Analysis of Epidermal Growth Factor Receptor Related Gene Expression Changes in a Cellular and Animal Model of Parkinson’s Disease
Abstract
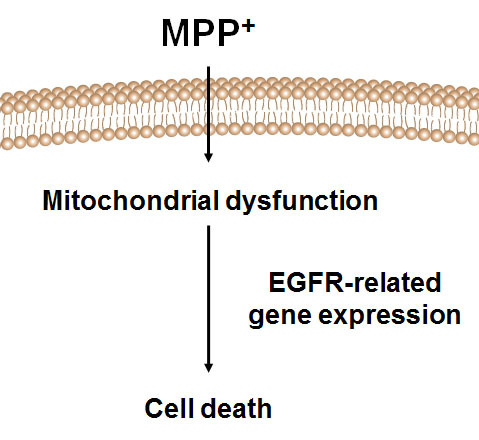
:1. Introduction
2. Results
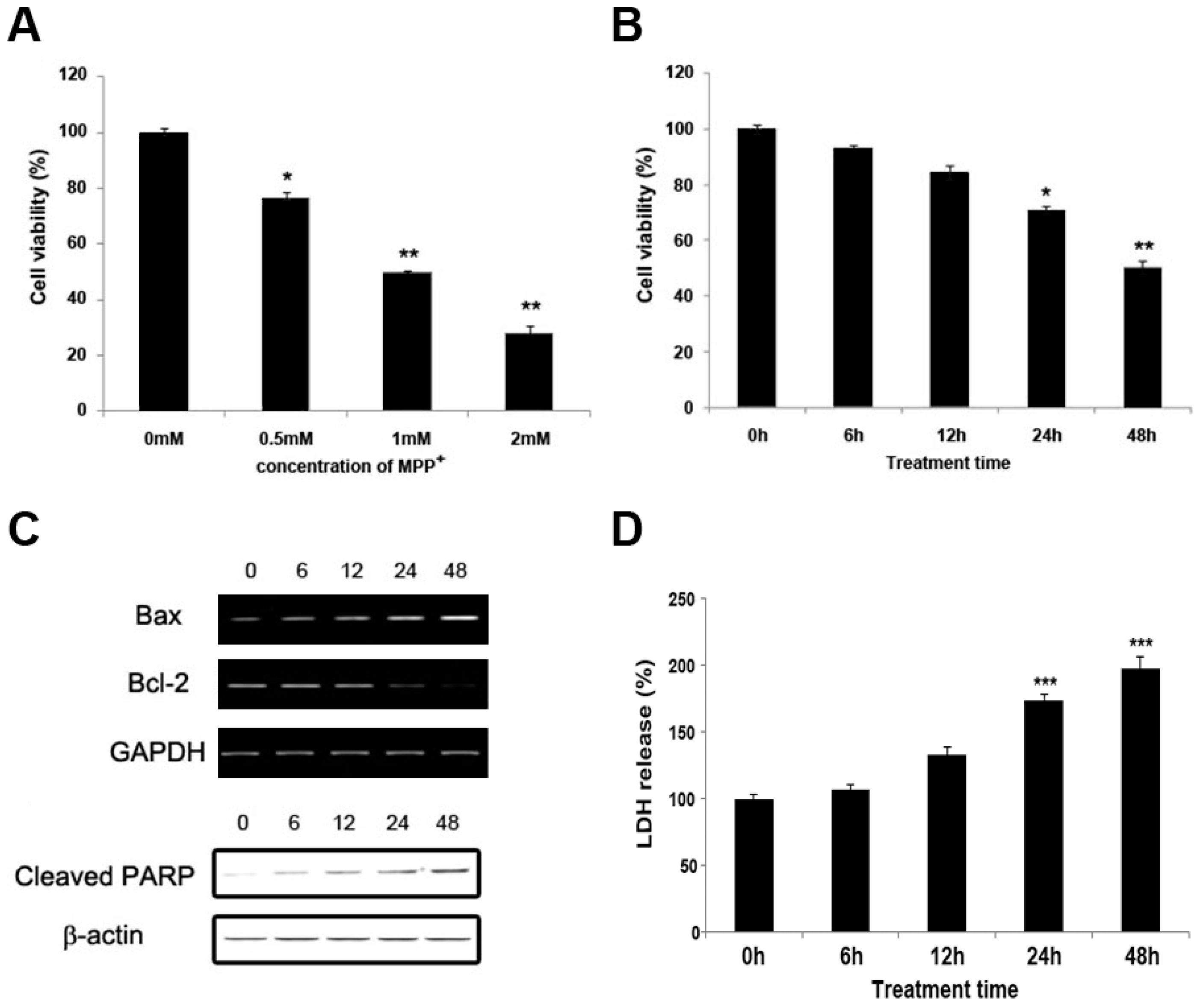
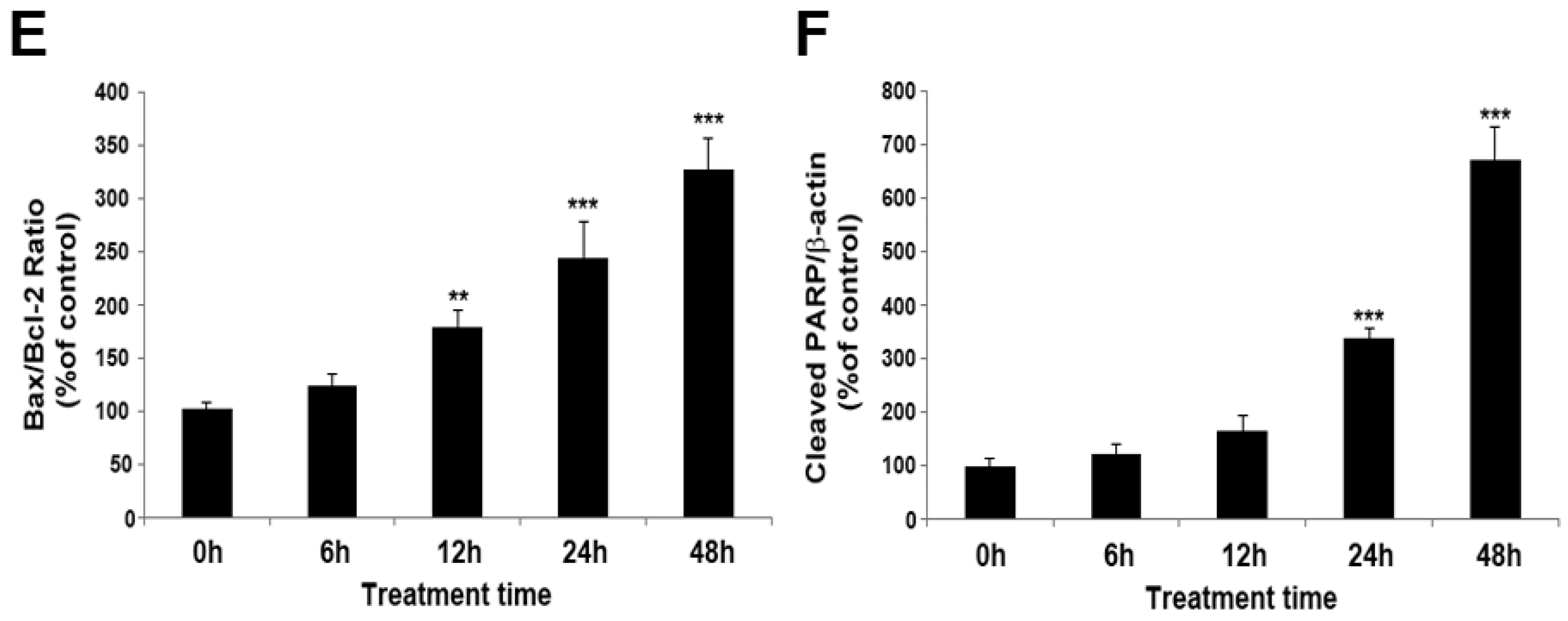
2.1. MPP+ Induces Dopaminergic Neuronal Cell Death in SH-SY5Y
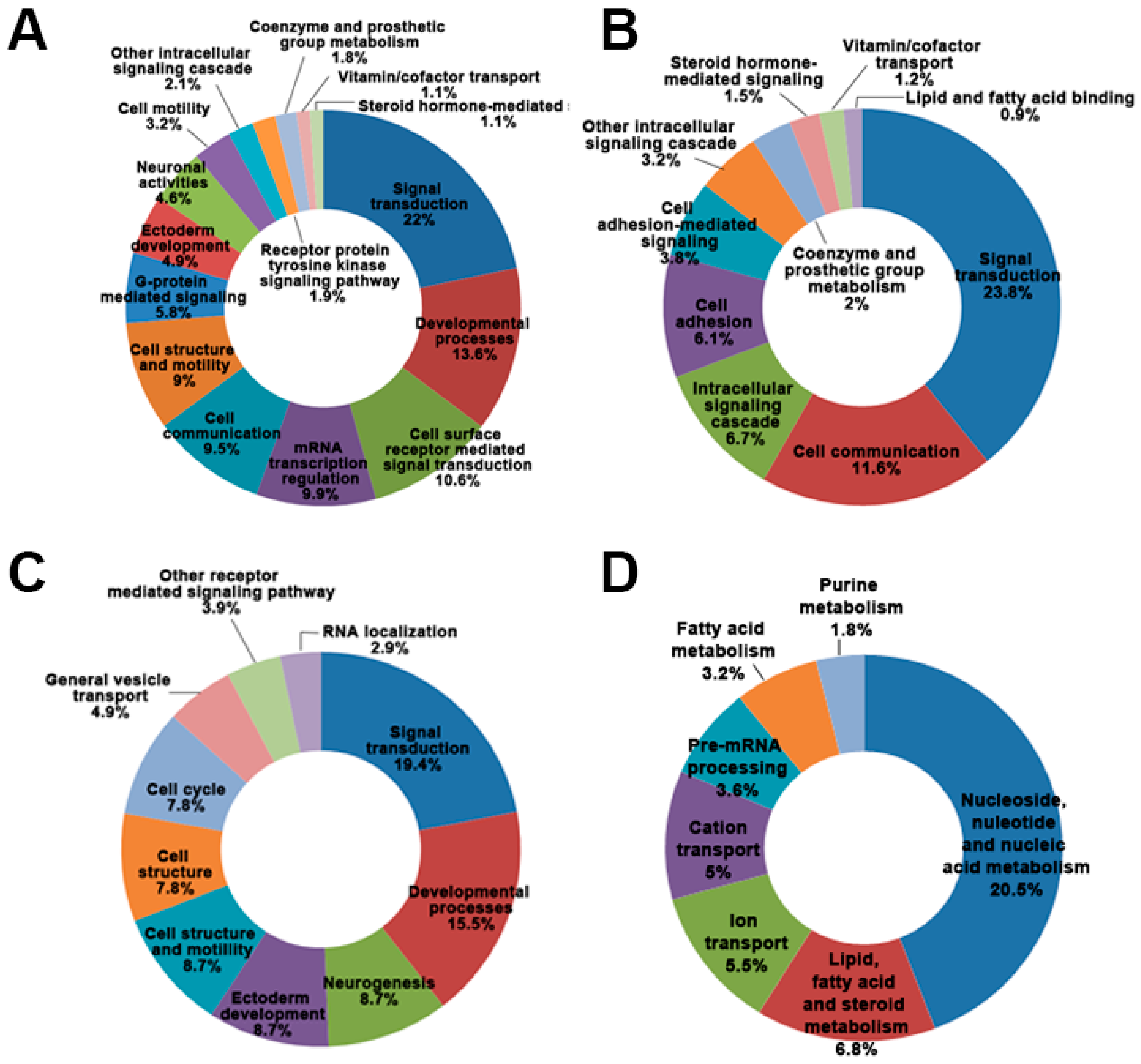
2.2. Differential Gene Expression and Pathway from MPP+-Treated SH-SY5Y Cells at Two Time Points (24 and 48 h)
2.3. Validation of Microarray Results
2.4. Analysis of EGFR Pathway-Related Gens in MPP+-Induced DAergic Neuronal Cell Death
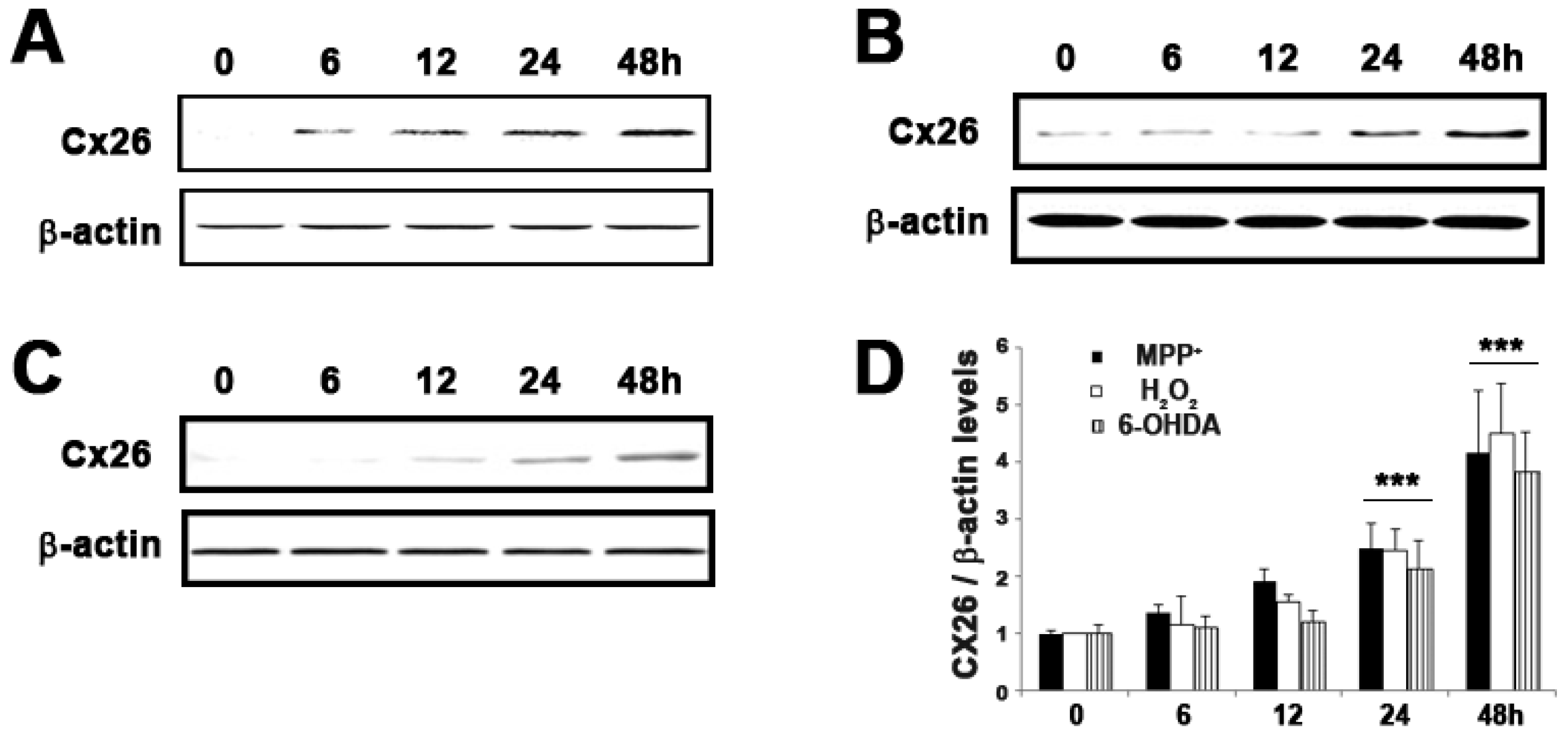
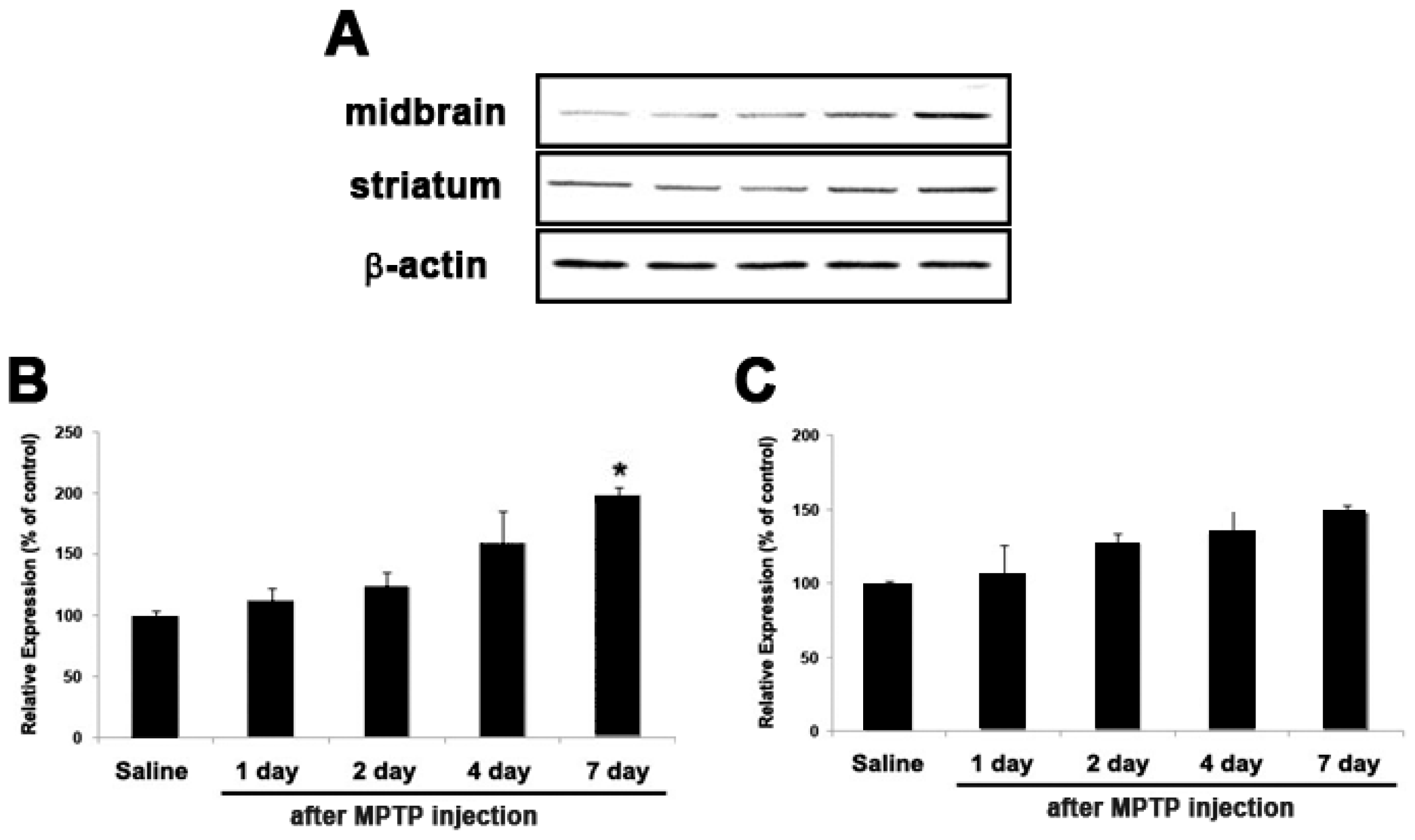
2.5. Expression of GJB2 (Cx26) in In Vitro and In Vivo Pakinson’s Model
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture and Treatments
4.3. Assessment of Cell Viability
4.4. Animals and Treatments
4.5. Microarray Analysis
4.6. Expression Analysis
4.7. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pieczenik, S.R.; Neustadt, J. Mitochondrial dysfunction and molecular pathways of disease. Exp. Mol. Pathol. 2007, 83, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Arribas, M.; Yakhine-Diop, S.M.; Pedro, J.M.; Gomez-Suaga, P.; Gomez-Sanchez, R.; Martinez-Chacon, G.; Fuentes, J.M.; Gonzalez-Polo, R.A.; Niso-Santano, M. Mitochondria-associated membranes (MAMs): Overview and its role in Parkinson’s disease. Mol. Neurobiol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Singer, T.P.; Ramsay, R.R. Mechanism of the neurotoxicity of MPTP. An update. FEBS Lett. 1990, 274, 1–8. [Google Scholar] [PubMed]
- Eberhardt, O.; Schulz, J.B. Apoptotic mechanisms and antiapoptotic therapy in the MPTP model of Parkinson’s disease. Toxicol. Lett. 2003, 139, 135–151. [Google Scholar] [CrossRef]
- Przedborski, S.; Tieu, K.; Perier, C.; Vila, M. MPTP as a mitochondrial neurotoxic model of Parkinson’s disease. J. Bioenerg. Biomembr. 2004, 36, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Blesa, J.; Przedborski, S. Parkinson’s disease: Animal models and dopaminergic cell vulnerability. Front. Neuroanat. 2014, 8, 155. [Google Scholar] [CrossRef] [PubMed]
