Erythroid Differentiation Regulator 1 as a Novel Biomarker for Hair Loss Disorders
Abstract
:1. Introduction
2. Results
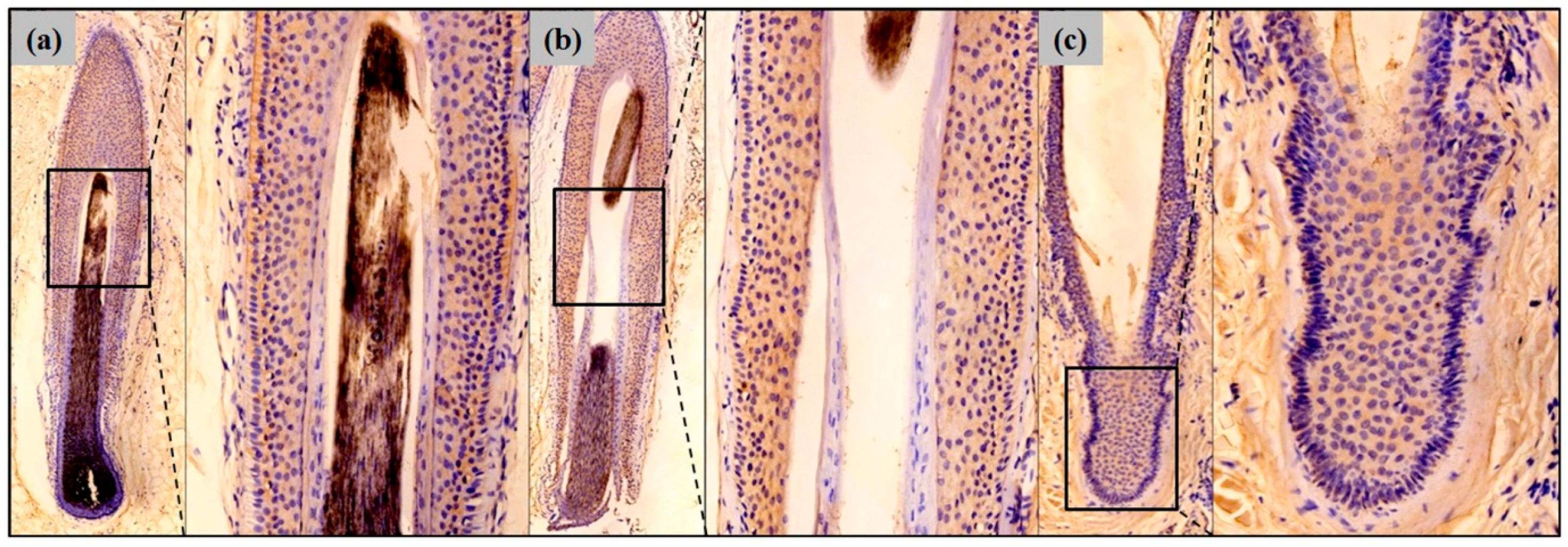
2.1. Erythroid Differentiation Regulator 1 (Erdr1) Is Expressed by Normal Hair Follicular Epithelium
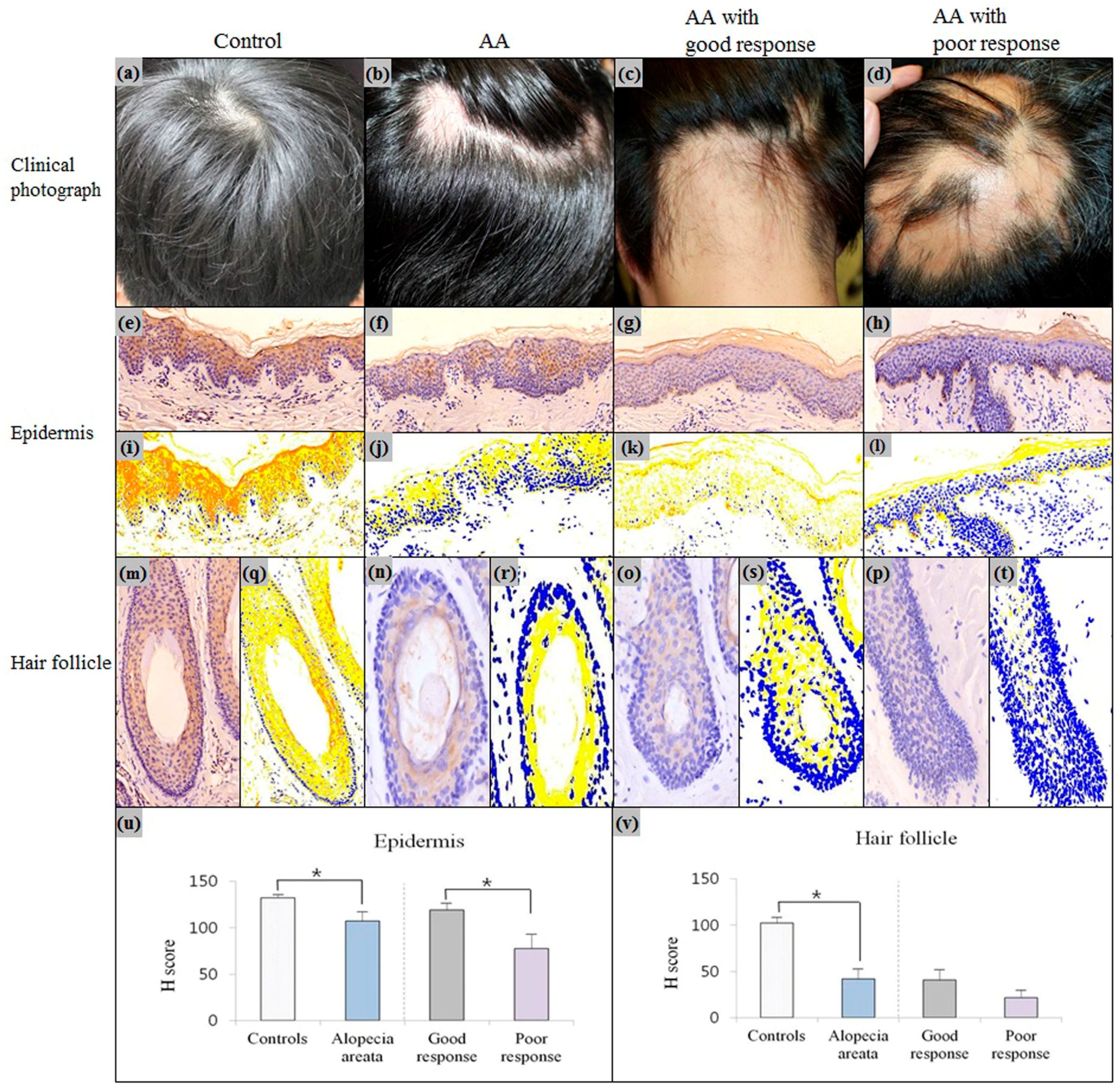
2.2. Downregulation of Erdr1 in Alopecia Areata
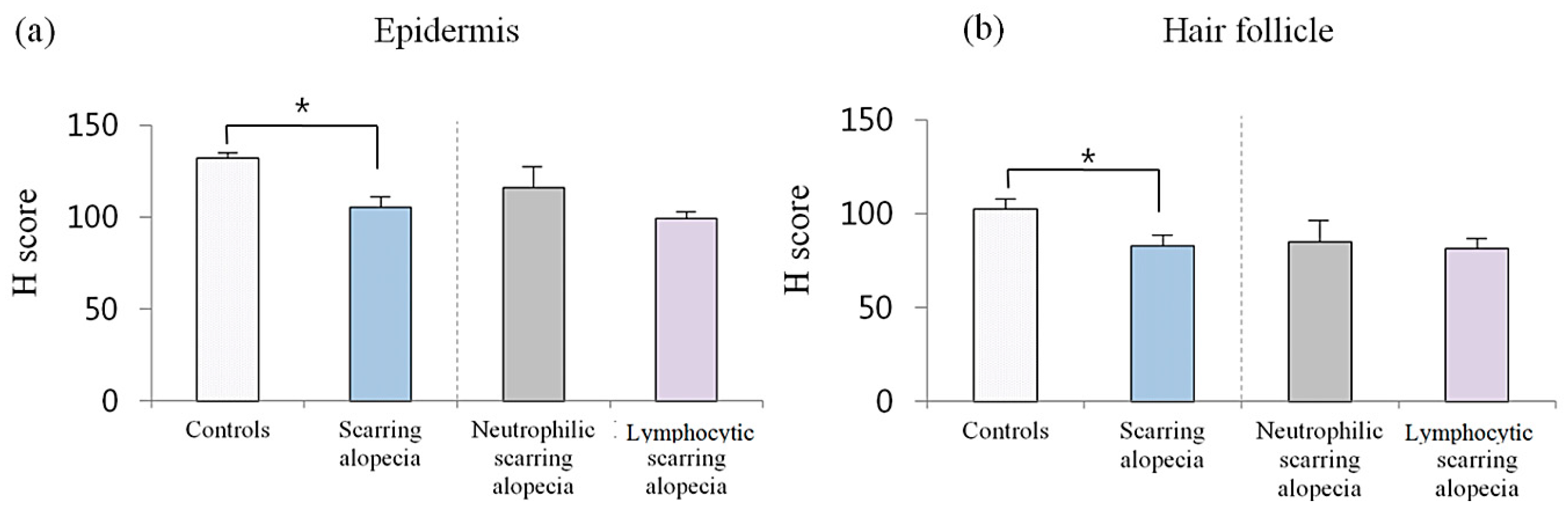
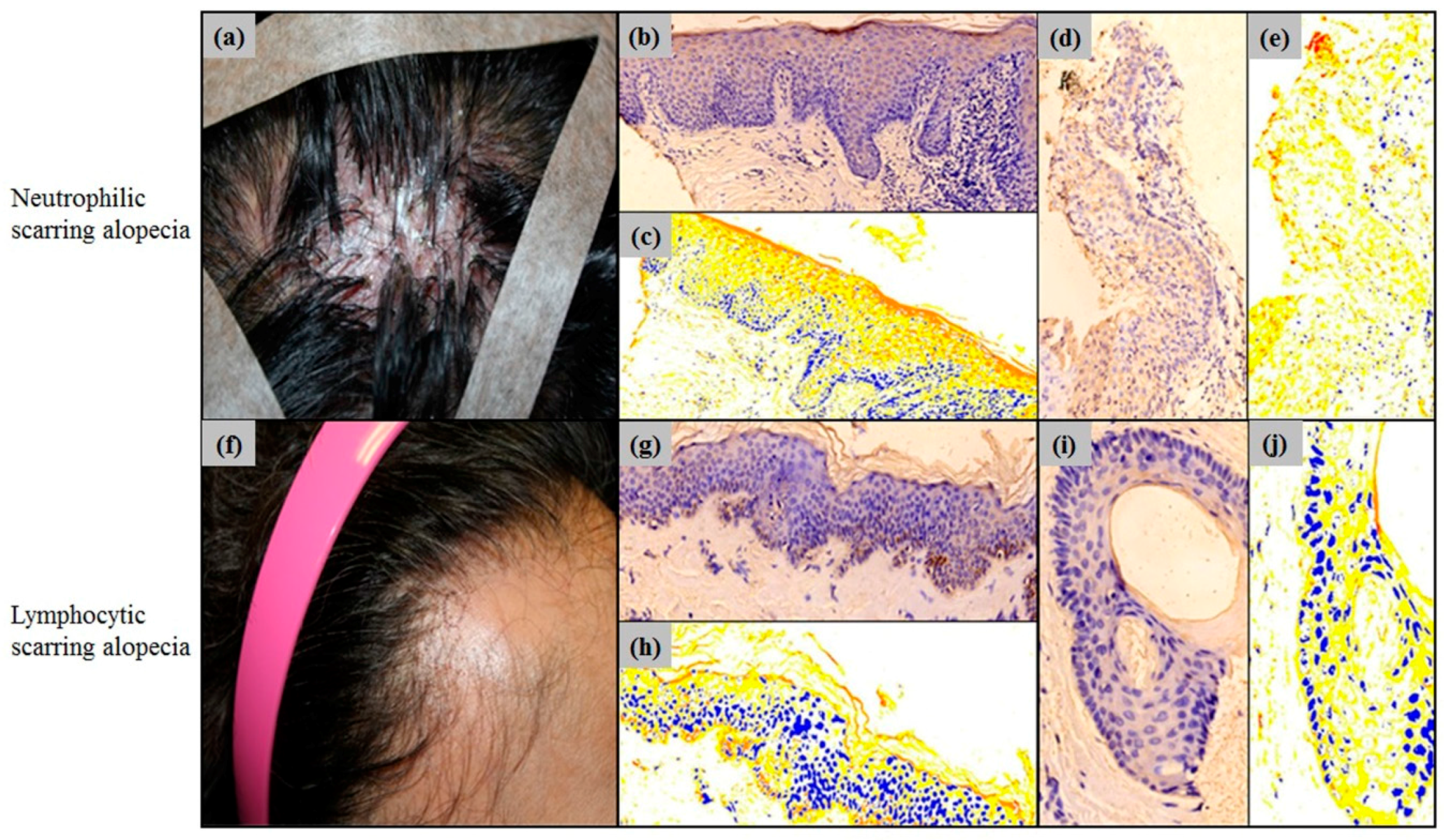
2.3. Erdr1 Downregulation in Scarring Alopecia
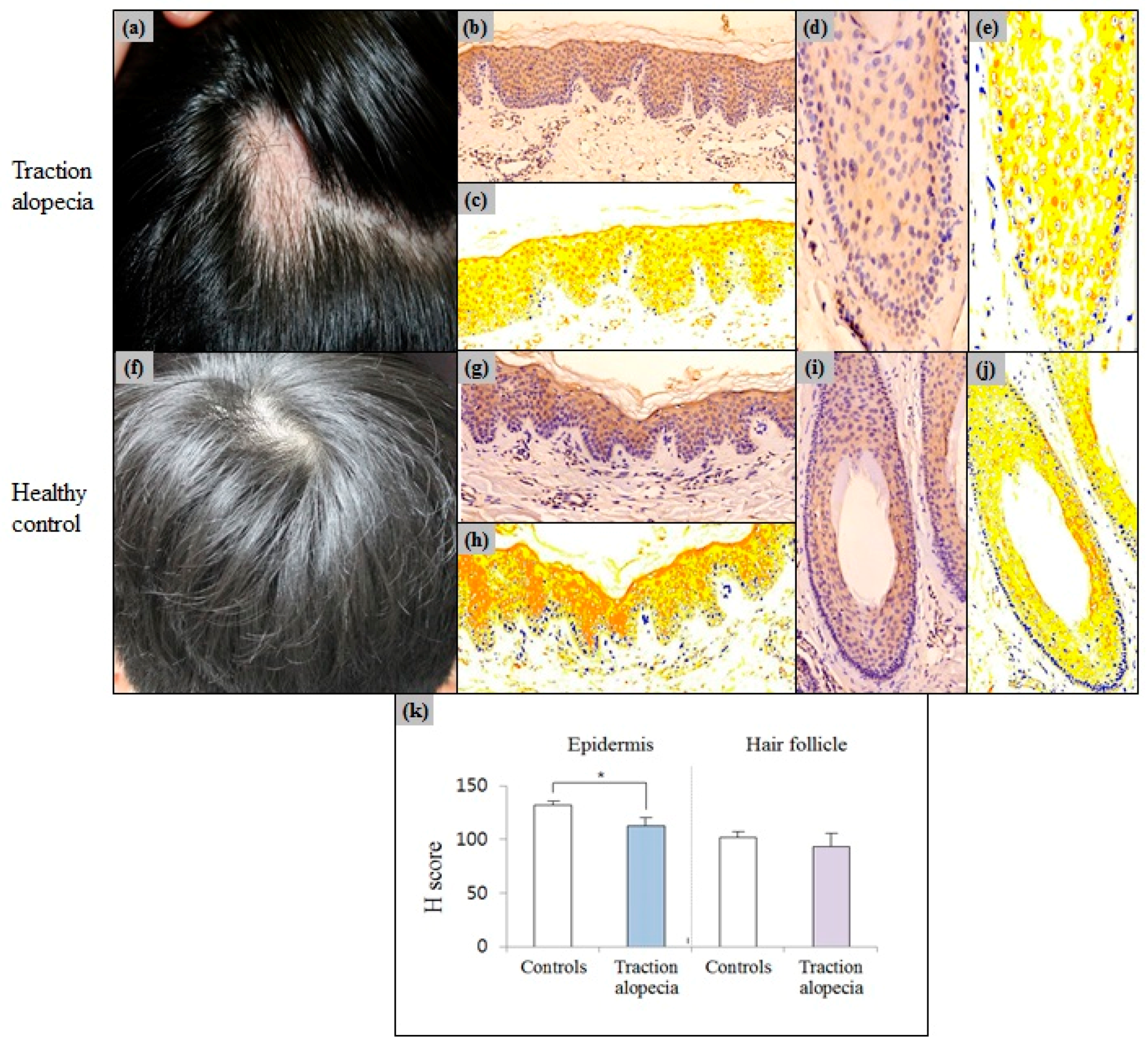
2.4. Epidermal Erdr1 Expression Was Downregulated in Traction Alopecia
2.5. Comparison of Erdr1 Expression in Alopecia Subgroups
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Immunohistochemical Analyses
4.3. Quantitative Digital Analysis for Erdr1 Expression
4.4. Statistics
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Alexis, A.F.; Dudda-Subramanya, R.; Sinha, A.A. Alopecia areata: Autoimmune basis of hair loss. Eur. J. Dermatol. 2004, 14, 364–370. [Google Scholar] [PubMed]
- Ross, E.K.; Tan, E.; Shapiro, J. Update on primary cicatricial alopecias. J. Am. Acad. Dermatol. 2005, 53, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Barahmani, N.; Lopez, A.; Babu, D.; Hernandez, M.; Donley, S.; Duvic, M. Serum T helper 1 cytokine levels are greater in patients with alopecia areata regardless of severity or atopy. Clin. Exp. Dermatol. 2010, 35, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Hantash, B.M.; Schwartz, R.A. Traction alopecia in children. Cutis 2003, 71, 18–20. [Google Scholar] [PubMed]
