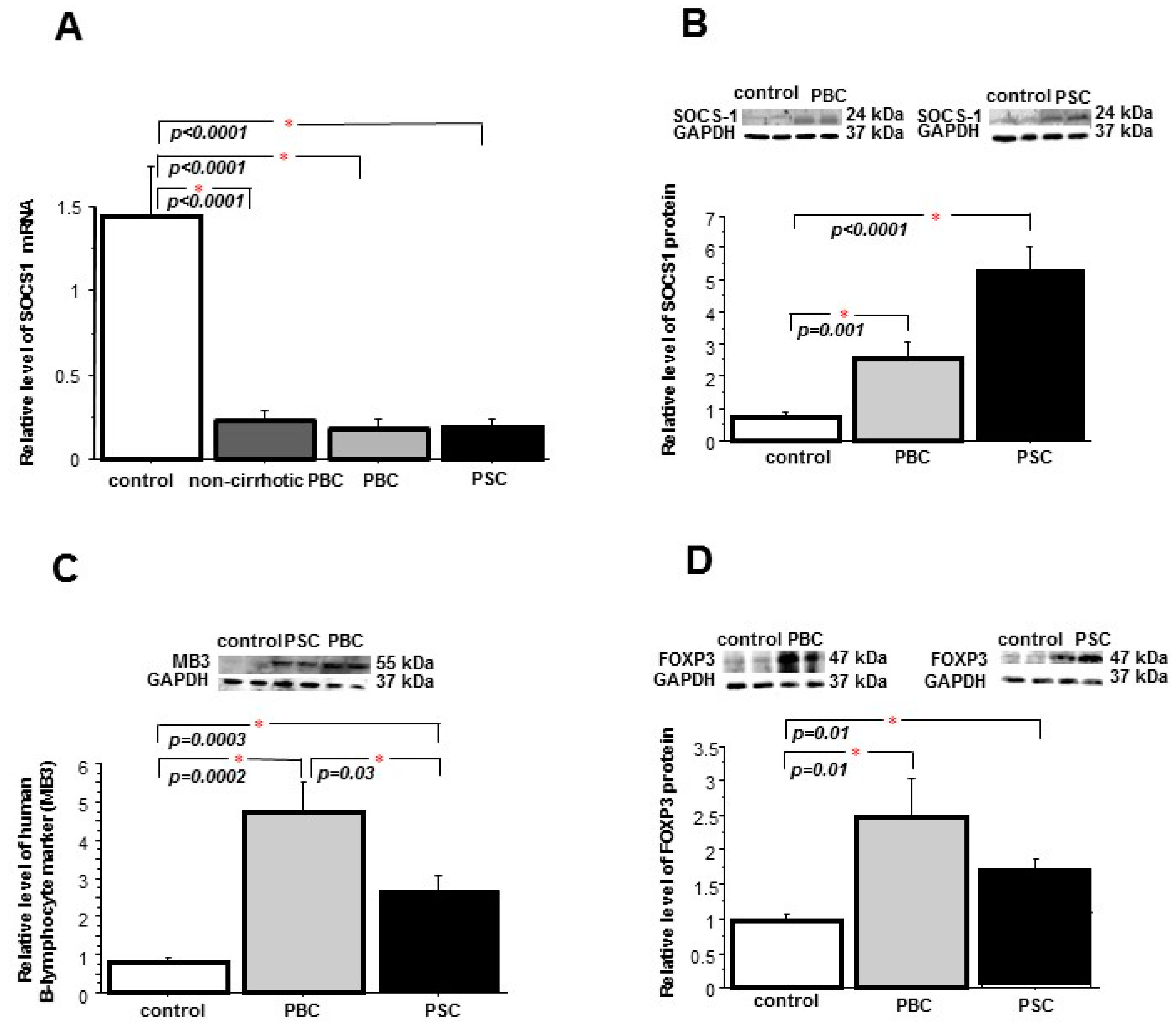
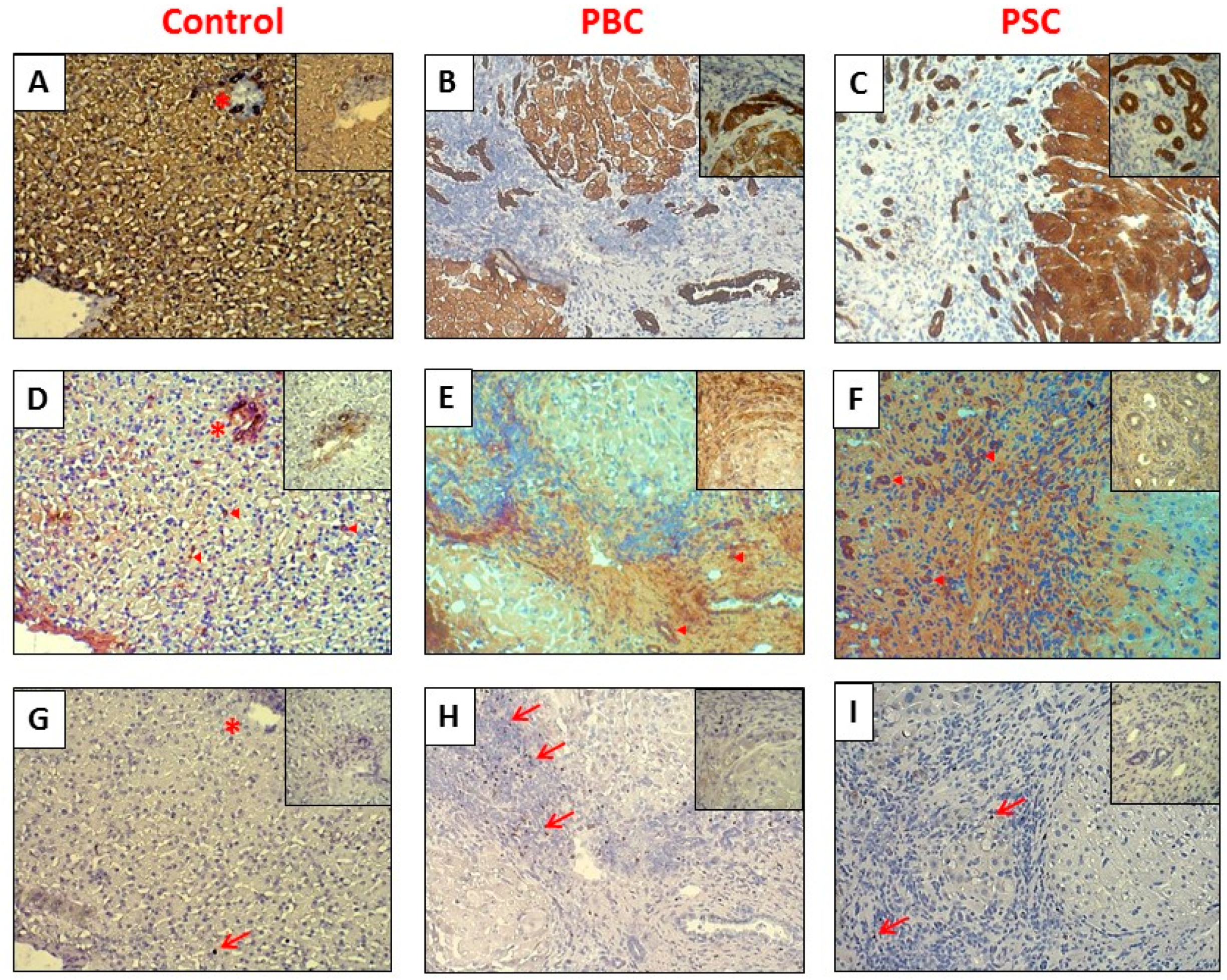
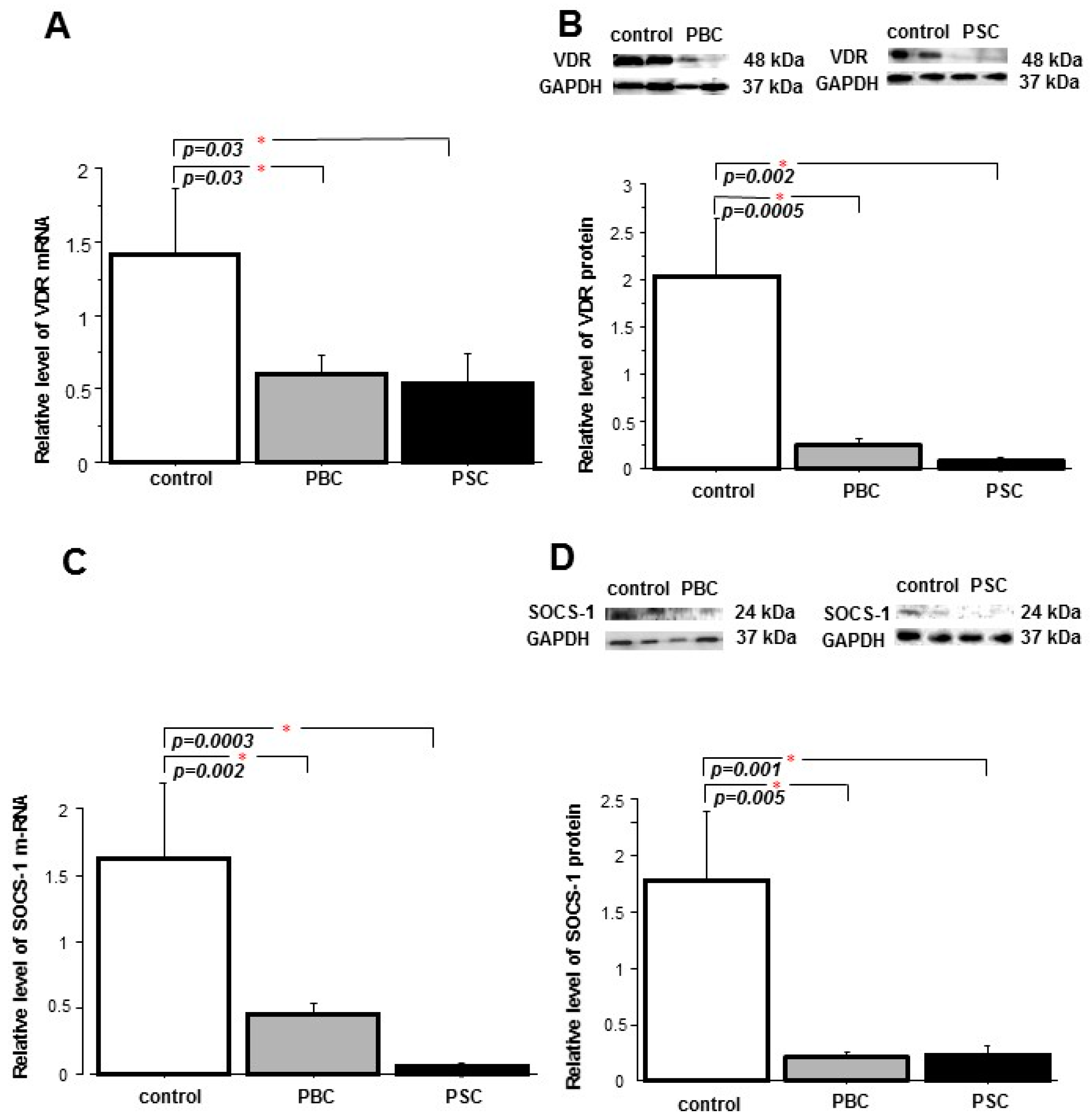
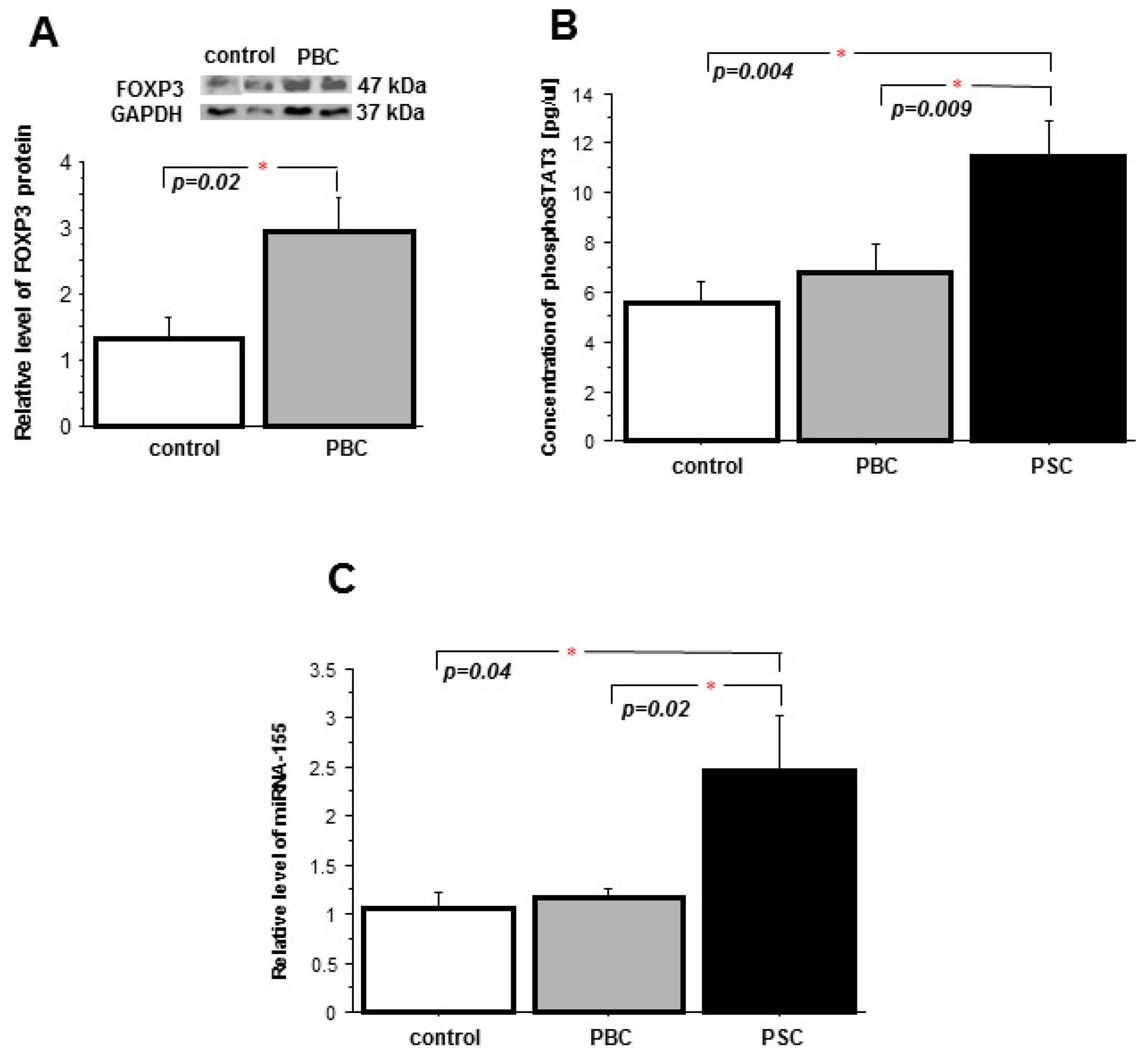
2.1. Liver
2.2. Peripheral Blood Mononuclear Cells (PBMCs)
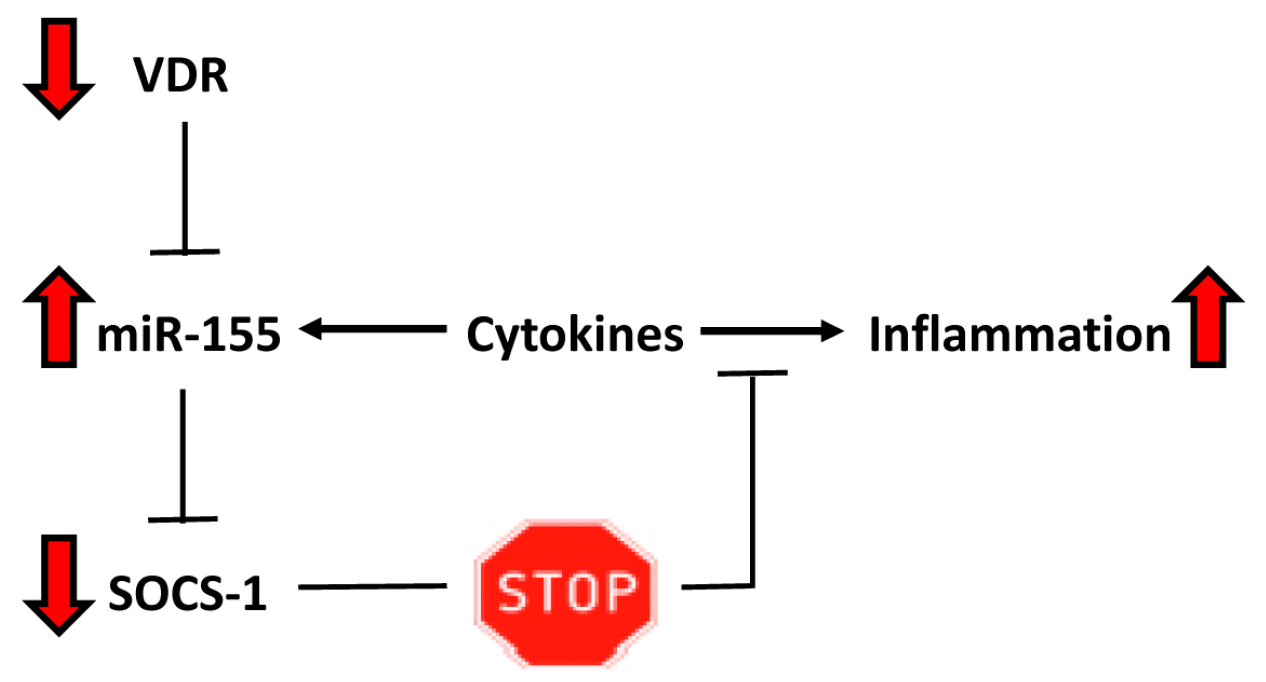
3. Discussion
4. Patients and Methods
4.1. Patients’ Characteristics and Tissue Specimens
4.2. RNA and miRNA Expression Analysis
4.3. Protein Expression Analysis
4.4. Immunohistochemistry
4.5. Statistics
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| PBC | Primary biliary cholangitis |
| VDR | Vitamin D receptor |
| SOCS1 | Suppressor of cytokine signaling1 |
| PSC | Primary sclerosing cholangitis |
| PBMC | Peripheral blood mononuclear cells |
| AMAs | Antimitochondrial antibodies |
| Treg | Regulatory T cells |
| FOXP3 | Forkhead family transcriptional regulatorbox-P3 |
| FOXP3+ Treg | FOXP3-positive regulatory T cells |
| STAT3 | Signal transducer and activator of transcription-3 |
References
- Kaplan, M.M.; Gershwin, M.E. Primary biliary cirrhosis. N. Engl. J. Med. 2005, 353, 1261–1273. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.E. Pathogenesis of primary biliary cirrhosis. Postgrad. Med. J. 2008, 84, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Kita, H.; Imawari, M.; Gershwin, M.E. Cellular immune response in primary biliary cirrhosis. Hepatol. Res. 2004, 28, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Lan, R.Y.; Salunga, T.L.; Tsuneyama, K.; Lian, Z.X.; Yang, G.X.; Hsu, W.; Moritoki, Y.; Ansari, A.A.; Kemper, C.; Price, J.; et al. Hepatic IL-17 responses in human and murine primary biliary cirrhosis. J. Autoimmun. 2009, 32, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.Y.; Ma, X.; Tsuneyama, K.; Huang, S.; Takahashi, T.; Chalasani, N.P.; Bowlus, C.L.; Yang, G.X.; Leung, P.S.; Ansari, A.A.; et al. IL-12/Th1 and IL-23/Th17 biliary microenvironment in primary biliary cirrhosis: implications for therapy. Hepatology 2014, 59, 1944–1953. [Google Scholar] [CrossRef] [PubMed]
