MicroRNAs: New Insight in Modulating Follicular Atresia: A Review
Abstract
:1. Introduction
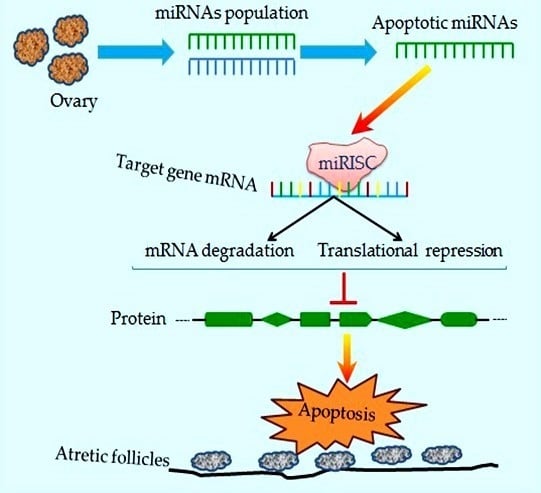
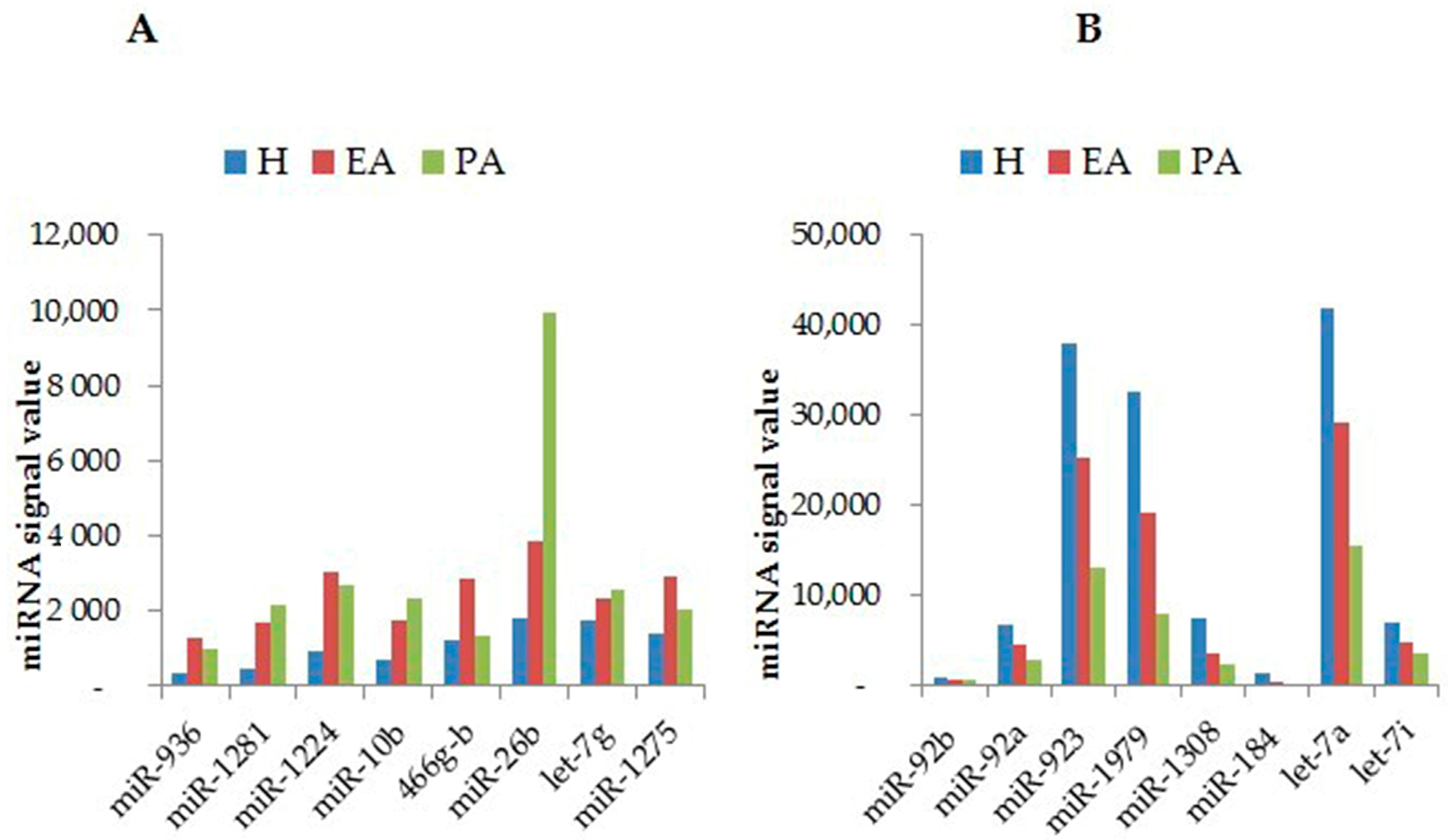
2. Profiling of miRNAs during Follicular Atresia
3. Functional Roles of miRNAs during Follicular Cellular Apoptosis
4. Molecular Signaling Pathways Enriched in miRNA-Mediated Follicular Atresia
5. miRNAs-Induced Autophagy Regulates Granulosa Apoptosis
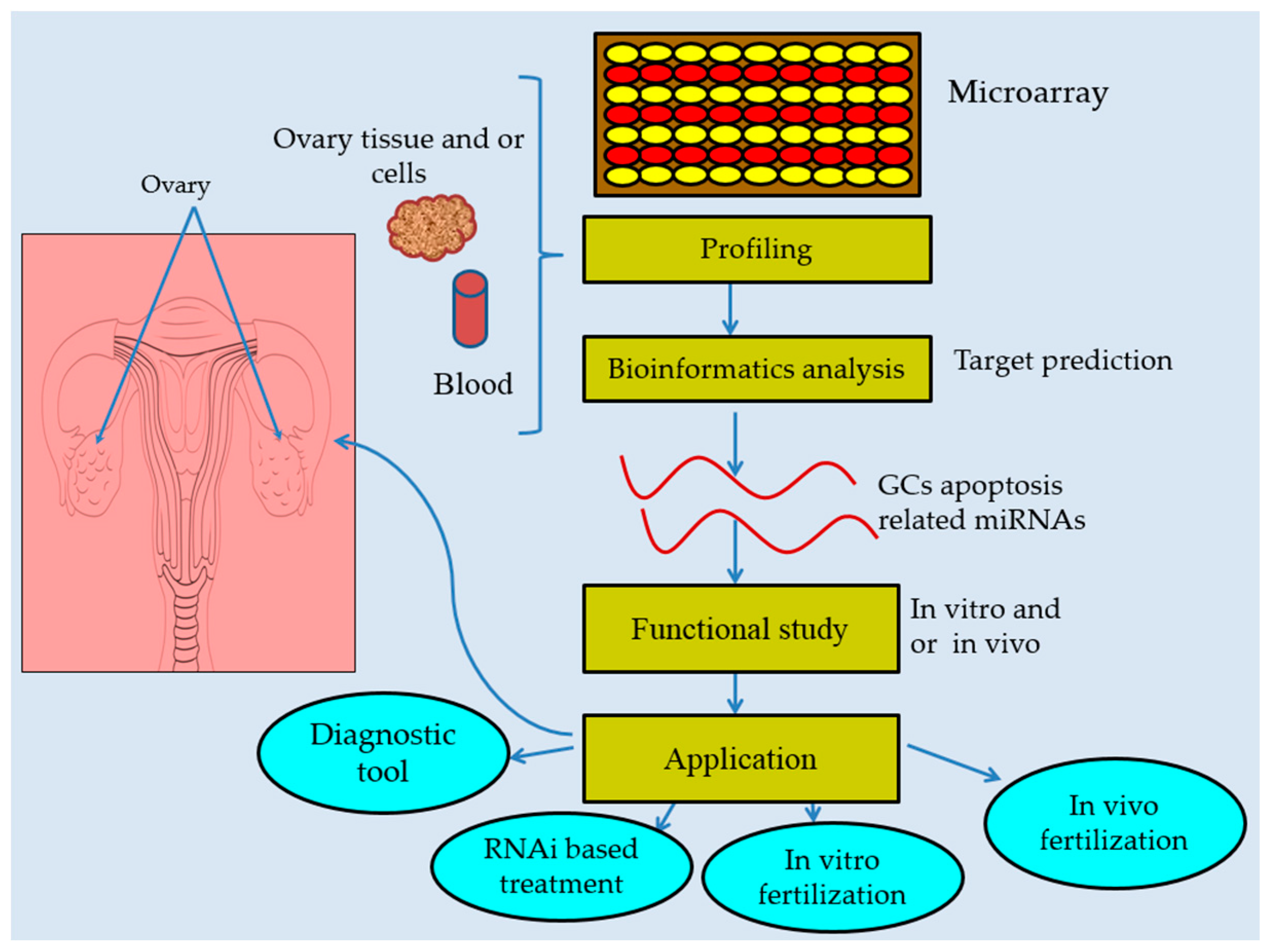
6. miRNAs Are Promising Therapeutic Agents and Biomarkers in Follicular Atresia
7. Concluding Remarks and Future Directions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Baker, T.G. A quantitative and cytological study of germ cells in human ovaries. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1963, 158, 417–433. [Google Scholar] [CrossRef]
- Ohno, S.; Smith, J.B. Role of fetal follicular cells in meiosis of mammalian ooecytes. Cytogenetics 1964, 3, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Himelstein-Braw, R.; Byskov, A.G.; Peters, H.; Faber, M. Follicular atresia in the infant human ovary. J. Reprod. Fertil. 1976, 46, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Peters, H.; Byskov, A.G.; Grinsted, J. Follicular growth in fetal and prepubertal ovaries of humans and other primates. Clin. Endocrinol. Metab. 1978, 7, 469–485. [Google Scholar] [CrossRef]
- Forabosco, A.; Sforza, C.; De Pol, A.; Vizzotto, L.; Marzona, L.; Ferrario, V.F. Morphometric study of the human neonatal ovary. Anat. Rec. 1991, 231, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Hirshfield, A.N. Theca cells may be present at the outset of follicular growth. Biol. Reprod. 1991, 44, 1157–1162. [Google Scholar] [CrossRef] [PubMed]
- Kaipia, A.; Hsueh, A.J. Regulation of ovarian follicle atresia. Annu. Rev. Physiol. 1997, 59, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Faddy, M.J.; Gosden, R.G.; Gougeon, A.; Richardson, S.J.; Nelson, J.F. Accelerated disappearance of ovarian follicles in mid-life: Implications for forecasting menopause. Hum. Reprod. 1992, 7, 1342–1346. [Google Scholar] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Ambros, V. The functions of animal microRNAs. Nature 2004, 431, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Yates, L.A.; Norbury, C.J.; Gilbert, R.J. The long and short of microRNA. Cell 2013, 153, 516–519. [Google Scholar] [CrossRef] [PubMed]
- Aravin, A.; Tuschl, T. Identification and characterization of small RNAs involved in RNA silencing. FEBS Lett. 2005, 579, 5830–5840. [Google Scholar] [CrossRef] [PubMed]