- Cotsarelis, G.; Millar, S.E. Towards a molecular understanding of hair loss and its treatment. Trends Mol. Med. 2001, 7, 293–301. [Google Scholar] [CrossRef]
- Kasumagic-Halilovic, E.; Prohic, A.; Karamehic, J. Serum concentrations of interferon-γ (IFN-γ) in patients with alopecia areata: Correlation with clinical type and duration of the disease. Med. Arch. 2010, 64, 212–214. [Google Scholar]
- Bodemer, C.; Peuchmaur, M.; Fraitaig, S.; Chatenoud, L.; Brousse, N.; de Prost, Y. Role of cytotoxic T cells in chronic alopecia areata. J. Invest. Dermatol. 2000, 114, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Cho, D.H.; Kim, H.J.; Lee, J.Y.; Cho, B.K.; Park, H.J. Immunoreactivity of corticotropin-releasing hormone, adrenocorticotropic hormone and α-melanocyte-stimulating hormone in alopecia areata. Exp. Dermatol. 2006, 15, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Ghoreishi, M.; Martinka, M.; Dutz, J. Type 1 interferon signature in the scalp lesions of alopecia areata. Br. J. Dermatol. 2010, 163, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Okamura, H.; Tsutsui, H.; Komatsu, T.; Yutsudo, M.; Hakura, A.; Tanimoto, T.; Torigoe, K.; Okura, T.; Nukada, Y.; Hattori, K. Cloning of a new cytokine that induces IFN-γ production by T cells. Nature 1995, 378, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. IL-18: A TH1-inducing, proinflammatory cytokine and new member of the IL-1 family. J. Allergy Clin. Immunol. 1999, 103, 11–24. [Google Scholar] [CrossRef]
