A Plant Proteinase Inhibitor from Enterolobium contortisiliquum Attenuates Pulmonary Mechanics, Inflammation and Remodeling Induced by Elastase in Mice
Abstract
:1. Introduction
2. Results
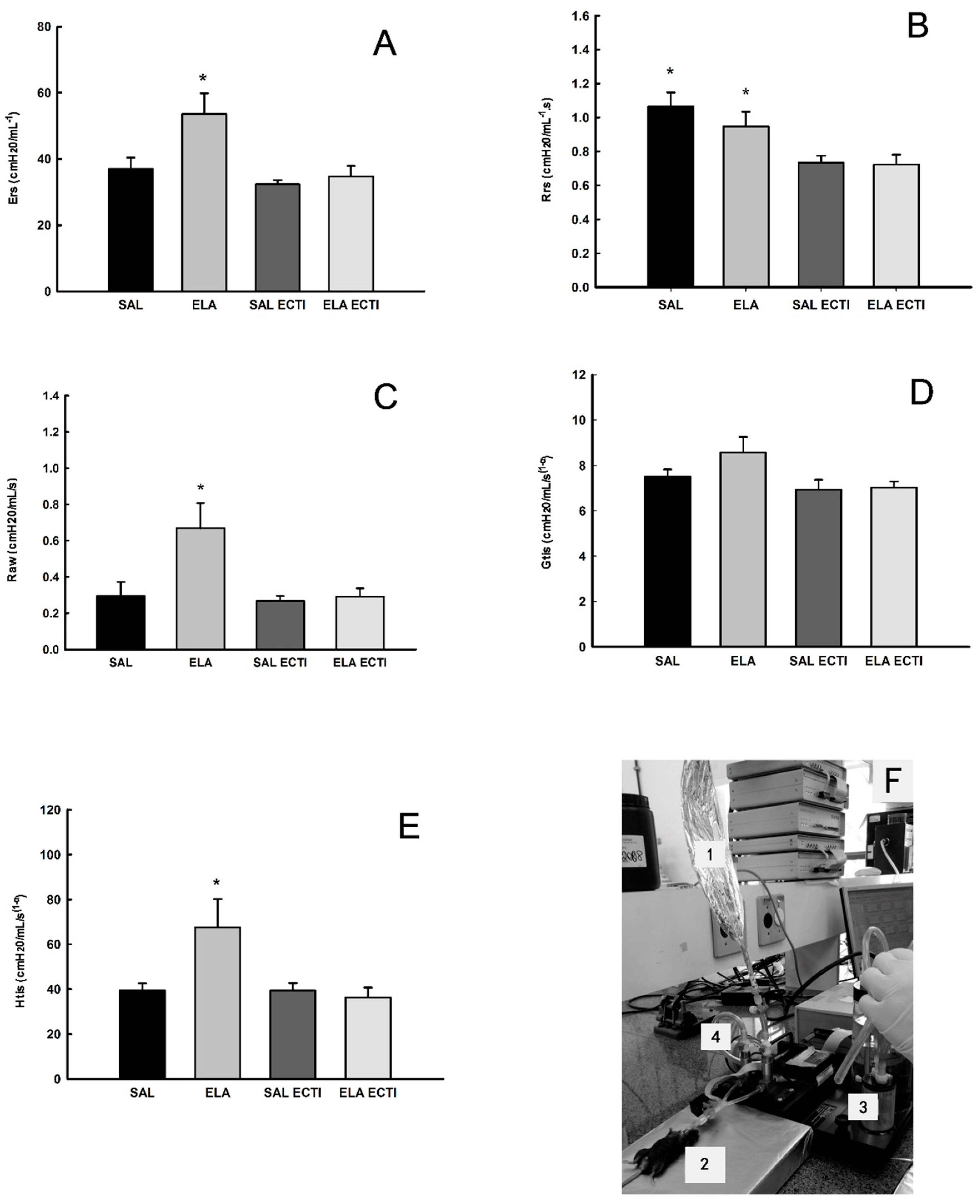
2.1. Lung Mechanics
2.2. Bronchoalveolar Lavage Fluid (BALF)
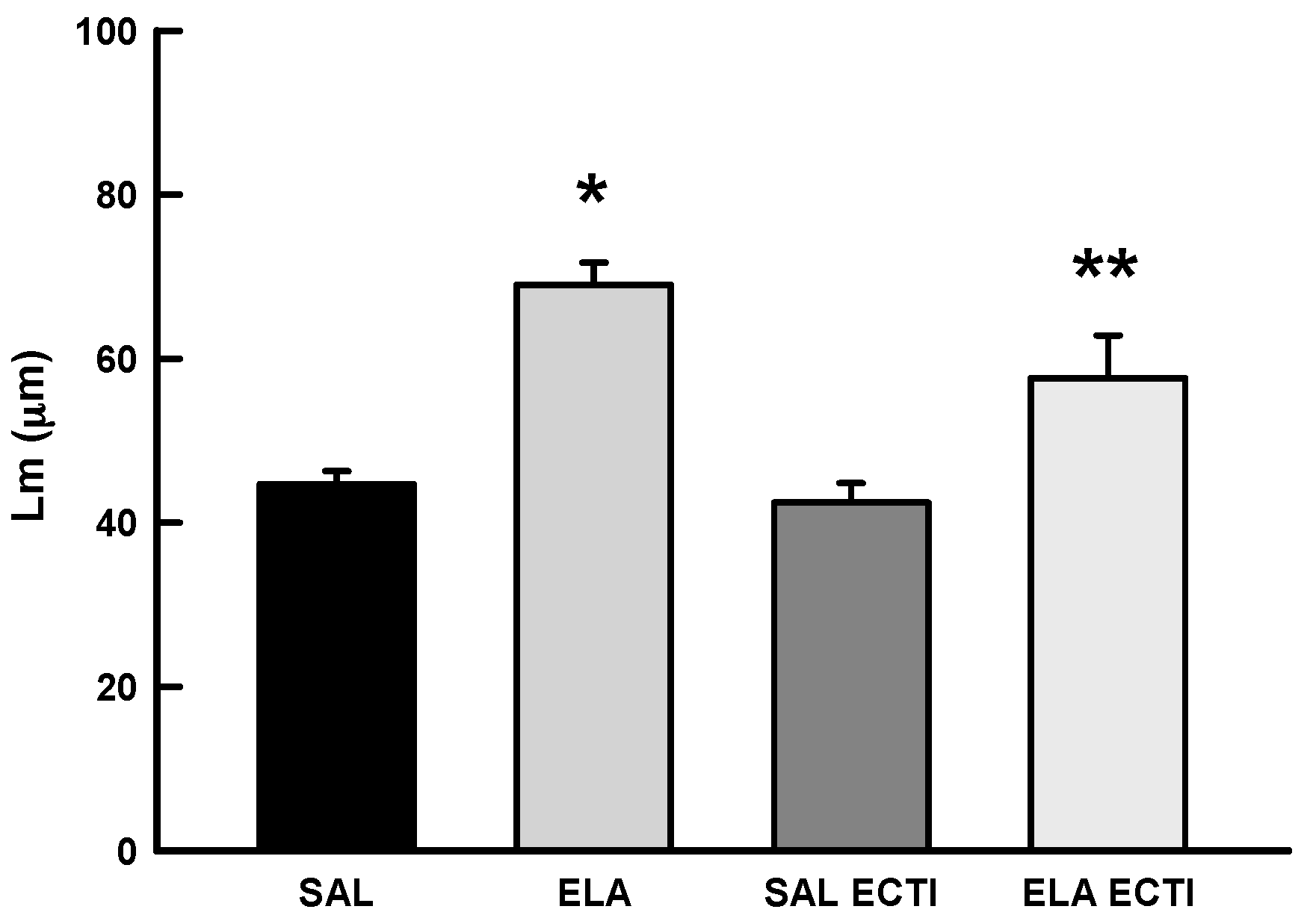
2.3. Morphometric Analysis
2.3.1. Mean Linear Intercept (Lm)
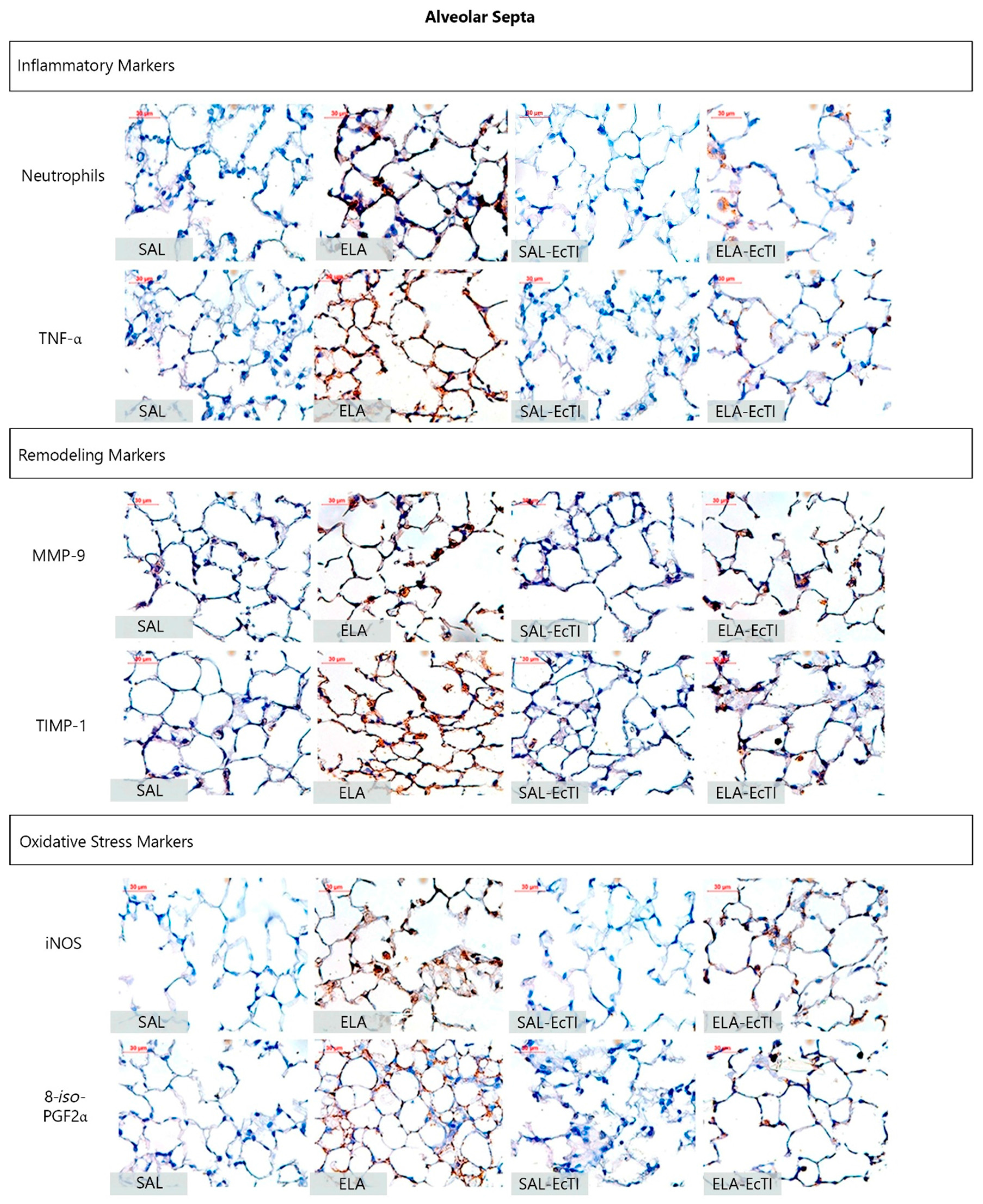
2.3.2. Lung Inflammation
2.3.3. Extracellular Matrix Remodeling
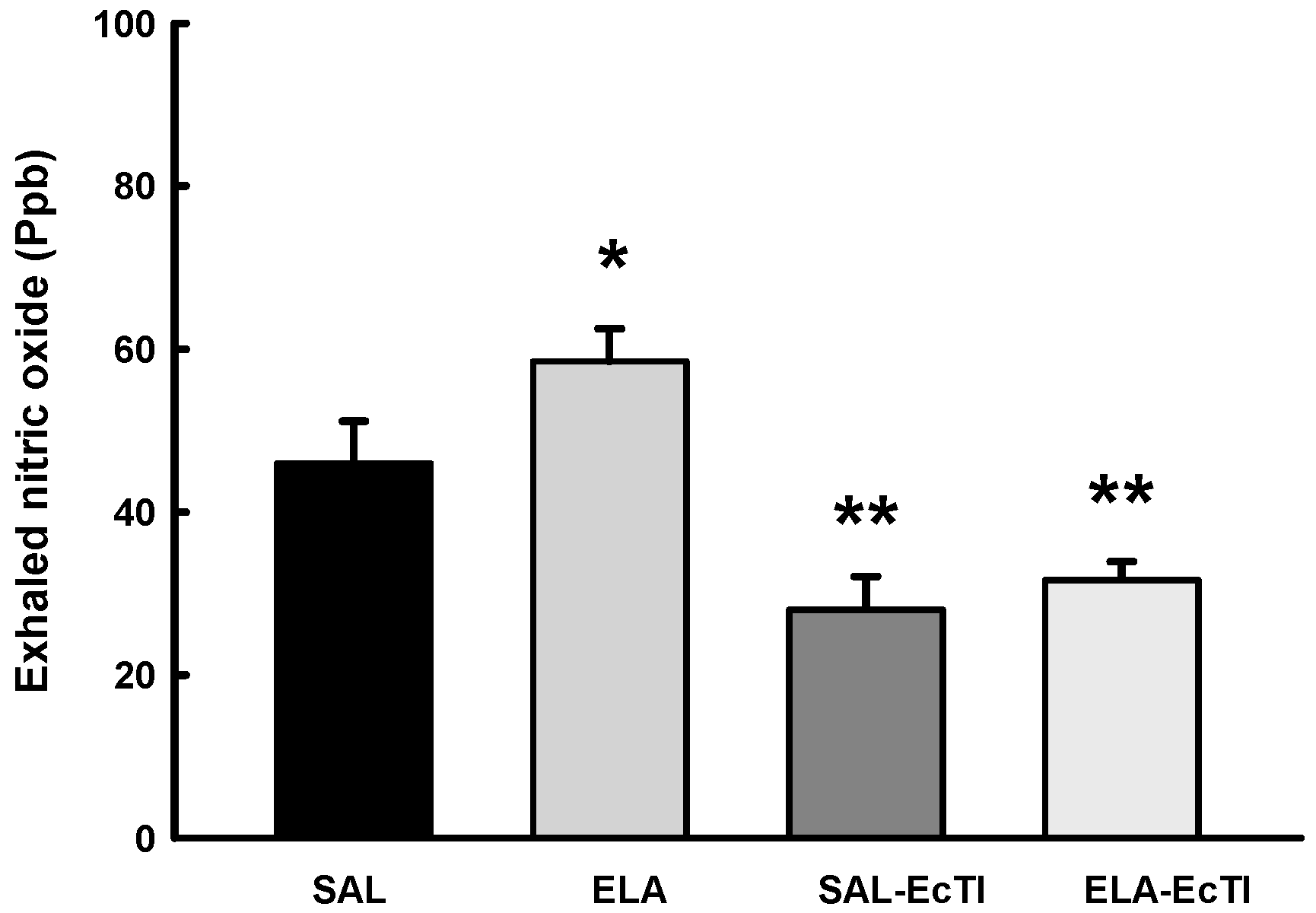
2.3.4. Oxidative Stress
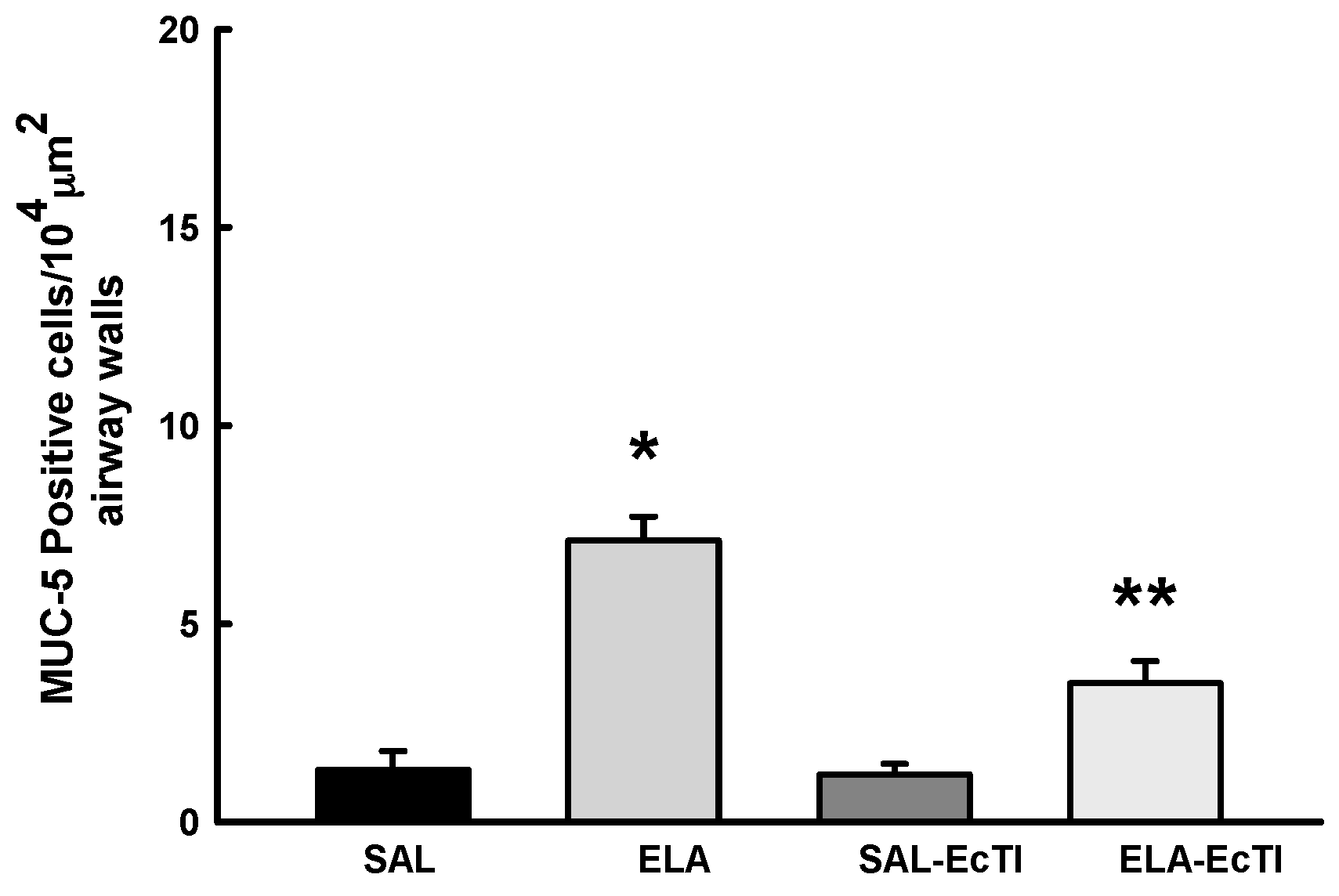
2.3.5. Tracheobronchial Positive Mucin Cells
2.4. Qualitative Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Protein
4.2. Animals
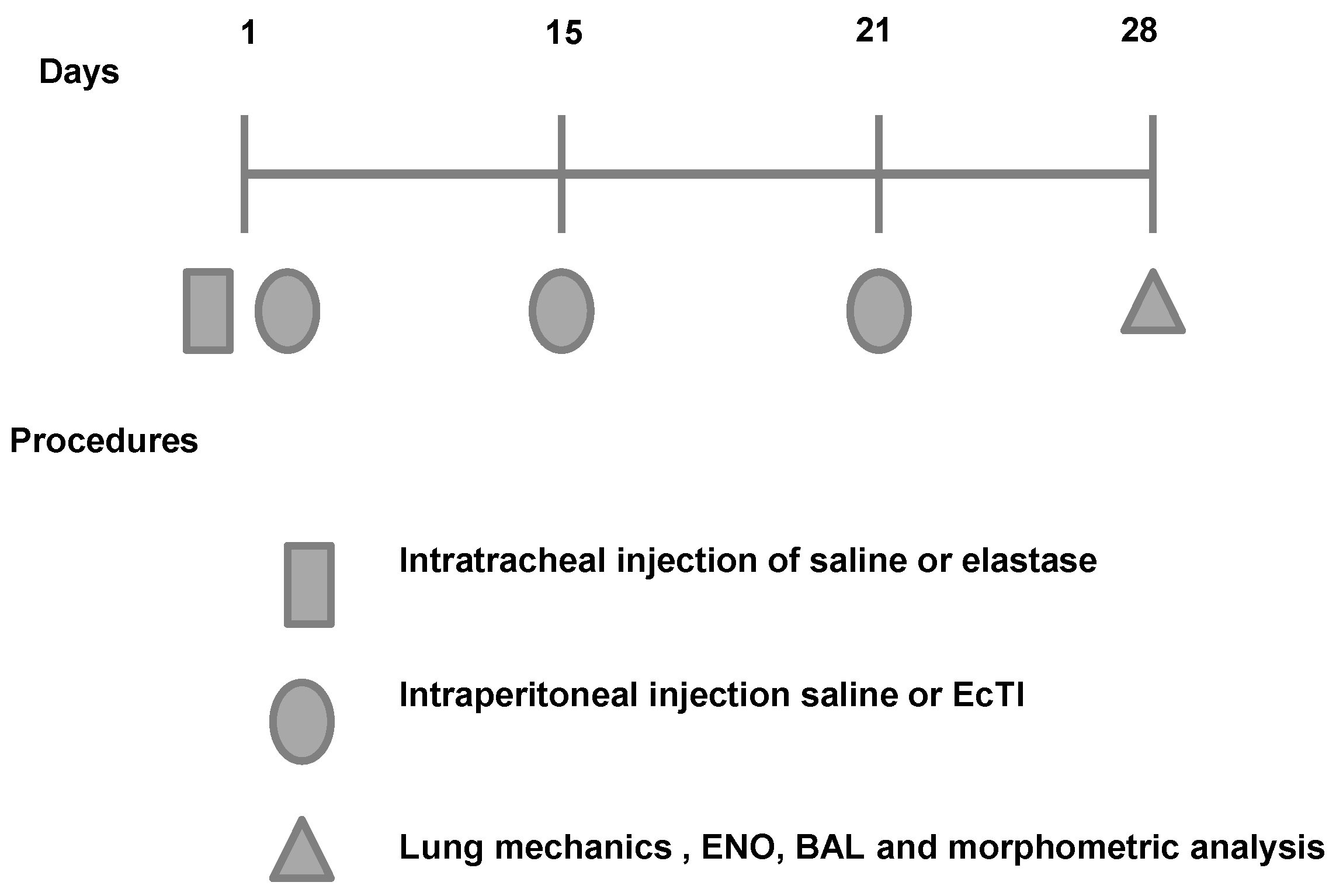
4.3. Animal Model
- (1)
- ELA group: the animals were anesthetized with isoflurane, and the neck region was shaved and disinfected with Povidone. An incision was made approximately 0.5 cm in the mid-region of the neck to expose the trachea. Using a 30-unit syringe, a single 50 µL dose of 0.025 mg of porcine pancreatic elastase (E-1250-100 mg elastase solution pancreatic porcine pancreas type 2x crystallized aqueous solution contains approximately 0.01% thymol 14.29 mL, 7 mg of protein/M 5.2 units/mg protein; Sigma, Carlsbad, CA, USA) was intratracheally administered between the cartilaginous rings. Afterward, the cervical incision was closed with a 5.0 silk suture and disinfected with Povidone [34];
- (2)
- ELA-EcTI group: 1 h after the intratracheal administration of elastase the mice received intraperitoneally injection (i.p.) of EcTI (2 mg/kg). On days 15, 21 and 28, the animals received two doses of EcTI (2 mg/kg i.p.) (n = 8);
- (3)
- SAL group: 1 h after the intratracheal administration of saline (50 µL). On days 15, 21 and 28, the animals received two doses of saline i.p. (n = 8);
- (4)
- SAL-EcTI: 1 h after the intratracheal administration of saline (50 µL), the mice received intraperitoneally injection of EcTI (2 mg/kg). On days 15, 21 and 28, the animals received two doses of EcTI (2 mg/kg i.p.) (n = 8).
4.4. Measurement of Exhaled Nitric Oxide (ENO) and Mechanical Evaluation