- Lan, R.Y.; Cheng, C.; Lian, Z.X.; Tsuneyama, K.; Yang, G.X.; Moritoki, Y.; Chuang, Y.H.; Nakamura, T.; Saito, S.; Shimoda, S.; et al. Liver-targeted and peripheral blood alterations of regulatory T cells in primary biliary cirrhosis. Hepatology 2006, 43, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Prietl, B.; Treiber, G.; Pieber, T.R.; Amrein, K. Vitamin D and immune function. Nutrients 2013, 5, 2502–2521. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Strugnell, S.A.; DeLuca, H.F. Current understanding of the molecular actions of vitamin D. Physiol. Rev. 1998, 78, 1193–1231. [Google Scholar] [PubMed]
- Zheng, Y.; Josefowicz, S.Z.; Kas, A.; Chu, T.T.; Gavin, M.A.; Rudensky, A.Y. Genome-wide analysis of Foxp3 target genes in developing and mature regulatory T cells. Nature 2007, 445, 936–940. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Carmeliet, G.; Verlinden, L.; van, E.E.; Verstuyf, A.; Luderer, H.F.; Lieben, L.; Mathieu, C.; Demay, M. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr. Rev. 2008, 29, 726–776. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.T.; Tavera-Mendoza, L.E.; Laperriere, D.; Libby, E.; MacLeod, N.B.; Nagai, Y.; Bourdeau, V.; Konstorum, A.; Lallemant, B.; Zhang, R.; et al. Large-scale in silico and microarray-based identification of direct 1,25-dihydroxyvitamin D3 target genes. Mol. Endocrinol. 2005, 19, 2685–2695. [Google Scholar] [CrossRef] [PubMed]
- Hewer, S.; Lucas, R.; van, D.M.I.; Taylor, B.V. Vitamin D and multiple sclerosis. J. Clin. Neurosci. 2013, 20, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Kostoglou-Athanassiou, I.; Athanassiou, P.; Lyraki, A.; Raftakis, I.; Antoniadis, C. Vitamin D and rheumatoid arthritis. Ther. Adv. Endocrinol. Metab. 