- Rasmy, H.; Mikhael, N.; Ismail, S. Interleukin-18 expression and the response to treatment in patients with psoriasis. Arch. Med. Sci. 2011, 7, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Kou, K.; Aihara, M.; Matsunaga, T.; Chen, H.; Taguri, M.; Morita, S.; Fujita, H.; Yamaguchi, Y.; Kambara, T.; Ikezawa, Z. Association of serum interleukin-18 and other biomarkers with disease severity in adults with atopic dermatitis. Arch. Dermatol. Res. 2012, 304, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Park, H.J.; Chung, J.-H.; Kim, J.W.; Seok, H.; Lew, B.-L.; Sim, W.-Y. Association between interleukin 18 polymorphisms and alopecia areata in Koreans. J. Interferon Cytokine Res. 2014, 34, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Hong, S.K.; Park, S.W.; Hur, D.Y.; Shon, J.H.; Shin, J.G.; Hwang, S.W.; Sung, H.S. Serum levels of IL-18 and sIL-2R in patients with alopecia areata receiving combined therapy with oral cyclosporine and steroids. Exp. Dermatol. 2010, 19, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.E.; Houh, Y.; Lee, J.; Kim, S.; Cho, D.; Park, H.J. Downregulation of erythroid differentiation regulator 1 (Erdr1) plays a critical role in psoriasis pathogenesis. Exp. Dermatol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, K.E.; Jung, H.Y.; Jo, H.; Jeong, S.W.; Lee, J.; Kim, C.H.; Kim, H.; Cho, D.; Park, H.J. Recombinant erythroid differentiation regulator 1 inhibits both inflammation and angiogenesis in a mouse model of rosacea. Exp. Dermatol. 