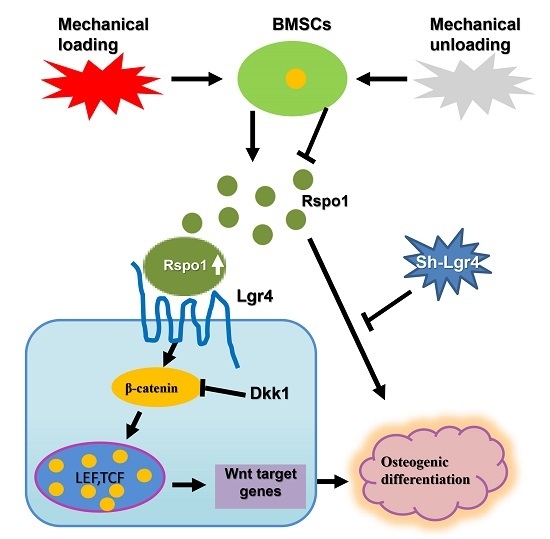
Evidence of the Role of R-Spondin 1 and Its Receptor Lgr4 in the Transmission of Mechanical Stimuli to Biological Signals for Bone Formation
Abstract
:1. Introduction
2. Results
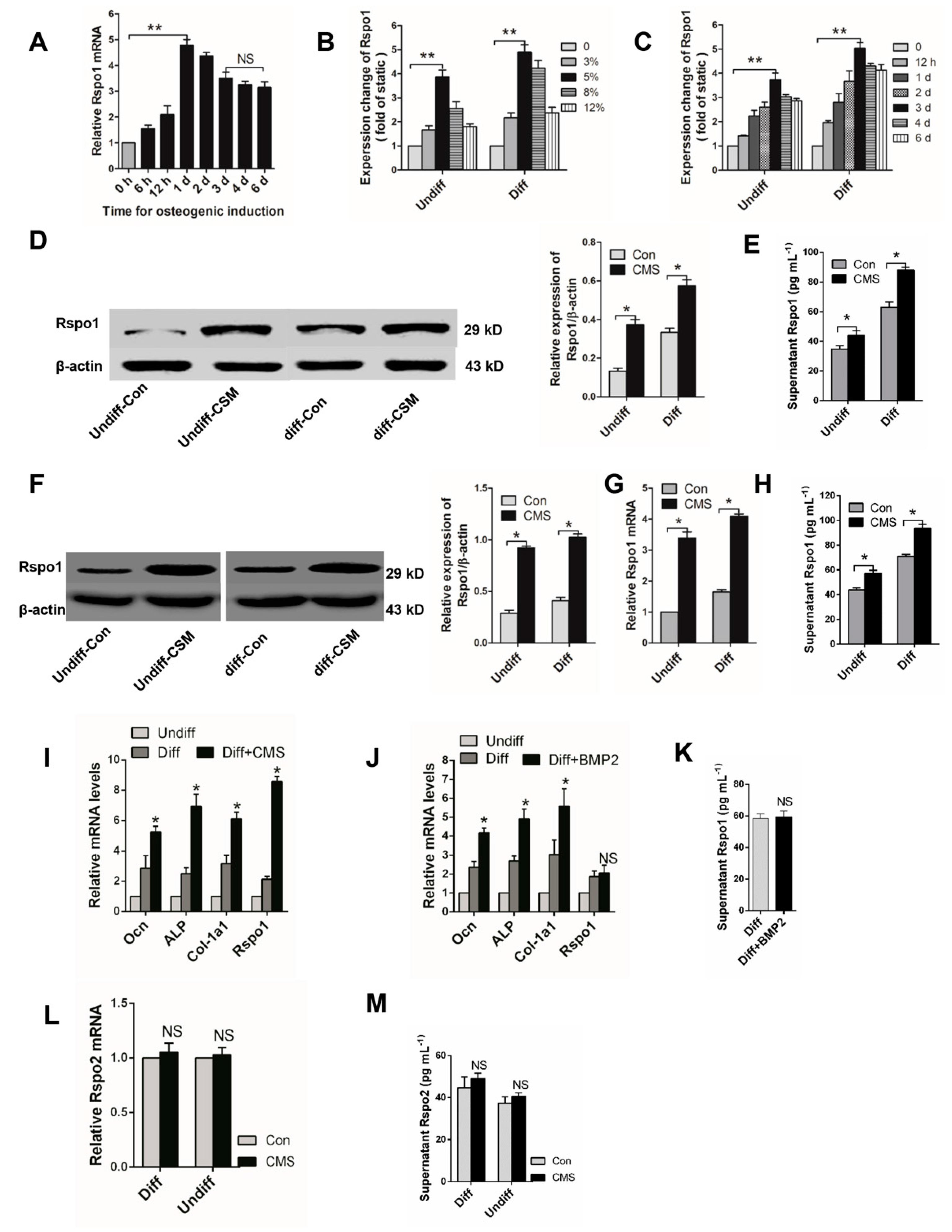
2.1. Mechanical Loading Upregulated the Expression of Rspo1 In Vitro
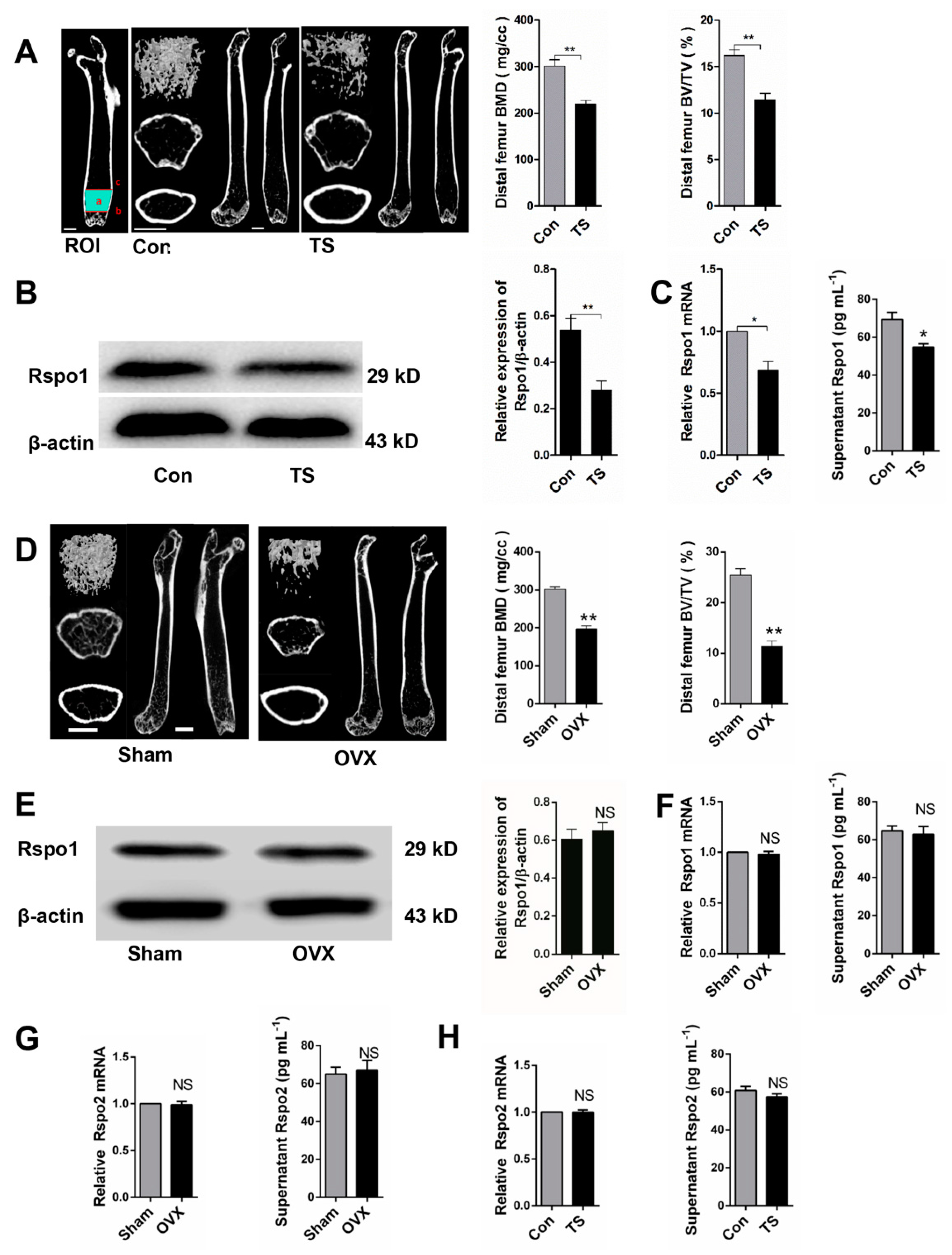