2012, 3, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- O’Connell, R.M.; Kahn, D.; Gibson, W.S.; Round, J.L.; Scholz, R.L.; Chaudhuri, A.A.; Kahn, M.E.; Rao, D.S.; Baltimore, D. MicroRNA-155 promotes autoimmune inflammation by enhancing inflammatory T cell development. Immunity 2010, 33, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Hanada, T.; Yoshida, H.; Kato, S.; Tanaka, K.; Masutani, K.; Tsukada, J.; Nomura, Y.; Mimata, H.; Kubo, M.; Yoshimura, A. Suppressor of cytokine signaling-1 is essential for suppressing dendritic cell activation and systemic autoimmunity. Immunity 2003, 19, 437–450. [Google Scholar] [CrossRef]
- Yoshimura, A.; Suzuki, M.; Sakaguchi, R.; Hanada, T.; Yasukawa, H. SOCS, Inflammation, and Autoimmunity. Front. Immunol. 2012, 3, 20. [Google Scholar] [CrossRef] [PubMed]
- Marine, J.C.; Topham, D.J.; McKay, C.; Wang, D.; Parganas, E.; Stravopodis, D.; Yoshimura, A.; Ihle, J.N. SOCS1 deficiency causes a lymphocyte-dependent perinatal lethality. Cell 1999, 98, 609–616. [Google Scholar] [CrossRef]
- Tanaka, K.; Ichiyama, K.; Hashimoto, M.; Yoshida, H.; Takimoto, T.; Takaesu, G.; Torisu, T.; Hanada, T.; Yasukawa, H.; Fukuyama, S.; et al. Loss of suppressor of cytokine signaling 1 in helper T cells leads to defective Th17 differentiation by enhancing antagonistic effects of IFN-gamma on STAT3 and Smads. J. Immunol. 2008, 180, 3746–3756. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Hiwatashi, K.; Ichiyama, K.; Morita, R.; Sekiya, T.; Kimura, A.; Sugiyama, Y.; Sibata, T.; Kuroda, K.; Takahashi, R.; et al. SOCS1 regulates type I/type II NKT cell balance by regulating IFNγ signaling. Int. Immunol. 2011, 23, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Hirschfield, G.M.; Xie, G.; Lu, E.; Sun, Y.; Juran, B.D.; Chellappa, V.; Coltescu, C.; Mason, A.L.; Milkiewicz, P.; Myers, R.P.; et al. Association of primary biliary cirrhosis with variants in the CLEC16A, SOCS1, SPIB and SIAE immunomodulatory genes. Genes Immun. 2012, 13, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, W.; Sun, T.; Huang, Y.; Wang, Y.; Deb, D.K.; Yoon, D.; Kong, J.; Thadhani, R.; Li, Y.C. 1,25-Dihydroxyvitamin D promotes negative feedback regulation of TLR signaling via targeting microRNA-155-SOCS1 in macrophages. J. Immunol. 