2015, 24, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Alkhalifah, A.; Alsantali, A.; Wang, E.; McElwee, K.J.; Shapiro, J. Alopecia areata update: Part II. Treatment. J. Am. Acad. Dermatol. 2010, 62, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Bayramgürler, D.; Demirsoy, E.O.; Aktürk, A.Ş.; Kıran, R. Narrowband ultraviolet B phototherapy for alopecia areata. Photodermatol. Photoimmunol. Photomed. 2011, 27, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, Z.; Bhouri, A.; Jallouli, A.; Fazaa, B.; Kamoun, M.; Mokhtar, I. Alopecia areata treatment with a phototoxic dose of UVA and topical 8-methoxypsoralen. J. Eur. Acad. Dermatol. Venereol. 2005, 19, 552–555. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.S.; Sachdeva, S. Alopecia areata: A review. J. Saudi Soc. Dermatol. Dermatol. Surg. 2013, 17, 37–45. [Google Scholar] [CrossRef]
- Dörmer, P.; Spitzer, E.; Frankenberger, M.; Kremmer, E. Erythroid differentiation regulator (EDR), a novel, highly conserved factor: I. Induction of haemoglobin synthesis in erythroleukaemic cells. Cytokine 2004, 26, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.B.; Kim, H.J.; Jung, H.Y.; Park, Y.G.; Kim, S.Y.; Cho, B.K.; Cho, D.; Park, H.J. Downregulation of erythroid differentiation regulator 1 as a novel marker of skin tumors. Int. J. Dermatol. 