4.5. Bronchoalveolar Lavage Fluid
4.6. Lung Histology
4.7. Immunohistochemistry
4.8. Mean Alveolar Diameter (Lm)
4.9. Data Analyses
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| EcTI | Enterolobium contortisiliquum trypsin inhibitor |
| MMP-9 | Matrix metalloproteinase-9 |
| MMP-12 | Matrix metalloproteinase-12 |
| TIMP-1 | Tissue inhibitor of matrix metalloproteinase-1 |
| iNOS | Inducible nitric oxide synthase |
| eNOS | Endothelial nitric oxide synthase |
| TNF-α | Tumor necrosis factor-α |
| 8-iso-PGF2α | 8-isoprostane-PGF2α |
| MUC-5AC | Mucin-5AC |
| Raw | Airway resistance greater |
| Gtis | Lower airway resistance or tissue |
| Htis | Lung tissue elastance |
References
- Global Strategy for Diagnosis, Management, and Prevention of COPD, Updated 2017. Available online: http://goldcopd.org/gold-2017-global-strategy-diagnosis-management-prevention-copd/ (accessed on 8 February 2017).
- Pauwels, R.A.; Rabe, K.F. Burden and clinical features of chronic obstructive pulmonary disease (COPD). Lancet 2004, 364, 613–620. [Google Scholar] [CrossRef]
- Shapiro, S.D. Proteolysis in the lung. Eur. Respir. J. Suppl. 2003, 44, 30s–32s. [Google Scholar] [CrossRef] [PubMed]
- Laurell, S. The electrophoretic α-1-globulin pattern of serum in α-1antitrypsin deficiency. Scand. J. Clin. Lab. Invest. 1963, 15, 132–140. [Google Scholar] [CrossRef]
- Pauwels, R.A.; Buist, A.S.; Calverley, P.M.; Jenkins, C.R.; Hurd, S.S. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO global initiative for chronic obstructive lung disease (GOLD) workshop summary. Am. J. Respir. Crit. Care Med. 2001, 163, 1256–1276. [Google Scholar] [CrossRef] [PubMed]
- Mahadeva, R.; Shapiro, S.D. Chronic obstructive pulmonary disease: Experimental animal models of pulmonary emphysema. Thorax 2002, 57, 908–914. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J.; Stockley, R.A. COPD: Current therapeutic interventions and future approaches. Eur. Respir. J. 2005, 25, 1084–1106. [Google Scholar] [CrossRef] [PubMed]
- Turk, B. Targeting proteases: Successes, failures and future prospects. Nat. Rev. Drug. Discov. 2006, 5, 785–799. [Google Scholar] [CrossRef] [PubMed]