- Fiskum, G.; Starkov, A.; Polster, B.M.; Chinopoulos, C. Mitochondrial mechanisms of neural cell death and neuroprotective interventions in Parkinson’s disease. Ann. N. Y. Acad. Sci. 2003, 991, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, R.K.; Flint Beal, M. Mitochondrial diseases of the brain. Free Radic. Biol. Med. 2013, 63, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Conn, K.J.; Ullman, M.D.; Larned, M.J.; Eisenhauer, P.B.; Fine, R.E.; Wells, J.M. cDNA microarray analysis of changes in gene expression associated with MPP+ toxicity in SH-SY5Y cells. Neurochem. Res. 2003, 28, 1873–1881. [Google Scholar] [CrossRef] [PubMed]
- Datki, Z.; Juhasz, A.; Galfi, M.; Soos, K.; Papp, R.; Zadori, D.; Penke, B. Method for measuring neurotoxicity of aggregating polypeptides with the MTT assay on differentiated neuroblastoma cells. Brain Res. Bull. 2003, 62, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Cory, S.; Adams, J.M. The BCL2 family: Regulators of the cellular life-or-death switch. Nat. Rev. Cancer 2002, 2, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Martire, S.; Mosca, L.; d’Erme, M. PARP-1 involvement in neurodegeneration: A focus on Alzheimer’s and Parkinson’s diseases. Mech. Ageing Dev. 2015, 146–148, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Perez, J.G.; Tran, N.L.; Rosenblum, M.G.; Schneider, C.S.; Connolly, N.P.; Kim, A.J.; Woodworth, G.F.; Winkles, J.A. The tweak receptor FN14 is a potential cell surface portal for targeted delivery of glioblastoma therapeutics. Oncogene 2016, 35, 2145–2155. [Google Scholar] [CrossRef] [PubMed]
- Crouch, P.J.; Harding, S.M.; White, A.R.; Camakaris, J.; Bush, A.I.; Masters, C.L. Mechanisms of a β mediated neurodegeneration in Alzheimer’s disease. Int. J. Biochem. Cell. Biol. 2008, 40, 181–198. [Google Scholar] [CrossRef] [PubMed]
- Hoe, H.S.; Lee, H.K.; Pak, D.T. The upside of APP at synapses. CNS Neurosci. Ther. 2012, 18, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Esposito, G.; Scuderi, C.; Steardo, L.; Delli-Bovi, L.C.; Hecht, J.L.; Dickinson, B.C.; Chang, C.J.; Mori, T.; Sheen, V. S100b and APP promote a gliocentric shift and impaired neurogenesis in down syndrome neural progenitors. PLoS ONE 2011, 6, e22126. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.Y.; Lin, T.Y.; Yang, W.H.; Wang, S.C.; Wang, K.T.; Su, Y.L.; Jiang, Y.W.; Chang, G.D.; Chang, C.J. Differential expression and functional analysis of the tristetraprolin family during early differentiation of 3T3-L1 preadipocytes. Int. J. Biol. Sci. 2012, 8, 761–777. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.F.; Nieh, S.; Jao, S.W.; Liu, C.L.; Wu, C.H.; Chang, Y.C.; Yang, C.Y.; Lin, Y.S. Quercetin suppresses drug-resistant spheres via the P38 MAPK-HSP27 apoptotic pathway in oral cancer cells. PLoS ONE 2012, 7, e49275. [Google Scholar] [CrossRef] [PubMed]
- Leibowitz, B.J.; Cohick, W.S. Endogenous IGFBP-3 is required for both growth factor-stimulated cell proliferation and cytokine-induced apoptosis in mammary epithelial cells. J. Cell. Physiol. 2009, 220, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Rash, J.E.; Yasumura, T.; Davidson, K.G.; Furman, C.S.; Dudek, F.E.; Nagy, J.I. Identification of cells expressing Cx43, Cx30, Cx26, Cx32 and Cx36 in gap junctions of rat brain and spinal cord. Cell. Commun. Adhes. 2001, 8, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Reactive oxygen species and the central nervous system. J. Neurochem. 1992, 59, 1609–1623. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, E.C. Does oxidative stress participate in nerve cell death in Parkinson’s disease? Eur. Neurol. 1993, 33, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Jackson-Lewis, V.; Przedborski, S. Protocol for the MPTP mouse model of Parkinson’s disease. Nat. Protoc. 2007, 2, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Riederer, P.; Jellinger, K. Morphological and biochemical changes in the aging brain: Pathophysiological and possible therapeutic consequences. Exp. Brain Res. 1982, 5, 158–166. [Google Scholar] [PubMed]
- Jorissen, R.N.; Walker, F.; Pouliot, N.; Garrett, T.P.; Ward, C.W.; Burgess, A.W. Epidermal growth factor receptor: Mechanisms of activation and signalling. Exp. Cell. Res. 2003, 284, 31–53. [Google Scholar] [CrossRef]
- Van der Geer, P.; Hunter, T.; Lindberg, R.A. Receptor protein-tyrosine kinases and their signal transduction pathways. Annu. Rev. Cell. Biol. 1994, 10, 251–337. [Google Scholar] [CrossRef] [PubMed]