- Zielak-Steciwko, A.E.; Browne, J.A.; McGettigan, P.A.; Gajewska, M.; Dzieciol, M.; Szulc, T.; Evans, A.C. Expression of microRNAs and their target genes and pathways associated with ovarian follicle development in cattle. Physiol. Genom. 2014, 46, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Gebremedhn, S.; Salilew-Wondim, D.; Ahmad, I.; Sahadevan, S.; Hossain, M.M.; Hoelker, M.; Rings, F.; Neuhoff, C.; Tholen, E.; Looft, C.; et al. MicroRNA expression profile in bovine granulosa cells of preovulatory dominant and subordinate follicles during the late follicular phase of the estrous cycle. PLoS ONE 2015, 10, e0125912. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.A.; Zhao, Y.; Wang, L.; Li, Q.; Du, Y.A.; Dan, Y.; Huo, L.J. Identification of miRNAs during mouse postnatal ovarian development and superovulation. J. Ovarian Res. 2015, 8, 44. [Google Scholar] [CrossRef] [PubMed]
- Donadeu, F.X.; Schauer, S.N.; Sontakke, S.D. Involvement of miRNAs in ovarian follicular and luteal development. J. Endocrinol. 2012, 215, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhou, Y.; Peng, S.; Wu, L.; Lin, H.Y.; Wang, S.; Wang, H. Differentially expressed plasma microRNAs in premature ovarian failure patients and the potential regulatory function of miR-23a in granulosa cell apoptosis. Reproduction 2012, 144, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wang, S.; Luo, A.; Ding, T.; Lai, Z.; Shen, W.; Ma, X.; Cao, C.; Shi, L.; Jiang, J.; et al. Expression patterns and regulatory functions of microRNAs during the initiation of primordial follicle development in the neonatal mouse ovary. Biol. Reprod. 2013, 89, 126. [Google Scholar] [CrossRef] [PubMed]
- Virant-Klun, I.; Stahlberg, A.; Kubista, M.; Skutella, T. MicroRNAs: From female fertility, germ cells, and stem cells to cancer in humans. Stem Cells Int. 2016, 2016, 3984937. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, M.; Hengartner, M.O. MiRNAs and apoptosis: RNAs to die for. Oncogene 2006, 25, 6176–6187. [Google Scholar] [CrossRef] [PubMed]
- Valadi, H.; Ekstrom, K.; Bossios, A.; Sjostrand, M.; Lee, J.J.; Lotvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Bueno, M.J.; de Castro, I.P.; Malumbres, M. Control of cell proliferation pathways by microRNAs. Cell Cycle 2008, 7, 3143–3148. [Google Scholar] [CrossRef] [PubMed]
- Chivukula, R.R.; Mendell, J.T. Circular reasoning: MicroRNAs and cell-cycle control. Trends Biochem. Sci. 2008, 33, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Ademokun, A.; Turner, M. Regulation of B-cell differentiation by microRNAs and RNA-binding proteins. Biochem. Soc. Trans. 2008, 36, 1191–1193. [Google Scholar] [CrossRef] [PubMed]
- Gangaraju, V.K.; Lin, H. MicroRNAs: Key regulators of stem cells. Nat. Rev. Mol. Cell Biol. 2009, 10, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Sirotkin, A.V.; Laukova, M.; Ovcharenko, D.; Brenaut, P.; Mlyncek, M. Identification of microRNAs controlling human ovarian cell proliferation and apoptosis. J. Cell. Physiol. 2010, 223, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Tsai-Morris, C.H.; Sato, H.; Villar, J.; Kang, J.H.; Zhang, J.; Dufau, M.L. Testis-specific miRNA-469 up-regulated in gonadotropin-regulated testicular RNA helicase (GRTH/DDX25)-null mice silences transition protein 2 and protamine 2 messages at sites within coding region: Implications of its role in germ cell development. J. Biol. Chem. 2011, 286, 44306–44318. [Google Scholar] [CrossRef] [PubMed]