- De Leo, F.; Volpicella, M.; Licciulli, F.; Liuni, S.; Gallerani, R.; Ceci, L.R. Plant-pis: A database for plant protease inhibitors and their genes. Nucleic. Acids. Res. 2002, 30, 347–348. [Google Scholar] [CrossRef] [PubMed]
- Oliva, M.L.; Sampaio, M.U. Action of plant proteinase inhibitors on enzymes of physiopathological importance. An. Acad. Cienc. 2009, 81, 615–621. [Google Scholar] [CrossRef]
- De Paula, C.A.; Coulson-Thomas, V.J.; Ferreira, J.G.; Maza, P.K.; Suzuki, E.; Nakahata, A.M.; Nader, H.B.; Sampaio, M.U.; Oliva, M.L. Enterolobium contortisiliquum trypsin inhibitor (EcTI), a plant proteinase inhibitor, decreases in vitro cell adhesion and invasion by inhibition of Src protein-focal adhesion kinase (FAK) signaling pathways. J. Biol. Chem. 2012, 287, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Batista, I.F.; Nonato, M.C.; Bonfadini, M.R.; Beltramini, L.M.; Oliva, M.L.; Sampaio, M.U.; Sampaio, C.A.; Garratt, R.C. Preliminary crystalographic studies of EcTI, a serine proteinase inhibitor from Enterolobium contortisiliquum seeds. Acta Crystallogr. D Biol. Crystallogr. 2001, 57, 602–604. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, S.D. Animal models for COPD. Chest 2000, 117, 223S–227S. [Google Scholar] [CrossRef] [PubMed]
- Selman, M.; Cisneros-Lira, J.; Gaxiola, M.; Ramirez, R.; Kudlacz, E.M.; Mitchell, P.G.; Pardo, A. Matrix metalloproteinases inhibition attenuates tobacco smoke-induced emphysema in guinea pigs. Chest 2003, 123, 1633–1641. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.L.; Farmer, S.G.; Churg, A. A neutrophil elastase inhibitor reduces cigarette smoke-induced remodelling of lung vessels. Eur. Respir. J. 2003, 22, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, F.; Spencer, J.L.; Lucey, E.C.; Nugent, M.A.; Stone, P.J. A human surfactant peptide-elastase inhibitor construct as a treatment for emphysema. Proc. Natl. Acad. Sci. USA 2010, 107, 10661–10666. [Google Scholar] [CrossRef] [PubMed]