2013, 190, 3687–3695. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Strassburg, C.P.; Manns, M.P. Genetic association of vitamin D receptor polymorphisms with primary biliary cirrhosis and autoimmune hepatitis. Hepatology 2002, 35, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Kempinska-Podhorecka, A.; Wunsch, E.; Jarowicz, T.; Raszeja-Wyszomirska, J.; Loniewska, B.; Kaczmarczyk, M.; Milkiewicz, M.; Milkiewicz, P. Vitamin D receptor polymorphisms predispose to primary biliary cirrhosis and severity of the disease in polish population. Gastroenterol. Res. Pract. 2012, 2012, 408723. [Google Scholar] [CrossRef] [PubMed]
- Gascon-Barre, M.; Demers, C.; Mirshahi, A.; Neron, S.; Zalzal, S.; Nanci, A. The normal liver harbors the vitamin D nuclear receptor in nonparenchymal and biliary epithelial cells. Hepatology 2003, 37, 1034–1042. [Google Scholar] [CrossRef] [PubMed]
- Barchetta, I.; Carotti, S.; Labbadia, G.; Gentilucci, U.V.; Muda, A.O.; Angelico, F.; Silecchia, G.; Leonetti, F.; Fraioli, A.; Picardi, A.; et al. Liver vitamin D receptor, CYP2R1, and CYP27A1 expression: relationship with liver histology and vitamin D3 levels in patients with nonalcoholic steatohepatitis or hepatitis C virus. Hepatology 2012, 56, 2180–2187. [Google Scholar] [CrossRef] [PubMed]
- O'Connell, R.M.; Rao, D.S.; Chaudhuri, A.A.; Baltimore, D. Physiological and pathological roles for microRNAs in the immune system. Nat. Rev. Immunol. 2010, 10, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Arteh, J.; Narra, S.; Nair, S. Prevalence of vitamin D deficiency in chronic liver disease. Dig. Dis. Sci. 2010, 55, 2624–2628. [Google Scholar] [CrossRef] [PubMed]