- Kedde, M.; Agami, R. Interplay between microRNAs and RNA-binding proteins determines developmental processes. Cell Cycle 2008, 7, 899–903. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Ku, S.Y.; Kim, Y.Y.; Liu, H.C.; Chi, S.W.; Kim, S.H.; Choi, Y.M.; Kim, J.G.; Moon, S.Y. MicroRNAs transfected into granulosa cells may regulate oocyte meiotic competence during in vitro maturation of mouse follicles. Hum. Reprod. 2013, 28, 3050–3061. [Google Scholar] [CrossRef] [PubMed]
- Assou, S.; Al-edani, T.; Haouzi, D.; Philippe, N.; Lecellier, C.H.; Piquemal, D.; Commes, T.; Ait-Ahmed, O.; Dechaud, H.; Hamamah, S. MicroRNAs: New candidates for the regulation of the human cumulus-oocyte complex. Hum. Reprod. 2013, 28, 3038–3049. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.R. Apoptosis in the ovary: Molecular mechanisms. Hum. Reprod. Updat. 2005, 11, 162–177. [Google Scholar] [CrossRef] [PubMed]
- Inoue, N.; Matsuda, F.; Goto, Y.; Manabe, N. Role of cell-death ligand-receptor system of granulosa cells in selective follicular atresia in porcine ovary. J. Reprod. Dev. 2011, 57, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Manabe, N.; Matsuda-Minehata, F.; Goto, Y.; Maeda, A.; Cheng, Y.; Nakagawa, S.; Inoue, N.; Wongpanit, K.; Jin, H.; Gonda, H.; et al. Role of cell death ligand and receptor system on regulation of follicular atresia in pig ovaries. Reprod. Domest. Anim. 2008, 43, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Matsuda-Minehata, F.; Inoue, N.; Goto, Y.; Manabe, N. The regulation of ovarian granulosa cell death by pro- and anti-apoptotic molecules. J. Reprod. Dev. 2006, 52, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Tilly, J.L.; Kowalski, K.I.; Johnson, A.L.; Hsueh, A.J. Involvement of apoptosis in ovarian follicular atresia and postovulatory regression. Endocrinology 1991, 129, 2799–2801. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lin, Y.; Li, H.; Liu, J.; Sheng, X.; Zhang, W. 2,5-Hexanedione induces human ovarian granulosa cell apoptosis through BCL-2, BAX, and caspase-3 signaling pathways. Arch. Toxicol. 2012, 86, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Quirk, S.M.; Cowan, R.G.; Harman, R.M. The susceptibility of granulosa cells to apoptosis is influenced by oestradiol and the cell cycle. J. Endocrinol. 2006, 189, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Quirk, S.M.; Cowan, R.G.; Joshi, S.G.; Henrikson, K.P. Fas antigen-mediated apoptosis in human granulosa/luteal cells. Biol. Reprod. 1995, 52, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Chun, S.Y.; Eisenhauer, K.M.; Minami, S.; Hsueh, A.J. Growth factors in ovarian follicle atresia. Semin. Reprod. Endocrinol. 1996, 14, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Manabe, N.; Goto, Y.; Matsuda-Minehata, F.; Inoue, N.; Maeda, A.; Sakamaki, K.; Miyano, T. Regulation mechanism of selective atresia in porcine follicles: Regulation of granulosa cell apoptosis during atresia. J. Reprod. Dev. 2004, 50, 493–514. [Google Scholar] [CrossRef] [PubMed]