2.2. Mechanical Unloading Downregulated the Expression of Rspo1 in BMSCs In Vivo
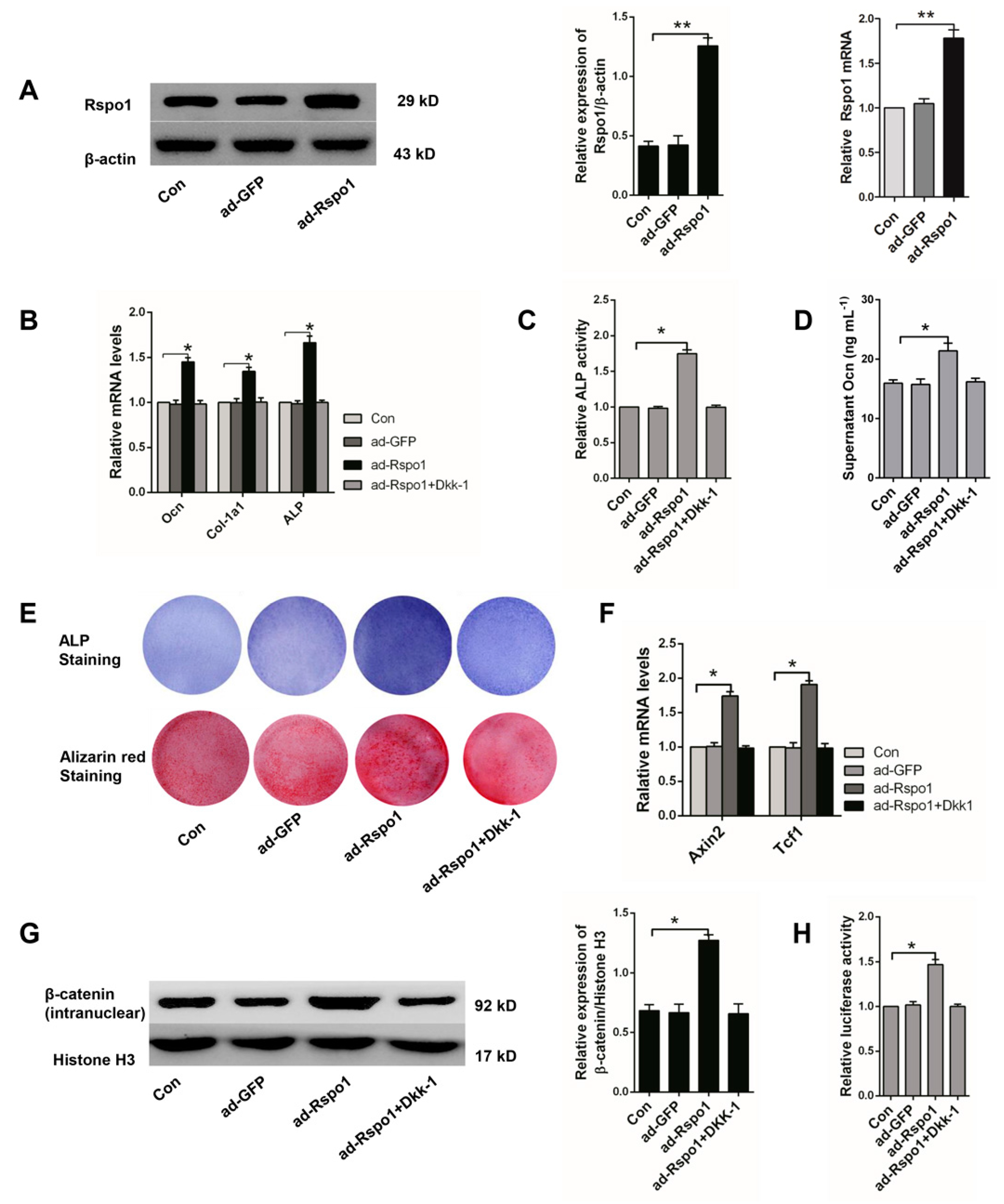
2.3. Rspo1 Promoted the Osteogenic Differentiation