- Daub, H.; Weiss, F.U.; Wallasch, C.; Ullrich, A. Role of transactivation of the EGF receptor in signalling by G-protein-coupled receptors. Nature 1996, 379, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Kogure, K.; Liu, X.H.; Araki, T.; Kato, K.; Itoyama, Y. Immunohistochemical localization of the low molecular weight stress protein HSP27 following focal cerebral ischemia in the rat. Brain Res. 1995, 679, 1–7. [Google Scholar] [CrossRef]
- Bruey, J.M.; Ducasse, C.; Bonniaud, P.; Ravagnan, L.; Susin, S.A.; Diaz-Latoud, C.; Gurbuxani, S.; Arrigo, A.P.; Kroemer, G.; Solary, E.; et al. HSP27 negatively regulates cell death by interacting with cytochrome C. Nat. Cell. Biol. 2000, 2, 645–652. [Google Scholar] [PubMed]
- Belluardo, N.; White, T.W.; Srinivas, M.; Trovato-Salinaro, A.; Ripps, H.; Mudo, G.; Bruzzone, R.; Condorelli, D.F. Identification and functional expression of HCX31.9, a novel gap junction gene. Cell. Commun. Adhes. 2001, 8, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Willecke, K.; Eiberger, J.; Degen, J.; Eckardt, D.; Romualdi, A.; Guldenagel, M.; Deutsch, U.; Sohl, G. Structural and functional diversity of connexin genes in the mouse and human genome. Biol. Chem. 2002, 383, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Matesic, D.F.; Nomata, K.; Kang, K.S.; Chang, C.C.; Trosko, J.E. Stimulation of cell proliferation and inhibition of gap junctional intercellular communication by linoleic acid. Cancer Lett. 1997, 112, 103–111. [Google Scholar] [CrossRef]
- Ogawa, T.; Hayashi, T.; Tokunou, M.; Nakachi, K.; Trosko, J.E.; Chang, C.C.; Yorioka, N. Suberoylanilide hydroxamic acid enhances gap junctional intercellular communication via acetylation of histone containing connexin 43 gene locus. Cancer Res. 2005, 65, 9771–9778. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.M.; Swanson, R.A. Astrocyte glutamate transport: Review of properties, regulation, and physiological functions. Glia 2000, 32, 1–14. [Google Scholar] [CrossRef]
- Ransom, B.R.; Brown, A.M. Intracellular Ca2+ release and ischemic axon injury: The Trojan horse is back. Neuron 2003, 40, 2–4. [Google Scholar] [CrossRef]
- Kelly, J.J.; Simek, J.; Laird, D.W. Mechanisms linking connexin mutations to human diseases. Cell. Tissue Res. 2015, 360, 701–721. [Google Scholar] [CrossRef] [PubMed]