- Luisetti, M.; Sturani, C.; Sella, D.; Madonini, E.; Galavotti, V.; Bruno, G.; Peona, V.; Kucich, U.; Dagnino, G.; Rosenbloom, J.; et al. MR889, a neutrophil elastase inhibitor, in patients with chronic obstructive pulmonary disease: A double-blind, randomized, placebo-controlled clinical trial. Eur. Respir. J. 1996, 9, 1482–1486. [Google Scholar] [CrossRef] [PubMed]
- Benetazzo, M.G.; Gilè, L.S.; Bombieri, C.; Malerba, G.; Massobrio, M.; Pignatti, P.F.; Luisetti, M. α1-Antitrypsin TAQ I polymorphism and α1-antichymotrypsin mutations in patients with obstructive pulmonary disease. Respir. Med. 1999, 93, 648–654. [Google Scholar] [CrossRef]
- Churg, A.; Wright, J.L. Proteases and emphysema. Curr. Opin. Pulm. Med. 2005, 11, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Owen, C.A. Roles for proteinases in the pathogenesis of chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulmon. Dis. 2008, 3, 253–268. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, S.D.; Goldstein, N.M.; Houghton, A.M.; Kobayashi, D.K.; Kelley, D.; Belaaouaj, A. Neutrophil elastase contributes to cigarette smoke-induced emphysema in mice. Am. J. Pathol. 2003, 163, 2329–2335. [Google Scholar] [CrossRef]
- Owen, C.A.; Campbell, E.J. The cell biology of leukocyte-mediated proteolysis. J. Leukoc. Biol. 1999, 65, 137–150. [Google Scholar] [PubMed]
- Deshmukh, H.S.; Case, L.M.; Wesselkamper, S.C.; Borchers, M.T.; Martin, L.D.; Hertzer, H.G.; Nadel, J.A.; Leikauf, G.D. Metalloproteinases mediate mucin 5AC expression by epidermal growth factor receptor activation. Am. J. Respir. Crit. Care Med. 2005, 171, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Hantos, Z.; Adamicza, A.; Janosi, T.Z.; Szabari, M.V.; Tolnai, J.; Suki, B. Lung volumes and respiratory mechanics in elastase-induced emphysema in mice. J. Appl. Physiol. 2008, 105, 1864–1872. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Ingenito, E.P.; Arold, S.P.; Parameswaran, H.; Tgavalekos, N.T.; Lutchen, K.R.; Suki, B. Tissue heterogeneity in the mouse lung: Effects of elastase treatment. J. Appl. Physiol. 2004, 97, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Scuri, M.; Forteza, R.; Lauredo, I.; Sabater, J.R.; Botvinnikova, Y.; Allegra, L.; Abraham, W.M. Inhaled porcine pancreatic elastase causes bronchoconstriction via a bradykinin-mediated mechanism. J. Appl. Physiol. 2000, 89, 1397–1402. [Google Scholar] [PubMed]