2014, 53, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.K.; Houh, Y.K.; Ha, S.; Yang, Y.; Kim, D.; Kim, T.S.; Yoon, S.R.; Bang, S.I.; Cho, B.J.; Lee, W.J. Recombinant Erdr1 suppresses the migration and invasion ability of human gastric cancer cells, SNU-216, through the JNK pathway. Immunol. Lett. 2013, 150, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.E.; Houh, Y.; Park, H.J.; Cho, D. Therapeutic Effects of Erythroid Differentiation Regulator 1 on Imiquimod-Induced Psoriasis-Like Skin Inflammation. Int. J. Mol. Sci. 2016, 17, 244. [Google Scholar] [CrossRef] [PubMed]
- Kossard, S. Lymphocytic mediated alopecia: Histological classification by pattern analysis. Clin. Dermatol. 2001, 19, 201–210. [Google Scholar] [CrossRef]
- Ito, T. Recent advances in the pathogenesis of autoimmune hair loss disease alopecia areata. Clin. Dev. Immunol. 2013, 2013, 348546. [Google Scholar] [CrossRef] [PubMed]
- Steck, W. Telogen effluvium: A clinically useful concept, with traction alopecia as an example. Cutis 1978, 21, 543–548. [Google Scholar] [PubMed]
- Gupta, M.A.; Gupta, A.K.; Watteel, G. Stress and alopecia areata: A psychodermatologic study. Acta Derm. Venereol. 1997, 77, 296–298. [Google Scholar] [PubMed]
- Trost, L.B.; Bergfeld, W.F.; Calogeras, E. The diagnosis and treatment of iron deficiency and its potential relationship to hair loss. J. Am. Acad. Dermatol. 2006, 54, 824–844. [Google Scholar] [CrossRef] [PubMed]