- Dube, E.; Dufresne, J.; Chan, P.T.; Cyr, D.G. Epidermal growth factor regulates connexin 43 in the human epididymis: Role of gap junctions in azoospermia. Hum. Reprod. 2012, 27, 2285–2296. [Google Scholar] [CrossRef] [PubMed]
- Kanczuga-Koda, L.; Sulkowski, S.; Koda, M.; Skrzydlewska, E.; Sulkowska, M. Connexin 26 correlates with Bcl-xl and Bax proteins expression in colorectal cancer. World J. Gastroenterol. 2005, 11, 1544–1548. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.S.; Ko, H.M.; Koppula, S.; Kim, B.W.; Choi, D.K. Protective effect of Chrysanthemum indicum Linne against 1-methyl-4-phenylpridinium ion and lipopolysaccharide-induced cytotoxicity in cellular model of Parkinson’s disease. Food Chem. Toxicol. 2009, 49, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.W.; Koppula, S.; Kumar, H.; Park, J.Y.; Kim, I.W.; More, S.V.; Kim, I.S.; Han, S.D.; Kim, S.K.; Yoon, S.H.; Choi, D.K. α-Asarone attenuates microglia-mediated neuroinflammation by inhibiting NF κB activation and mitigates MPTP-induced behavioral deficits in a mouse model of Parkinson’s disease. Neuropharmacology 2015, 97, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.S.; Park, J.H.; Do, J.H.; Ko, H.M.; Park, E.J.; Suk, K.; Choi, D.K. Expression profiling of neuroinflammatory genes in LPS and LPS/IFNγ-stimulated BV-2 microglia cells. Biochip J. 2009, 3, 299–305. [Google Scholar]
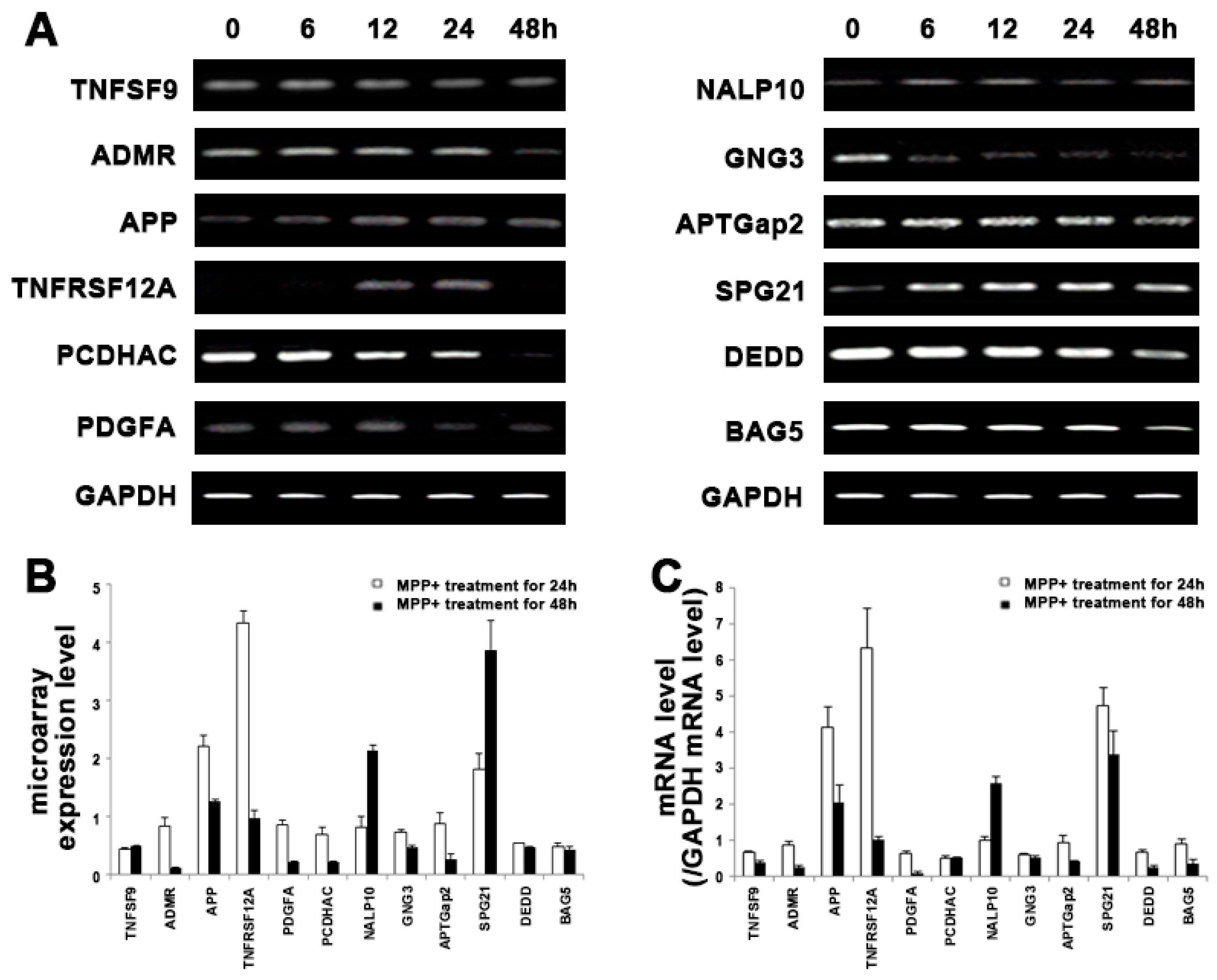

| GenBank Accession No. | Gene Symbol | Description | Fold Change | KEGG Pathways | p-Value | |
|---|---|---|---|---|---|---|
| 24 h | 48 h | |||||
| NM_001775 | CD38 | ADP-ribosyl cyclase 1 | 0.49 | 0.47 | Calcium signaling pathway | 6.48 × 10−4 |
| NM_000957 | PTGER3 | Prostaglandin E2 receptor, EP3 subtype | 0.48 | 0.44 | 1.58- × 10−4 | |
| NM_000833 | GRIN2A | Glutamate receptor subunit epsilon 1 precursor | 0.49 | 0.09 | 2.62 × 10−5 | |
| NM_004160 | PYY | Peptide YY precursor | 0.49 | 0.50 | Neuroactive ligand-receptor interaction | 2.36 × 10−4 |
| BC045651 | P2RY5 | P2Y purinoceptor 5 | 0.43 | 0.47 | 1.54 × 10−4 | |
| BC036030 | GABRG2 | Gamma-aminobutyric-acid receptor γ-2 subunit precursor | 0.46 | 0.43 | 5.56 × 10−5 | |