- Suzuki, M.; Betsuyaku, T.; Ito, Y.; Nagai, K.; Odajima, N.; Moriyama, C.; Nasuhara, Y.; Nishimura, M. Curcumin attenuates elastase- and cigarette smoke-induced pulmonary emphysema in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009, 296, L614–L623. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.F.; Adcock, I.M. Multifaceted mechanisms in COPD: Inflammation, immunity, and tissue repair and destruction. Eur. Respir. J. 2008, 31, 1334–1356. [Google Scholar] [CrossRef] [PubMed]
- Neuhof, C.; Oliva, M.L.; Maybauer, D.; Maybauer, M.; de Oliveira, C.; Sampaio, U.M.; Sampaio, C.A.; Neuhof, F. Effect of plant kunitz inhibitors from bauhinia bauhinioides and bauhinia rufa on pulmonary edema caused by activated neutrophils. Biol. Chem. 2003, 384, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Plantier, L.; Marchand-Adam, S.; Antico, V.G.; Boyer, L.; de Coster, C.; Marchal, J.; Bachoual, R.; Mailleux, A.; Boczkowski, J.; Crestani, B. Keratinocyte growth factor protects against elastase-induced pulmonary emphysema in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 293, L1230–L1239. [Google Scholar] [CrossRef] [PubMed]
- Lopes, F.D.; Toledo, A.C.; Olivo, C.R.; Prado, C.M.; Leick, E.A.; Medeiros, M.C.; Santos, A.B.; Garippo, A.; Martins, M.A.; Mauad, T. A comparative study of extracellular matrix remodelling in two murine models of emphysema. Histol. Histopathol. 2013, 28, 269–276. [Google Scholar] [PubMed]
- Hanaoka, M.; Droma, Y.; Chen, Y.; Agatsuma, T.; Kitaguchi, Y.; Voelkel, N.F.; Kubo, K. Carbocisteine protects against emphysema induced by cigarette smoke extract in rats. Chest 2011, 139, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, Q.; Zhou, X.; Kolosov, V.P.; Perelman, J.M. Human airway trypsin-like protease induces mucin5ac hypersecretion via a protease-activated receptor 2-mediated pathway in human airway epithelial cells. Arch. Biochem. Biophys. 2013, 535, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.M.; Leick-Maldonado, E.; Kasahara, D.I.; Capellozi, V.L.; Martins, M.A.; Tibério, I.F. Effects of acute and chronic nitric oxide inhibition in an experimental model of chronic pulmonary allergic inflammation in guinea pigs. Am. J. Physiol. Lung. Cell. Mol. Physiol. 2005, 289, L677–L683. [Google Scholar] [CrossRef] [PubMed]