- Sirotkin, A.V.; Ovcharenko, D.; Grossmann, R.; Laukova, M.; Mlyncek, M. Identification of microRNAs controlling human ovarian cell steroidogenesis via a genome-scale screen. J. Cell. Physiol. 2009, 219, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Linher-Melville, K.; Yang, B.B.; Wu, D.; Li, J. Micro-RNA378 (miR-378) regulates ovarian estradiol production by targeting aromatase. Endocrinology 2011, 152, 3941–3951. [Google Scholar] [CrossRef] [PubMed]
- Schauer, S.N.; Sontakke, S.D.; Watson, E.D.; Esteves, C.L.; Donadeu, F.X. Involvement of miRNAs in equine follicle development. Reproduction 2013, 146, 273–282. [Google Scholar] [CrossRef] [PubMed]
- McBride, D.; Carre, W.; Sontakke, S.D.; Hogg, C.O.; Law, A.; Donadeu, F.X.; Clinton, M. Identification of miRNAs associated with the follicular-luteal transition in the ruminant ovary. Reproduction 2012, 144, 221–233. [Google Scholar] [CrossRef] [PubMed]
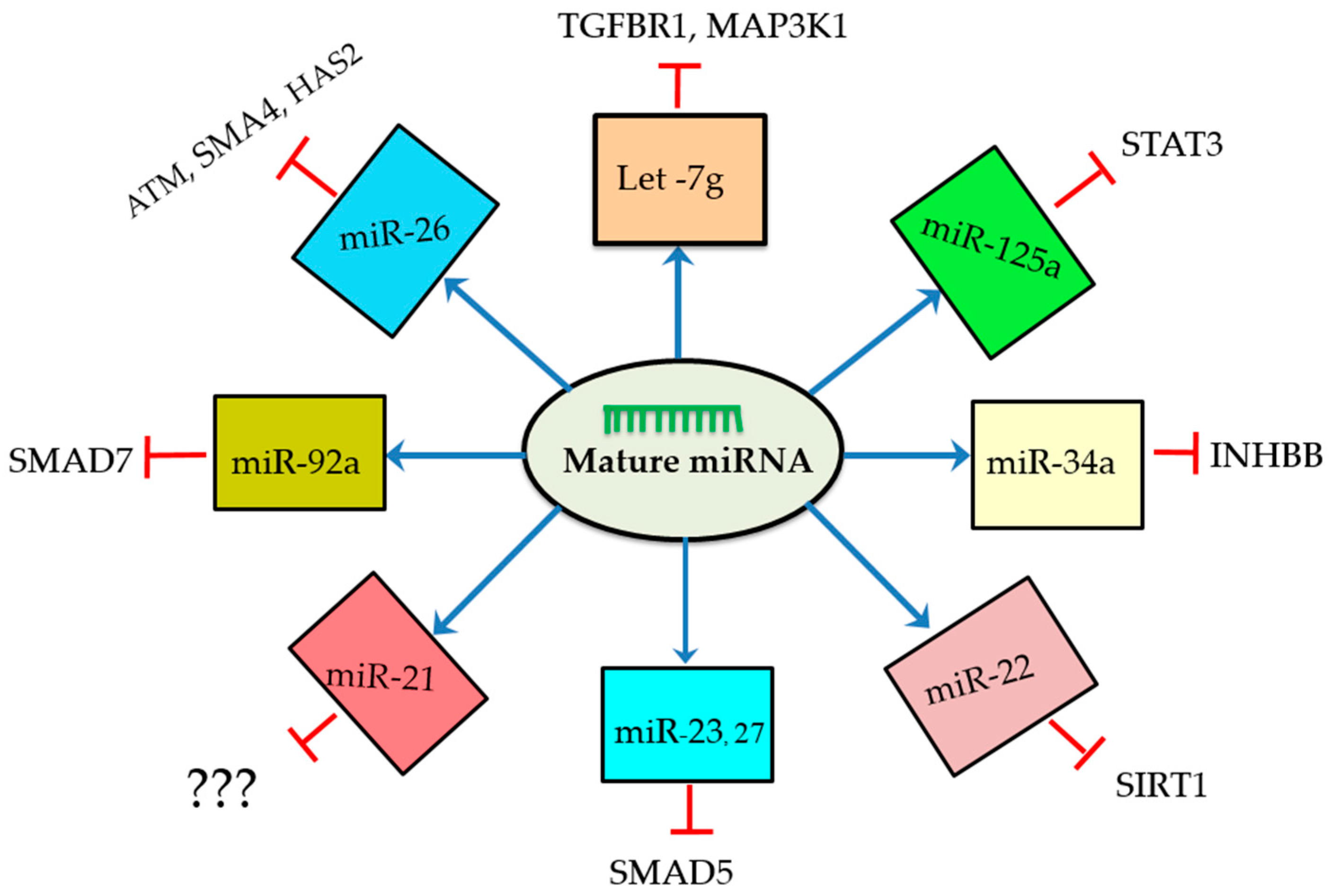
- Cao, R.; Wu, W.; Zhou, X.; Liu, K.; Li, B.; Huang, X.; Zhang, Y.; Liu, H. Let-7g induces granulosa cell apoptosis by targeting MAP3K1 in the porcine ovary. Int. J. Biochem. Cell Biol. 2015, 68, 148–157. [Google Scholar] [CrossRef] [PubMed]
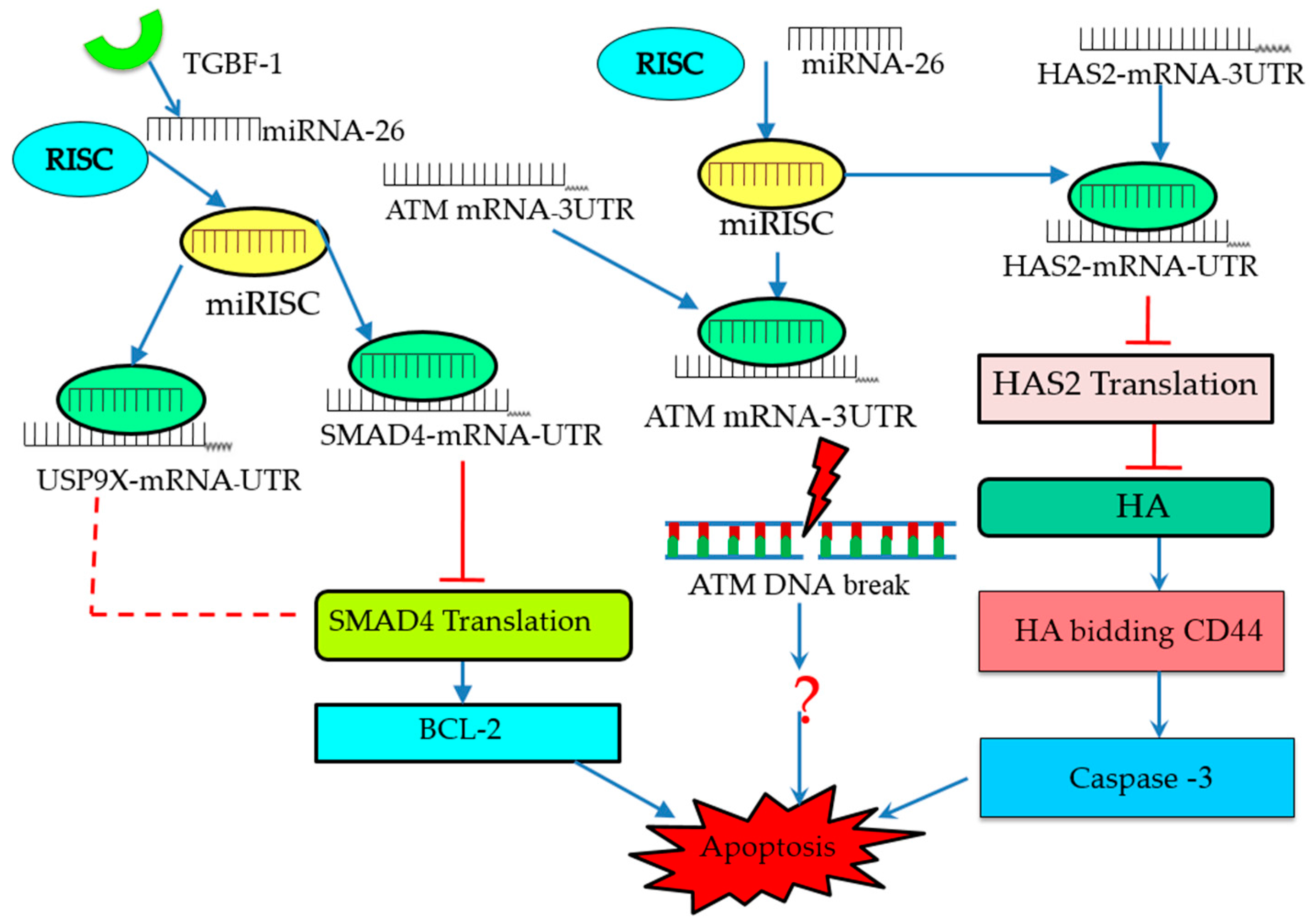
- Lin, F.; Li, R.; Pan, Z.X.; Zhou, B.; Yu, D.B.; Wang, X.G.; Ma, X.S.; Han, J.; Shen, M.; Liu, H.L. miR-26b promotes granulosa cell apoptosis by targeting atm during follicular atresia in porcine ovary. PLoS ONE 2012, 7, e38640. [Google Scholar] [CrossRef] [PubMed]
- Sontakke, S.D.; Mohammed, B.T.; McNeilly, A.S.; Donadeu, F.X. Characterization of microRNAs differentially expressed during bovine follicle development. Reproduction 2014, 148, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yao, W.; Yao, Y.; Du, X.; Zhou, J.; Ma, B.; Liu, H.; Li, Q.; Pan, Z. miR-92a inhibits porcine ovarian granulosa cell apoptosis by targeting SMAD7 gene. FEBS Lett. 2014, 588, 4497–4503. [Google Scholar] [CrossRef] [PubMed]
- Nie, M.; Yu, S.; Peng, S.; Fang, Y.; Wang, H.; Yang, X. miR-23a and miR-27a promote human granulosa cell apoptosis by targeting SMAD5. Biol. Reprod. 2015, 93, 98. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Liu, J.; Pan, Z.; Du, X.; Li, X.; Ma, B.; Yao, W.; Li, Q.; Liu, H. The let-7g microRNA promotes follicular granulosa cell apoptosis by targeting transforming growth factor-β type 1 receptor. Mol. Cell. Endocrinol. 