| AY358893 | CCL26 | Small inducible cytokine A26 precursor | 0.45 | 0.49 | Cytokine-cytokine receptor interaction | 4.66 × 10−5 |
| NM_003811 | TNFSF9 | Tumor necrosis factor ligand superfamily member 9 | 0.44 | 0.49 | 2.05 × 10−4 | |
| BM555452 | TNFRSF12A | Tumor necrosis factor receptor superfamily member Fn14 precursor | 4.33 | 0.98 | 8.36 × 10−5 | |
| X06374 | PDGFA | Splice Isoform Short of Platelet-derived growth factor, A chain precursor | 0.69 | 0.22 | 2.43 × 10−4 | |
| NM_000565 | IL6R | Interleukin-6 receptor α chain precursor | 0.44 | 0.42 | 2.23 × 10−4 | |
| NM_006917 | RXRG | Retinoic acid receptor RXR-γ | 0.46 | 0.44 | Adipocytokine signaling pathway | 1.63 × 10−4 |
| NM_004457 | ACSL3 | Long-chain-fatty-acid--CoA ligase 3 | 0.46 | 0.06 | 3.37 × 10−4 | |
| BC030529 | PSMA4 | Proteasome subunit α type 4 | 2.25 | 6.75 | Proteasome | 8.35 × 10−4 |
| NM_002790 | PSMA5 | Proteasome subunit α type 5 | 0.45 | 0.47 | 4.39 × 10−4 | |
| AF097935 | DSG1 | Desmoglein-1 precursor | 0.46 | 0.44 | Cell Communication | 2.58 × 10−8 |
| BC034761 | ADMR | Adrenomedullin receptor | 0.84 | 0.11 | G-protein coupled receptor protein signaling pathway | 5.33 × 10−4 |
| NM_012202 | GNG3 | Guanine nucleotide-binding protein G(I)/G(S)/G(O) γ-3 subunit. | 0.73 | 0.47 | 3.68 × 10−4 | |
| BC055286 | A4GALT | Lactosylceramide 4-α-galactosyltransferase | 0.43 | 0.45 | Globoside metabolism | 4.71 × 10−4 |
| AY643499 | HEXB | β-Hexosaminidase β chain precursor | 0.43 | 0.49 | 8.27 × 10−4 | |
| CR627398 | MAML2 | Mastermind-like 2 (Drosophila) | 5.90 | 2.96 | Notch signaling pathway | 6.79 × 10−4 |
| BC018937 | APP | Amyloid β A4 protein precursor | 2.21 | 1.27 | 2.11 × 10−4 | |
| AK057791 | METTL6 | Hypothetical protein MGC24132 | 4.60 | 2.07 | Histidine metabolism | 4.55 × 10−6 |
| NM_020997 | LEFTY1 | Left-right determination factor B precursor | 0.25 | 0.30 | TGF-β signaling pathway | 8.39 × 10−4 |
| L04751 | CYP4A11 | Cytochrome P450 4A11 precursor | 0.48 | 0.45 | Fatty acid metabolism | 6.27 × 10−4 |
| BC073828 | PPM1J | Protein phosphatase 2a, catalytic subunit, isoform | 0.46 | 0.45 | Tight junction | 2.61 × 10−4 |
| NM_001015049 | BAG5 | B-cell CLL/lymphoma 7C | 0.49 | 0.43 | Anti-apoptosis | 7.38 × 10−4 |
| NM_004323 | BAG1 | BCL-2 binding athanogene-1 | 0.48 | 0.32 | 6.83 × 10−4 | |