- Nakahata, A.M.; Mayer, B.; Ries, C.; de Paula, C.A.; Karow, M.; Neth, P.; Sampaio, M.U.; Jochum, M.; Oliva, M.L. The effects of a plant proteinase inhibitor from Enterolobium contortisiliquum on human tumor cell lines. Biol. Chem. 2011, 392, 327–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martins-Olivera, B.T.; Almeida-Reis, R.; Theodoro-Júnior, O.A.; Oliva, L.V.; Neto Dos Santos Nunes, N.; Olivo, C.R.; Vilela de Brito, M.; Prado, C.M.; Leick, E.A.; Martins, M.A.; et al. The plant-derived bauhinia bauhinioides kallikrein proteinase inhibitor (rBbKI) attenuates elastase-induced emphysema in mice. Mediators Inflamm. 2016, 2016, 5346574. [Google Scholar] [CrossRef] [PubMed]
- Weibel, E.R. Principles and methods for the morphometric study of the lung and other organs. Lab. Investig. 1963, 12, 131–155. [Google Scholar] [PubMed]
- Righetti, R.F.; Pigati, P.A.; Possa, S.S.; Habrum, F.C.; Xisto, D.G.; Antunes, M.A.; Leick, E.A.; Prado, C.M.; Martins, M.A.; Rocco, P.R.; et al. Effects of Rho-kinase inhibition in lung tissue with chronic inflammation. Respir. Physiol. Neurobiol. 2014, 192, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Pigati, P.A.; Righetti, R.F.; Possa, S.S.; Romanholo, B.S.; Rodrigues, A.P.; dos Santos, A.S.; Xisto, D.G.; Antunes, M.A.; Prado, C.M.; Leick, E.A.; et al. Y-27632 is associated with corticosteroid-potentiated control of pulmonary remodeling and inflammation in guinea pigs with chronic allergic inflammation. BMC Pulm. Med. 2015, 15, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakoda, C.P.; de Toledo, A.C.; Perini, A.; Pinheiro, N.M.; Hiyane, M.I.; Grecco, S.S.; Tibério, I.F.L.C.; Câmara, N.O.; Martins, M.A.; Lago, J.H.; et al. Sakuranetin reverses vascular peribronchial and lung parenchyma remodeling in a murine model of chronic allergic pulmonary inflammation. Acta Histochem. 2016, 118, 615–624. [Google Scholar] [CrossRef] [PubMed]