- Rushton, D.; Norris, M.; Dover, R.; Busuttil, N. Causes of hair loss and the developments in hair rejuvenation. Int. J. Cosmet. Sci. 2002, 24, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Kantor, J.; Kessler, L.J.; Brooks, D.G.; Cotsarelis, G. Decreased serum ferritin is associated with alopecia in women. J. Investig. Dermatol. 2003, 121, 985–988. [Google Scholar] [CrossRef] [PubMed]
- Myung, P.S.; Takeo, M.; Ito, M.; Atit, R.P. Epithelial Wnt ligand secretion is required for adult hair follicle growth and regeneration. J. Investig. Dermatol. 2013, 133, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Huelsken, J.; Vogel, R.; Erdmann, B.; Cotsarelis, G.; Birchmeier, W. β-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell 2001, 105, 533–545. [Google Scholar] [CrossRef]
- Fuentes-Duculan, J.; Gulati, N.; Bonifacio, K.M.; Kunjravia, N.; Zheng, X.; Suárez-Fariñas, M.; Shemer, A.; Guttman-Yassky, E.; Krueger, J.G. Biomarkers of alopecia areata disease activity and response to corticosteroid treatment. Exp. Dermatol. 2016, 25, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, M.; Pretzlaff, M.; Sulk, M.; Bahr, M.; Gherardini, J.; Uchida, Y.; Reibelt, M.; Kinori, M.; Rossi, A.; Biro, T.; et al. Vasoactive intestinal peptide, whose receptor-mediated signalling may be defective in alopecia areata, provides protection from hair follicle immune privilege collapse. Br. J. Dermatol. 2016, 175, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, M.; Makino, T.; Kagoura, M.; Morohashi, M. Expression of neuropeptide-degrading enzymes in alopecia areata: An immunohistochemical study. Br. J. Dermatol. 2001, 144, 46–54. [Google Scholar] [CrossRef] [PubMed]