2015, 409, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, D.; Zhang, S.; Xing, Y.; Gao, Y.; Wu, J. MicroRNA-125a-5p induces mouse granulosa cell apoptosis by targeting signal transducer and activator of transcription 3. Menopause 2016, 23, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Xiong, F.; Hu, L.; Zhang, Y.; Xiao, X.; Xiao, J. miR-22 inhibits mouse ovarian granulosa cell apoptosis by targeting SIRT1. Biol. Open 2016, 5, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tu, F.; Yao, W.; Li, X.; Xie, Z.; Liu, H.; Li, Q.; Pan, Z. Conserved miR-26b enhances ovarian granulosa cell apoptosis through HAS2-HA-CD44-caspase-3 pathway by targeting HAS2. Sci. Rep. 2016, 6, 21197. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Wu, W.J.; Zhou, X.L.; Xiao, P.; Wang, Y.; Liu, H.L. Expression and preliminary functional profiling of the let-7 family during porcine ovary follicle atresia. Mol. Cells 2015, 38, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.H.; Ren, C.H.; Guo, X.F.; Xu, L.N.; Huang, Y.F.; Luo, J.C.; Zhang, Y.H.; Zhang, X.R.; Zhang, Z.J. Identification and characterization of microRNAs in the ovaries of multiple and uniparous goats (Capra hircus) during follicular phase. BMC Genom. 2014, 15, 339. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, Y.; Wang, T.; Guan, J.; Luo, Z.; Chen, H.; Wang, X.; Chen, L.; Ma, J.; Mu, Z.; et al. Repertoire of porcine microRNAs in adult ovary and testis by deep sequencing. Int. J. Biol. Sci. 2011, 7, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- Carletti, M.Z.; Fiedler, S.D.; Christenson, L.K. MicroRNA 21 blocks apoptosis in mouse periovulatory granulosa cells. Biol. Reprod. 2010, 83, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Tu, F.; Pan, Z.X.; Yao, Y.; Liu, H.L.; Liu, S.R.; Xie, Z.; Li, Q.F. miR-34a targets the inhibin β B gene, promoting granulosa cell apoptosis in the porcine ovary. Genet. Mol. Res. 2014, 13, 2504–2512. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Du, X.; Zhou, J.; Pan, Z.; Liu, H.; Li, Q. MicroRNA-26b functions as a proapoptotic factor in porcine follicular granulosa cells by targeting SMA-and MAD-related protein 4. Biol. Reprod. 2014, 91, 146. [Google Scholar] [CrossRef] [PubMed]
- Jovicic, A.; Zaldivar Jolissaint, J.F.; Moser, R.; Silva Santos Mde, F.; Luthi-Carter, R. MicroRNA-22 (miR-22) overexpression is neuroprotective via general anti-apoptotic effects and may also target specific huntington’s disease-related mechanisms. PLoS ONE 2013, 8, e54222. [Google Scholar] [CrossRef] [PubMed]
- Pasquinelli, A.E.; McCoy, A.; Jimenez, E.; Salo, E.; Ruvkun, G.; Martindale, M.Q.; Baguna, J. Expression of the 22 nucleotide let-7 heterochronic RNA throughout the metazoa: A role in life history evolution? Evol. Dev. 2003, 5, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.T.; Risom, T.; Strauss, W.M. Evolutionary conservation of microRNA regulatory circuits: An examination of microRNA gene complexity and conserved microRNA-target interactions through metazoan phylogeny. DNA Cell Biol. 2007, 26, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.M.; Martinez, N.J.; Thornton, J.E.; Hagan, J.P.; Nguyen, K.D.; Gregory, R.I. Trim71 cooperates with microRNAs to repress Cdkn1a expression and promote embryonic stem cell proliferation. Nat. Commun. 