| Inflammatory Markers | SAL | ELA | SAL-EcTI | ELA-EcTI |
|---|---|---|---|---|
| Total cells (104 cells/mL) | 0.51 ± 0.05 | 1.83 ± 0.06 * | 0.61 ± 0.07 | 0.92 ± 0.3 ** |
| Macrophages (104 cells/mL) | 0.46 ± 0.11 | 1.57 ± 0.17 * | 0.57 ± 0.09 | 0.64 ± 0.12 ** |
| Neutrophils (104 cells/mL) | 0.00 ± 0.00 | 0.05 ± 0.17 * | 0.01 ± 0.09 | 0.00 ± 0.00 ** |
| Lymphocytes (104 cells/mL) | 0.00 ± 0.00 | 0.36 ± 0.01 * | 0.00 ± 0.00 | 0.00 ± 0.00 ** |
| Eosinophils (104 cells/mL) | 0.00 ± 0.00 | 0.15 ± 0.01 * | 0.00 ± 0.00 | 0.00 ± 0.00 ** |
| Inflammatory Markers | SAL | ELA | SAL-EcTI | ELA-EcTI |
| Neutrophils (cells/104 μm2) | 0.12 ± 0.05 | 0.51 ± 0.06 * | 0.21 ± 0.07 | 0.22 ± 0.3 ** |
| Macrophages (cells/104 μm2) | 0.30 ± 0.11 | 1.28 ± 0.17 * | 0.74 ± 0.0.09 | 0.87 ± 0.12 ** |
| TNF-α (cells/104 μm2) | 0.98 ± 0.19 | 3.92 ± 0.43 * | 1.13 ± 0.23 | 2.11 ± 0.31 ** |
| Remodeling Markers | SAL | ELA | SAL-EcTI | ELA-EcTI |
| Collagen Fibers (%) | 9.69 ± 0.07 | 11.62 ± 0.37 * | 9.3 ± 0.9 | 9.06 ± 0.7 ** |
| Elastic Fibers (%) | 0.29 ± 0.03 | 0.47 ± 0.03 * | 0.34 ± 0.02 | 0.41 ± 0.04 |
| MMP-9 (cells/104 μm2) | 3.17 ± 0.5 | 12.48 ± 0.86 * | 4.69 ± 0.47 | 8.7 ± 1.42 */** |
| MMP-12 (cells/104 μm2) | 5.03 ± 0.63 | 16.29 ± 1.07 * | 5.93 ± 0.69 | 9.49 ± 0.37 */** |
| TIMP-1 (cells/104 μm2) | 3.4 ± 0.33 | 14.04 ± 0.75 * | 4.34 ± 0.4 | 11.3 ± 1.08 */** |
| Oxidative Stress Markers | SAL | ELA | SAL-EcTI | ELA-EcTI |
| iNOS (cells/104 μm2) | 0.7 ± 0.15 | 3.33 ± 0.37 * | 1.08 ± 0.23 | 1.94 ± 0.29 ** |
| eNOS (cells/104 μm2) | 0.63 ± 0.18 | 3.01 ± 0.45 * | 0.58 ± 0.12 | 1.51 ± 0.26 ** |
| 8-iso-PGF2α (%) | 5.93 ± 0.58 | 13.89 ± 1.07 * | 7.16 ± 0.9 | 11.08 ± 0.64 */** |
| Inflammatory Markers | SAL | ELA | SAL-EcTI | ELA-EcTI |
| Neutrophils (cells/104 μm2) | 0.89 ± 0.27 | 4.02 ± 0.38 * | 0.87 ± 0.25 | 2.35 ± 0.40 */** |
| TNF-α (cells/104 μm2) | 0.84 ± 0.38 | 4.01 ± 0.54 * | 1.16 ± 0.22 | 2.45 ± 0.45 ** |
| Remodeling Markers | SAL | ELA | SAL-EcTI | ELA-EcTI |
| Collagen Fibers (%) | 4.46 ± 0.51 | 8.84 ± 0.44 * | 3.15 ± 0.29 | 6.11 ± 0.7 ** |
| Elastic Fibers (%) | 0.30 ± 0.06 | 2.47 ± 0.36 * | 0.91 ± 0.17 | 1.53 ± 0.35 ** |