- Agmon-Levin, N.; Kopilov, R.; Selmi, C.; Nussinovitch, U.; Sanchez-Castanon, M.; Lopez-Hoyos, M.; Amital, H.; Kivity, S.; Gershwin, E.M.; Shoenfeld, Y. Vitamin D in primary biliary cirrhosis, a plausible marker of advanced disease. Immunol. Res. 2015, 61, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Horino, J.; Fujimoto, M.; Terabe, F.; Serada, S.; Takahashi, T.; Soma, Y.; Tanaka, K.; Chinen, T.; Yoshimura, A.; Nomura, S.; et al. Suppressor of cytokine signaling-1 ameliorates dextran sulfate sodium-induced colitis in mice. Int. Immunol. 2008, 20, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Torisu, T.; Nakaya, M.; Watanabe, S.; Hashimoto, M.; Yoshida, H.; Chinen, T.; Yoshida, R.; Okamoto, F.; Hanada, T.; Torisu, K.; et al. Suppressor of cytokine signaling 1 protects mice against concanavalin A-induced hepatitis by inhibiting apoptosis. Hepatology 2008, 47, 1644–1654. [Google Scholar] [CrossRef] [PubMed]
- Pene, J.; Chevalier, S.; Preisser, L.; Venereau, E.; Guilleux, M.H.; Ghannam, S.; Moles, J.P.; Danger, Y.; Ravon, E.; Lesaux, S.; et al. Chronically inflamed human tissues are infiltrated by highly differentiated Th17 lymphocytes. J. Immunol. 2008, 180, 7423–7430. [Google Scholar] [CrossRef] [PubMed]
- Rong, G.; Zhou, Y.; Xiong, Y.; Zhou, L.; Geng, H.; Jiang, T.; Zhu, Y.; Lu, H.; Zhang, S.; Wang, P.; et al. Imbalance between T helper type 17 and T regulatory cells in patients with primary biliary cirrhosis: The serum cytokine profile and peripheral cell population. Clin. Exp. Immunol. 2009, 156, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Fenoglio, D.; Bernuzzi, F.; Battaglia, F.; Parodi, A.; Kalli, F.; Negrini, S.; de, P.R.; Invernizzi, P.; Filaci, G. Th17 and regulatory T lymphocytes in primary biliary cirrhosis and systemic sclerosis as models of autoimmune fibrotic diseases. Autoimmun. Rev. 2012, 12, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.; Jiang, T.; Zhang, W.; Ren, C.; Wang, Q.; Qin, Q.; Chen, J.; Deng, A.; Zhong, R. Increased IL-23 and IL-17 expression by peripheral blood cells of patients with primary biliary cirrhosis. Cytokine 2013, 64, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Ichiyama, K.; Sekiya, T.; Inoue, N.; Tamiya, T.; Kashiwagi, I.; Kimura, A.; Morita, R.; Muto, G.; Shichita, T.; Takahashi, R.; et al. Transcription factor Smad-independent T helper 17 cell induction by transforming-growth factor-beta is mediated by suppression of eomesodermin. Immunity 2011, 34, 741–754. [Google Scholar] [CrossRef] [PubMed]