2012, 3, 923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurtan, A.M.; Ravi, A.; Rahl, P.B.; Bosson, A.D.; JnBaptiste, C.K.; Bhutkar, A.; Whittaker, C.A.; Young, R.A.; Sharp, P.A. Let-7 represses Nr6a1 and a mid-gestation developmental program in adult fibroblasts. Genes Dev. 2013, 27, 941–954. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.M.; Triboulet, R.; Thornton, J.E.; Gregory, R.I. A role for the perlman syndrome exonuclease Dis3l2 in the Lin28-let-7 pathway. Nature 2013, 497, 244–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sha, D.; Lee, A.M.; Shi, Q.; Alberts, S.R.; Sargent, D.J.; Sinicrope, F.A.; Diasio, R.B. Association study of the let-7 miRNA-complementary site variant in the 3′ untranslated region of the KRAS gene in stage III colon cancer (NCCTG N0147 clinical trial). Clin. Cancer Res. 2014, 20, 3319–3327. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Nakahara, K.; Pham, J.W.; Kim, K.; He, Z.; Sontheimer, E.J.; Carthew, R.W. Distinct roles for Drosophila Dicer-1 and Dicer-2 in the siRNA/miRNA silencing pathways. Cell 2004, 117, 69–81. [Google Scholar] [CrossRef]
- Richards, J.S.; Russell, D.L.; Ochsner, S.; Hsieh, M.; Doyle, K.H.; Falender, A.E.; Lo, Y.K.; Sharma, S.C. Novel signaling pathways that control ovarian follicular development, ovulation, and luteinization. Recent Prog. Horm. Res. 2002, 57, 195–220. [Google Scholar] [CrossRef] [PubMed]
- Knight, P.G.; Glister, C. Local roles of TGF-β superfamily members in the control of ovarian follicle development. Anim. Reprod. Sci. 2003, 78, 165–183. [Google Scholar] [CrossRef]
- Knight, P.G.; Glister, C. TGF-β superfamily members and ovarian follicle development. Reproduction 2006, 132, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, T.; Saito, H.; Toya, M.; Satio, T.; Nakahara, K.; Hiroi, M. Hyaluronic acid inhibits apoptosis in granulosa cells via CD44. J. Assist. Reprod. Genet. 2000, 17, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Tunjung, W.A.; Yokoo, M.; Hoshino, Y.; Miyake, Y.; Kadowaki, A.; Sato, E. Effect of hyaluronan to inhibit caspase activation in porcine granulosa cells. Biochem. Biophys. Res. Commun. 2009, 382, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Tsuchihara, K.; Fujii, S.; Sugiyama, M.; Goya, T.; Atomi, Y.; Ueno, T.; Ochiai, A.; Esumi, H. Autophagy is activated in colorectal cancer cells and contributes to the tolerance to nutrient deprivation. Cancer Res. 2007, 67, 9677–9684. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.B.; Chen, Y.; Henson, E.S.; Cizeau, J.; McMillan-Ward, E.; Israels, S.J.; Gibson, S.B. Hypoxia induces autophagic cell death in apoptosis-competent cells through a mechanism involving BNIP3. Autophagy 2008, 4, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.L.; Wen, X.; Bao, J.K.; Liu, B. MicroRNA-modulated autophagic signaling networks in cancer. Int. J. Biochem. Cell Biol. 2012, 44, 733–736. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Jo, M.W.; Lee, E.Y.; Yoon, B.K.; Choi, D.S. The role of autophagy in follicular development and atresia in rat granulosa cells. Fertil. Steril. 2010, 93, 2532–2537. [Google Scholar] [CrossRef] [PubMed]
- Fimia, G.M.; Piacentini, M. Regulation of autophagy in mammals and its interplay with apoptosis. Cell. Mol. Life Sci. 2010, 67, 1581–1588. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yao, W.; Liu, K.; Wen, Q.; Wu, W.; Liu, H.; Li, Q. MicroRNA let-7g regulates mouse granulosa cell autophagy by targeting insulin-like growth factor 1 receptor. Int. J. Biochem. Cell Biol. 2016, 78, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Pal, M.K.; Jaiswar, S.P.; Dwivedi, V.N.; Tripathi, A.K.; Dwivedi, A.; Sankhwar, P. MicroRNA: A new and promising potential biomarker for diagnosis and prognosis of ovarian cancer. Cancer Biol. Med. 2015, 12, 328–341. [Google Scholar] [PubMed]
- Cho, W.C. MicroRNAs: Potential biomarkers for cancer diagnosis, prognosis and targets for therapy. Int. J. Biochem. Cell Biol. 2010, 42, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.W.; Bae, H.S.; Song, J.Y.; Lee, J.K.; Lee, N.W.; Kim, T.; Lee, K.W. Detection of microRNA as novel biomarkers of epithelial ovarian cancer from the serum of ovarian cancer patients. Int. J. Gynecol. Cancer 2013, 23, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Scalici, E.; Traver, S.; Mullet, T.; Molinari, N.; Ferrieres, A.; Brunet, C.; Belloc, S.; Hamamah, S. Circulating microRNAs in follicular fluid, powerful tools to explore in vitro fertilization process. Sci. Rep. 2016, 6, 24976. [Google Scholar] [CrossRef] [PubMed]
- Traver, S.; Assou, S.; Scalici, E.; Haouzi, D.; Al-Edani, T.; Belloc, S.; Hamamah, S. Cell-free nucleic acids as non-invasive biomarkers of gynecological cancers, ovarian, endometrial and obstetric disorders and fetal aneuploidy. Hum. Reprod. Updat. 2014, 20, 905–923. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhou, H.; Lin, H.; Qi, J.; Zhu, C.; Gao, Z.; Wang, H. Circulating microRNAs are elevated in plasma from severe preeclamptic pregnancies. Reproduction 2012, 143, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Gong, F.; Zhang, S.; Zhang, C.Y.; Zen, K.; Chen, X. The origin, function, and diagnostic potential of extracellular microRNAs in human body fluids. Wiley Interdiscip. Rev. 2014, 5, 285–300. [Google Scholar] [CrossRef] [PubMed]