| MMP-9 (cells/104 μm2) | 4.61 ± 0.78 | 13.85 ± 1.53 * | 4.4 ± 0.52 | 9.85 ± 0.82 */** |
| MMP-12 (cells/104 μm2) | 5.25 ± 0.49 | 15.94 ± 0.95 * | 6.05 ± 0.45 | 9.59 ± 0.48 */** |
| TIMP-1 (cells/104 μm2) | 3.49 ± 0.65 | 13.13 ± 0.98 * | 3.64 ± 0.79 | 11.82 ± 0.87 * |
| Oxidative Stress Markers | SAL | ELA | SAL-EcTI | ELA-EcTI |
| iNOS (cells/104 μm2) | 0.84 ± 0.37 | 5.13 ± 038 * | 1.34 ± 0.27 | 2.39 ± 0.39 ** |
| eNOS (cells/104 μm2) | 0.86 ± 0.28 | 3.09 ± 0.43 * | 1.13 ± 0.2 | 1.99 ± 0.37 ** |
| 8-iso-PGF2α (%) | 6.75 ± 0.39 | 16.12 ± 1.17 * | 6.67 ± 0.89 | 12.04 ± 1.14 */** |
| Mucus Production | SAL | ELA | SAL-EcTI | ELA-EcTI |
| MUC-5AC (cells/104 μm2) | 1.31 ± 0.48 | 7.09 ± 0.61 * | 1.19 ± 0.27 | 3.5 ± 0.6 */** |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Theodoro-Júnior, O.A.; Righetti, R.F.; Almeida-Reis, R.; Martins-Oliveira, B.T.; Oliva, L.V.; Prado, C.M.; Saraiva-Romanholo, B.M.; Leick, E.A.; Pinheiro, N.M.; Lobo, Y.A.; et al. A Plant Proteinase Inhibitor from Enterolobium contortisiliquum Attenuates Pulmonary Mechanics, Inflammation and Remodeling Induced by Elastase in Mice. Int. J. Mol. Sci. 2017, 18, 403. https://doi.org/10.3390/ijms18020403
Theodoro-Júnior OA, Righetti RF, Almeida-Reis R, Martins-Oliveira BT, Oliva LV, Prado CM, Saraiva-Romanholo BM, Leick EA, Pinheiro NM, Lobo YA, et al. A Plant Proteinase Inhibitor from Enterolobium contortisiliquum Attenuates Pulmonary Mechanics, Inflammation and Remodeling Induced by Elastase in Mice. International Journal of Molecular Sciences. 2017; 18(2):403. https://doi.org/10.3390/ijms18020403
Chicago/Turabian StyleTheodoro-Júnior, Osmar Aparecido, Renato Fraga Righetti, Rafael Almeida-Reis, Bruno Tadeu Martins-Oliveira, Leandro Vilela Oliva, Carla Máximo Prado, Beatriz Mangueira Saraiva-Romanholo, Edna Aparecida Leick, Nathalia Montouro Pinheiro, Yara Aparecida Lobo, and et al. 2017. "A Plant Proteinase Inhibitor from Enterolobium contortisiliquum Attenuates Pulmonary Mechanics, Inflammation and Remodeling Induced by Elastase in Mice" International Journal of Molecular Sciences 18, no. 2: 403. https://doi.org/10.3390/ijms18020403