- Katt, J.; Schwinge, D.; Schoknecht, T.; Quaas, A.; Sobottka, I.; Burandt, E.; Becker, C.; Neurath, M.F.; Lohse, A.W.; Herkel, J.; et al. Increased T helper type 17 response to pathogen stimulation in patients with primary sclerosing cholangitis. Hepatology 2013, 58, 1084–1093. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S.; Powrie, F. Emerging challenges in regulatory T cell function and biology. Science 2007, 317, 627–629. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu. Rev. Immunol. 2004, 22, 531–562. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhou, X. Regulatory T cells turn pathogenic. Cell. Mol. Immunol. 2015, 12, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, R.; Nishimoto, S.; Muto, G.; Sekiya, T.; Tamiya, T.; Kimura, A.; Morita, R.; Asakawa, M.; Chinen, T.; Yoshimura, A. SOCS1 is essential for regulatory T cell functions by preventing loss of Foxp3 expression as well as IFN-γ and IL-17A production. J. Exp. Med. 2011, 208, 2055–2067. [Google Scholar] [CrossRef] [PubMed]
- Rushbrook, S.M.; Hoare, M.; Alexander, G.J. T-regulatory lymphocytes and chronic viral hepatitis. Expert. Opin. Biol. Ther. 2007, 7, 1689–1703. [Google Scholar] [CrossRef] [PubMed]
- Unitt, E.; Rushbrook, S.M.; Marshall, A.; Davies, S.; Gibbs, P.; Morris, L.S.; Coleman, N.; Alexander, G.J. Compromised lymphocytes infiltrate hepatocellular carcinoma: The role of T-regulatory cells. Hepatology 2005, 41, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Bernuzzi, F.; Fenoglio, D.; Battaglia, F.; Fravega, M.; Gershwin, M.E.; Indiveri, F.; Ansari, A.A.; Podda, M.; Invernizzi, P.; Filaci, G. Phenotypical and functional alterations of CD8 regulatory T cells in primary biliary cirrhosis. J. Autoimmun. 