| NM_000633 | BCL2 | B-cell CLL/lymphoma 2 | 0.48 | 0.21 | 2.1 × 10−6 | |
| AK001497 | DEDD | Death effector domain-containing protein | 0.54 | 0.47 | Induction of apoptosis | 7.85 × 10−4 |
| NM_031882 | PCDHAC1 | Protocadherin α subfamily C, 1 | 0.85 | 0.23 | Cell adhesion | 3.67 × 10−5 |
| CR622836 | NALP10 | NACHT-, LRR- and PYD-containing protein 10 | 0.81 | 2.13 | Unknown | 8.90 × 10−6 |
| AK075382 | ATP6AP2 | Vacuolar ATP synthase membrane sector associated protein M8-9 | 0.88 | 0.26 | 3.47 × 10−4 | |
| NM_016630 | SPG21 | Spastic paraplegia 21 | 1.81 | 3.88 | 1.24 × 10−4 | |
| BC039110 | SMNDC1 | Survival of motor neuron-related splicing factor 30 | 2 | 2.7 | 1.54 × 10−5 | |
| GenBank Accession No. | Gene Symbol | Description | Fold Change | p-Value | |
|---|---|---|---|---|---|
| 24 h | 48 h | ||||
| BM907768 | HSPB1 | Heat-shock protein β-1 | 0.48 ± 0.09 | 0.58 ± 0.16 | 3.47 × 10−4 |
| NM_001013398 | IGFBP3 | Insulin-like growth factor binding protein 3 precursor | 0.49 ± 0.08 | 0.64 ± 0.14 | 1.54 × 10−5 |
| NM_014791 | MELK | Maternal embryonic leucine zipper kinase | 1.96 ± 0.24 | 2.15 ± 0.09 | 1.18 × 10−4 |
| AL832618 | IFI44L | interferon induced protein 44-like | 1.76 ± 0.09 | 2.31 ± 0.33 | 1.20 × 10−4 |
| AK055053 | SHMT2 | Serine hydroxymethyltransferase | 1.59 ± 0.12 | 2.12 ± 0.29 | 3.47 × 10−4 |
| NM_004004 | GJB2 | Connexin 26 | 2.1 ± 0.30 | 9.5 ± 0.46 | 4.12 × 10−5 |
| NM_006887 | ZFP36L2 | Butyrate response factor 2 | 0.95 ± 0.24 | 2.77 ± 0.42 | 4.51 × 10−4 |
| NM_001753 | CAV1 | Caveolin-1 | 1.33 ± 0.06 | 2.38 ± 0.18 | 1.20 × 10−4 |
| NM_003979 | GPRC5A | G-protein coupled receptor family C group 5 member A | 0.42 ± 0.04 | 0.41 ± 0.02 | 3.66 × 10−4 |
| NM_005558 | LAD1 | Ladinin 1 | 1.86 ± 0.14 | 3.7 ± 0.29 | 5.54 × 10−4 |
| NM_181865 | ACOT7 | Cytosolicacyl coenzyme A thioester hydrolase | 1.36 ± 0.19 | 2.14 ± 0.16 | 1.20 × 10−4 |
| AY358893 | SAA4 | Serum amyloid A-4 protein precursor | 2.09 ± 0.26 | 1.02 ± 0.16 | 4.62 × 10−4 |
| Gene Symbol | Primer | |
|---|---|---|
| Forward | Reverse | |
| Bcl-2 | ACGACTTCTCCCGCCGCTAC | CCCAGCCTCCGTTATCCTGG |
| Bax | CACCAAGGTGCCGGAACTGA | AATGCCCATGTCCCCCAATC |
| TNFSF9 | ACTCGGCCTTCGGTTTCCAG | AAGGGGAGGTCAGCAGCAGG |