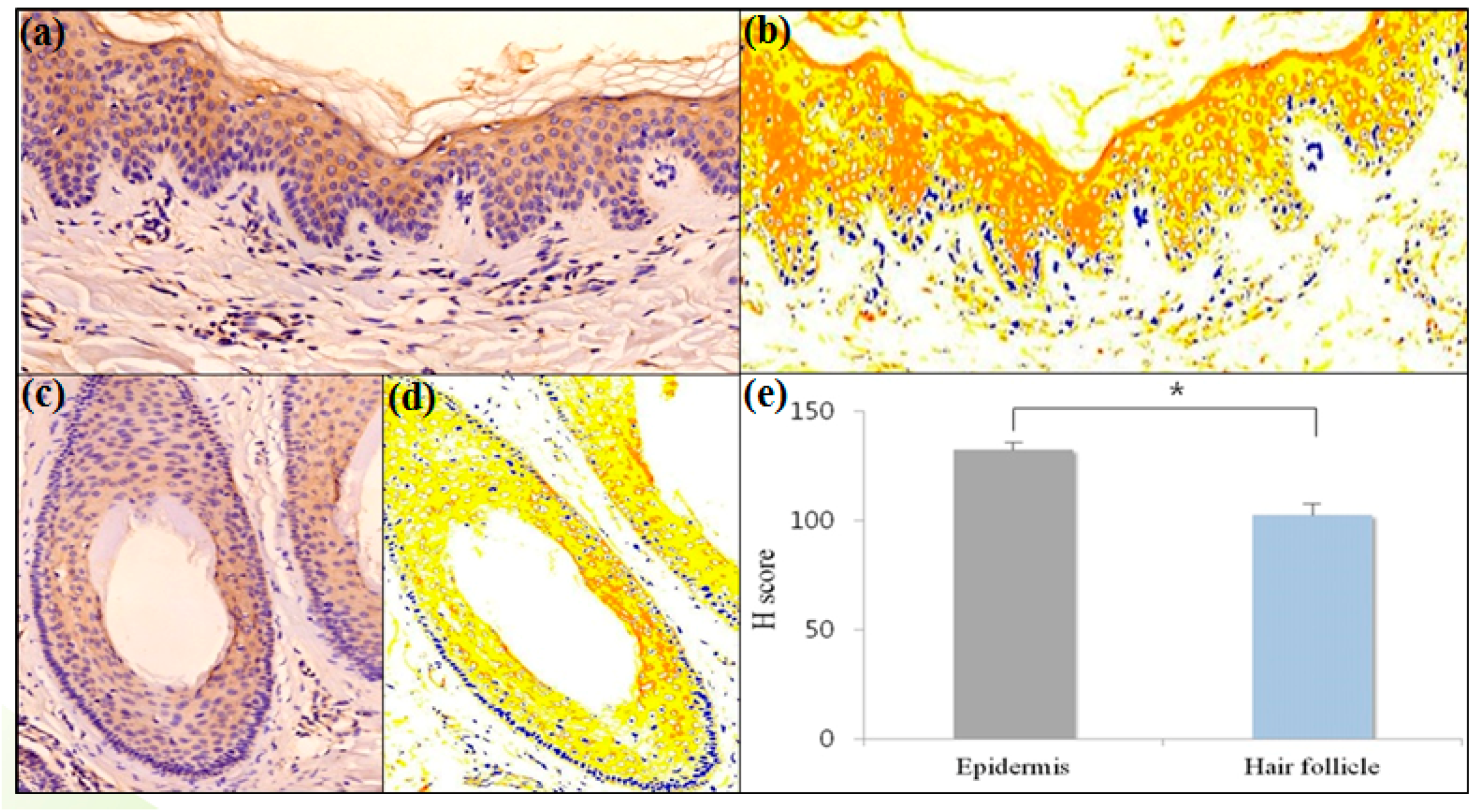

| Ratio of Positive Immunoreactivity | Epithelium | Hair Follicle | p-Value |
|---|---|---|---|
| Ratio of negative pixels to total pixels | 7.76 ± 2.15 | 13.57 ± 3.81 | 0.310 |
| Ratio of weak-positive pixels to total pixels | 53.65 ± 3.92 | 70.60 ± 3.24 | 0.032 |
| Ratio of moderate-positive pixels to total pixels | 37.16 ± 2.50 | 15.65 ± 2.66 | 0.008 |
| Ratio of strong-positive pixels to total pixels | 1.43 ± 0.39 | 0.18 ± 0.05 | 0.008 |
| H score | 132.24 ± 3.15 | 102.45 ± 5.68 | 0.008 |
| Disorders | Clinical Findings | Histopathology | Associated Biomarkers |
|---|---|---|---|
| Alopecia areata | - Smooth oval or round patch on the hair bearing area | - Peribulbar lymphocytic infiltration of affected follicles - Increased numbers of miniaturized follicles | Inflammation; ↓ Erdr1 in the epidermis; ↓ Erdr1 in the HF; ↑ IFN-γ, CXCL10, CXCL9, IL-13, CCL18, CCL26, TSLP [35] around the HF; stress; ↑ CRH, α-MSH, ACTH [8] in the epidermis and HF; neuromediators; ↓ VIP receptor [36] in the HF; ↑ Substance P [37] in the perifollicular nerve fibers; keratin; ↓ KRT83–86, KRT35, KRT81, KRT40, KRT75, KRTAP1 [34] in the HF |