2010, 35, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.O.; Housley, W.J.; Bhowmick, S.; Cone, R.E.; Rajan, T.V.; Forouhar, F.; Clark, R.B. Cbl-b(−/−) T cells demonstrate in vivo resistance to regulatory T cells but a context-dependent resistance to TGF-β. J. Immunol. 2010, 185, 2051–2058. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.F.; Thai, T.H.; Calado, D.P.; Chaudhry, A.; Kubo, M.; Tanaka, K.; Loeb, G.B.; Lee, H.; Yoshimura, A.; Rajewsky, K.; et al. Foxp3-dependent microRNA155 confers competitive fitness to regulatory T cells by targeting SOCS1 protein. Immunity 2009, 30, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Starr, R.; Metcalf, D.; Elefanty, A.G.; Brysha, M.; Willson, T.A.; Nicola, N.A.; Hilton, D.J.; Alexander, W.S. Liver degeneration and lymphoid deficiencies in mice lacking suppressor of cytokine signaling-1. Proc. Natl. Acad. Sci. USA 1998, 95, 14395–14399. [Google Scholar] [CrossRef] [PubMed]
- Kopycinska, J.; Kempinska-Podhorodecka, A.; Haas, T.; Elias, E.; DePinho, R.A.; Paik, J.; Milkiewicz, P.; Milkiewicz, M. Activation of FoxO3a/Bim axis in patients with Primary Biliary Cirrhosis. Liver Int. 2013, 33, 231–238. [Google Scholar] [CrossRef] [PubMed]



| Parameters | PBC | PSC | |||
|---|---|---|---|---|---|
| Rho | p-Value | Rho | p-Value | ||
| VDR protein | SOCS-1 protein | −0.252 | 0.147 | −0.319 | 0.202 |
| SOCS-1 mRNA | 0.402 | 0.017 | 0.319 | 0.154 | |
| miR-155 | 0.151 | 0.441 | 0.454 | 0.089 | |
| VDR mRNA | SOCS-1 protein | 0.069 | 0.697 | −0.118 | 0.637 |
| SOCS-1 mRNA | 0.234 | 0.159 | 0.469 | 0.027 | |
| miR-155 | 0.714 | 0.058 | −0.028 | 0.926 | |
| miR-155 | SOCS-1 protein | 0.694 | 0.001 | 0.421 | 0.167 |
| SOCS-1 mRNA | −0.344 | 0.169 | −0.286 | 0.449 | |
| Variables | Liver | Peripheral Blood Mononuclear Cells | |||
|---|---|---|---|---|---|
| PBC non-cirrhotic n = 22 | PBC cirrhotic n = 22 | PSC n = 13 | PBC n = 16 | PSC n = 10 | |
| Number (male/female) | 0/22 | 1/21 | 8/5 | 1/15 | 14/6 |