| ADMR | GACCGCTATGTCACCCTCAC | GGTGCTGTACGTTTCAAAAGGT |
| APP | AGCTTCTGGCCACTGGGAGG | AGTGGGACATGCGGGGAAGT |
| TNFRSF12A | CTCCTCCAACCACACAAGGGG | TCCCCTCCAAACTCTCCCCA |
| PDGFA | CCAGCGACTCCTGGAGATAGA | CGTCCTGGTCTTGCAGACAG |
| PCDHAC1 | TGTGGGGTGGCAGTTTTATGT | GAAAGGAAGCGAAAGTTCCG |
| NALP10 | CTACTTACGGGATATGACCCTGT | GCTGGTCCACAAGTTCCAACA |
| GNG3 | CCGGTGAACAGCACTATGAGT | GGCATCACAGTAAGTCATCAGG |
| ATP6AP2 | AGCTCCGTAATCGCCTGTTTC | GATGCTTATGACGAGACAGCAA |
| SPG21 | TCTGACTGGATGGGGTTACCG | GAAGCGCCAAAAAGATGAACTT |
| DEDD | GCACCGCATGTTTGACATCG | TCACGTCCATTTCGGATGAGT |
| BAG5 | AATGCAAACCACCCACACCG | ATCAGCCCGAGAGCACACA |
| HSPB1 | GTCCCTGGATGTCAACCACT | CTTTACTTGGCGGCAGTCTC |
| IGFBP3 | CCAGCTCCAGGTGAGCC | GGTCATGTCCTTGGCAGTCT |
| MELK | CCATGTGCTAGAGACAGCCA | ATGCTACTGGGAGAGAGCCA |
| IFI44L | TCCTAGCCATGTGTCCTTCC | GGCCTACCTCCTCAATTTCC |
| SHMT2 | AGACTCAGACTGGGGAAGCA | TCAGTGCCAGCTGGTTGTAG |
| GJB2 | CTACTTCCCCATCTCCCACA | GGGAAATGCTAGCGACTGAG |
| ZFP36L2 | CACCTTTCATACCATCGGCT | GCAAAGTTGTGGGTCTGGAT |
| CAV1 | TGAGATTCAGTGCATCAGCC | AACTTGAAATTGGCACCAGG |
| GPRC5A | TCGTCGCAATGAAGACTTTG | TGGTTCTGCAGCTGAAAATG |
| LAD1 | GACCATCTCCTTTCGGATGA | CCCTTGAGACGAAGACTTGC |
| ACOT7 | CGTCCTCAGAAAGGAAGTCG | GTTCCTCCACTTGGTCTCCA |
| SAA4 | CAGCACAATGAGGCTTTTCA | AGTGACCCTGTGTCCCTGTC |
| GAPDH | GTCAGTGGTGGACCTGACCT | TGTGAGGAGGGGAGATTCAG |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, I.-S.; Koppula, S.; Park, S.-Y.; Choi, D.-K. Analysis of Epidermal Growth Factor Receptor Related Gene Expression Changes in a Cellular and Animal Model of Parkinson’s Disease. Int. J. Mol. Sci. 2017, 18, 430. https://doi.org/10.3390/ijms18020430
Kim I-S, Koppula S, Park S-Y, Choi D-K. Analysis of Epidermal Growth Factor Receptor Related Gene Expression Changes in a Cellular and Animal Model of Parkinson’s Disease. International Journal of Molecular Sciences. 2017; 18(2):430. https://doi.org/10.3390/ijms18020430
Chicago/Turabian StyleKim, In-Su, Sushruta Koppula, Shin-Young Park, and Dong-Kug Choi. 2017. "Analysis of Epidermal Growth Factor Receptor Related Gene Expression Changes in a Cellular and Animal Model of Parkinson’s Disease" International Journal of Molecular Sciences 18, no. 2: 430. https://doi.org/10.3390/ijms18020430