- Zubakov, D.; Boersma, A.W.; Choi, Y.; van Kuijk, P.F.; Wiemer, E.A.; Kayser, M. MicroRNA markers for forensic body fluid identification obtained from microarray screening and quantitative RT-PCR confirmation. Int. J. Legal Med. 2010, 124, 217–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.J.; Ku, S.Y.; Rosenwaks, Z.; Liu, H.C.; Chi, S.W.; Kang, J.S.; Lee, W.J.; Jung, K.C.; Kim, S.H.; Choi, Y.M.; et al. MicroRNA expression profiles are altered by gonadotropins and vitamin C status during in vitro follicular growth. Reprod. Sci. 2010, 17, 1081–1089. [Google Scholar] [PubMed]
| Gene Symbol | Functions and Changes during Apoptosis/Atresia | Target Genes | Model Species | Study Types | Reference |
|---|---|---|---|---|---|
| let-7g | Inhibits TGB-β1 and induces GC apoptosis | TGBR1 | pig | In vitro | [50] |
| Inhibits MAPK1 induces GC apoptosis | MAP3K1 | pig | In vitro | [45] | |
| miR-125a | Enhances cleaved caspase-3 and promotes GC apoptosis | STAT3 | mice | In vitro | [51] |
| miR-34a | Represses INHBB and promotes GC apoptosis | INHBB | pig | In vitro | [58] |
| miR-22 | Suppresses SIRT1 and inhibits apoptosis | SIRT1 | mice | In vitro | [52] |
| miR-23a and miR-27a | Increases expression of cleaved caspase-8, cleaved caspase-3, promotes GC apoptosis | SMAD5 | human | In vitro | [49] |
| miR-23a | Increased cleaved caspase-3, decrease caspase-3 protein and promote GC apoptosis | XIAP (protein) | human | In vitro | [17] |
| miR-26b | Increases DNA break, inhibits ATM, and promotes GC apoptosis | ATM | pig | In vitro | [46] |
| Inhibits BCL-2, suppresses SMAD4, and promotes GC apoptosis | SMAD4 | pig | In vitro | [59] | |
| Suppresses HAS2, enhances caspase-3 and promotes GC apoptosis | HAS2 | pig | In vitro | [53] | |
| miR-92a | Inhibits SMAD7 and anti-apoptotic | SMAD7 | pig | In vitro | [48] |
| miR-21 | Decreases cleaved caspase 3, inhibits apoptotic | ? | mice | in vivo and in vitro | [57] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Worku, T.; Rehman, Z.U.; Talpur, H.S.; Bhattarai, D.; Ullah, F.; Malobi, N.; Kebede, T.; Yang, L. MicroRNAs: New Insight in Modulating Follicular Atresia: A Review. Int. J. Mol. Sci. 2017, 18, 333. https://doi.org/10.3390/ijms18020333
Worku T, Rehman ZU, Talpur HS, Bhattarai D, Ullah F, Malobi N, Kebede T, Yang L. MicroRNAs: New Insight in Modulating Follicular Atresia: A Review. International Journal of Molecular Sciences. 2017; 18(2):333. https://doi.org/10.3390/ijms18020333
Chicago/Turabian StyleWorku, Tesfaye, Zia Ur Rehman, Hira Sajjad Talpur, Dinesh Bhattarai, Farman Ullah, Ngabu Malobi, Tesfaye Kebede, and Liguo Yang. 2017. "MicroRNAs: New Insight in Modulating Follicular Atresia: A Review" International Journal of Molecular Sciences 18, no. 2: 333. https://doi.org/10.3390/ijms18020333
APA StyleWorku, T., Rehman, Z. U., Talpur, H. S., Bhattarai, D., Ullah, F., Malobi, N., Kebede, T., & Yang, L. (2017). MicroRNAs: New Insight in Modulating Follicular Atresia: A Review. International Journal of Molecular Sciences, 18(2), 333. https://doi.org/10.3390/ijms18020333