| Age, years | 52 ± 11 | 56 ± 9 | 44 ± 14.1 | 52.6 ± 7.3 | 35.1 ± 10.8 |
| AST IU/L | 62.6 ± 56.1 | 147.9 ± 128.0 | 260. ± 348.4 | 83 ± 63.6 | 67.6 ± 33.9 |
| ALP IU/L | 265.4 ± 182.9 | 447.4 ± 296.4 | 543.7±454.3 | 111 ± 109.5 | 306.6 ± 44.9 |
| Bilirubin (µmol/L) | 21.6 ± 28.8 | 114.0 ± 112.4 | 64.8 ± 91.9 | 15.1 ± 9.6 | 85.5 ± 67.5 |
| 1,25-(OH)2-Vitamin D (pmol/L) 1,25-(OH)2-Vitamin D (range) | 66.6 ± 20.5 (36.8−117.1) | 85.1 ± 9.8 (67.8−114.3) | - | 62.6 ± 4.7 (44.2−112.9) | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kempinska-Podhorodecka, A.; Milkiewicz, M.; Wasik, U.; Ligocka, J.; Zawadzki, M.; Krawczyk, M.; Milkiewicz, P. Decreased Expression of Vitamin D Receptor Affects an Immune Response in Primary Biliary Cholangitis via the VDR-miRNA155-SOCS1 Pathway. Int. J. Mol. Sci. 2017, 18, 289. https://doi.org/10.3390/ijms18020289
Kempinska-Podhorodecka A, Milkiewicz M, Wasik U, Ligocka J, Zawadzki M, Krawczyk M, Milkiewicz P. Decreased Expression of Vitamin D Receptor Affects an Immune Response in Primary Biliary Cholangitis via the VDR-miRNA155-SOCS1 Pathway. International Journal of Molecular Sciences. 2017; 18(2):289. https://doi.org/10.3390/ijms18020289
Chicago/Turabian StyleKempinska-Podhorodecka, Agnieszka, Malgorzata Milkiewicz, Urszula Wasik, Joanna Ligocka, Michał Zawadzki, Marek Krawczyk, and Piotr Milkiewicz. 2017. "Decreased Expression of Vitamin D Receptor Affects an Immune Response in Primary Biliary Cholangitis via the VDR-miRNA155-SOCS1 Pathway" International Journal of Molecular Sciences 18, no. 2: 289. https://doi.org/10.3390/ijms18020289
APA StyleKempinska-Podhorodecka, A., Milkiewicz, M., Wasik, U., Ligocka, J., Zawadzki, M., Krawczyk, M., & Milkiewicz, P. (2017). Decreased Expression of Vitamin D Receptor Affects an Immune Response in Primary Biliary Cholangitis via the VDR-miRNA155-SOCS1 Pathway. International Journal of Molecular Sciences, 18(2), 289. https://doi.org/10.3390/ijms18020289