| Scarring alopecia | - Permanent loss of hair follicle manifested by absence of follicular ostia | - Destruction of hair follicle | Inflammation; ↓ Erdr1 in the epidermis; ↓ Erdr1 in the HF |
| Traction alopecia | - History for the application of tensile force to the hair follicle | - Absence of the inflammatory infiltration around hair follicle - Marked damage of inner root sheath | Inflammation; ↓ Erdr1 in the epidermis |
| Characteristics | Alopecia (n = 21) | Normal Controls (n = 5) |
|---|---|---|
| Age (range) | 32 (12–68) | 43 (25–60) |
| Male/Female | 12/9 | 2/3 |
| Types of alopecia | 21 | - |
| Alopecia areata | 9 (42.6) | - |
| Scarring alopecia | 8 (38.1) | - |
| Lymphocytic | 5 | - |
| Neutrophilic | 3 | - |
| Traction alopecia | 4 (19.3) | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woo, Y.R.; Hwang, S.; Jeong, S.W.; Cho, D.H.; Park, H.J. Erythroid Differentiation Regulator 1 as a Novel Biomarker for Hair Loss Disorders. Int. J. Mol. Sci. 2017, 18, 316. https://doi.org/10.3390/ijms18020316
Woo YR, Hwang S, Jeong SW, Cho DH, Park HJ. Erythroid Differentiation Regulator 1 as a Novel Biomarker for Hair Loss Disorders. International Journal of Molecular Sciences. 2017; 18(2):316. https://doi.org/10.3390/ijms18020316
Chicago/Turabian StyleWoo, Yu Ri, Sewon Hwang, Seo Won Jeong, Dae Ho Cho, and Hyun Jeong Park. 2017. "Erythroid Differentiation Regulator 1 as a Novel Biomarker for Hair Loss Disorders" International Journal of Molecular Sciences 18, no. 2: 316. https://doi.org/10.3390/ijms18020316