of BMSCs through the Wnt/β-Catenin Signaling Pathway
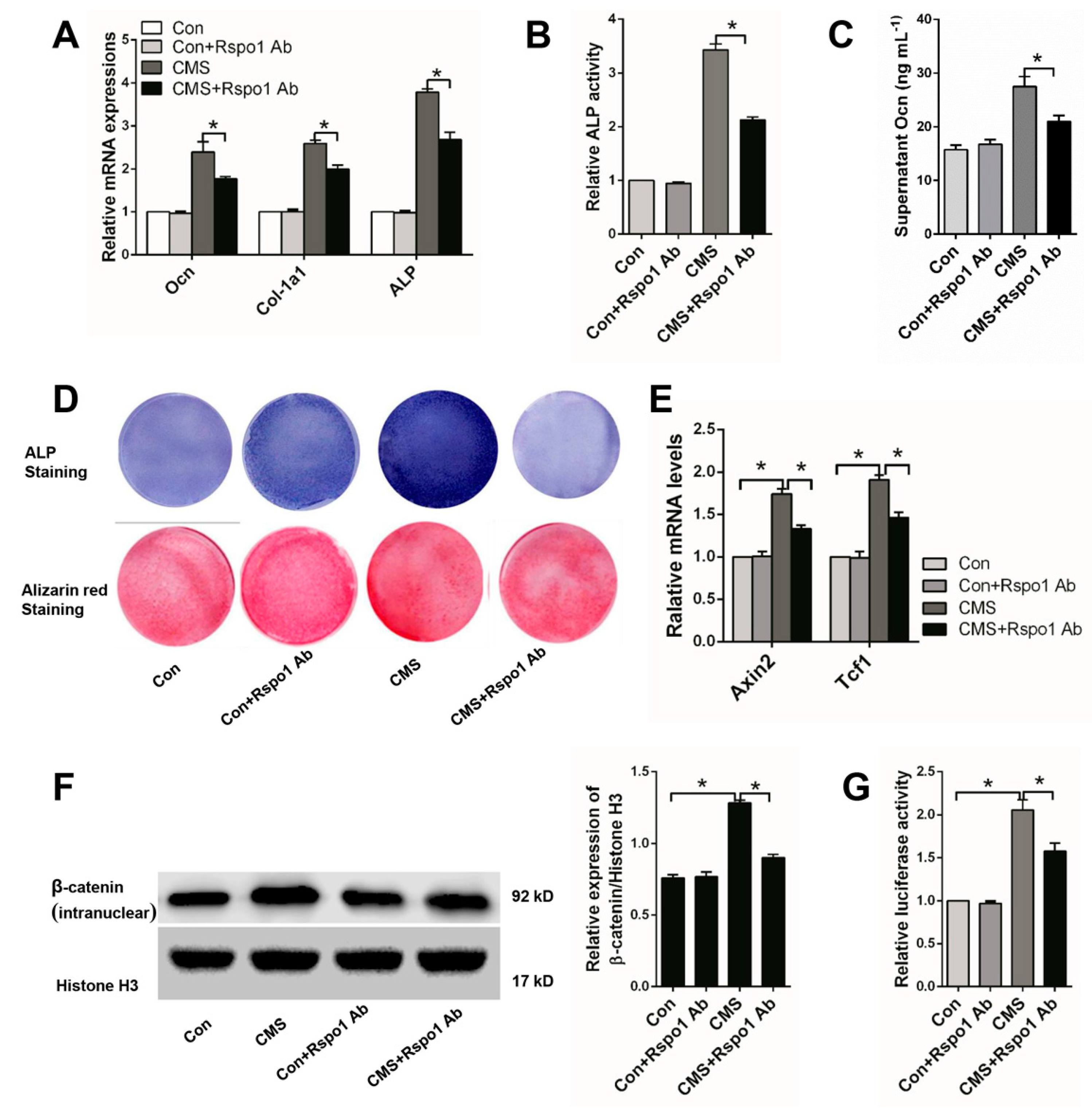
2.4. Rspo1 Was Essential in CMS-Promoted Osteogenic Differentiation of BMSCs
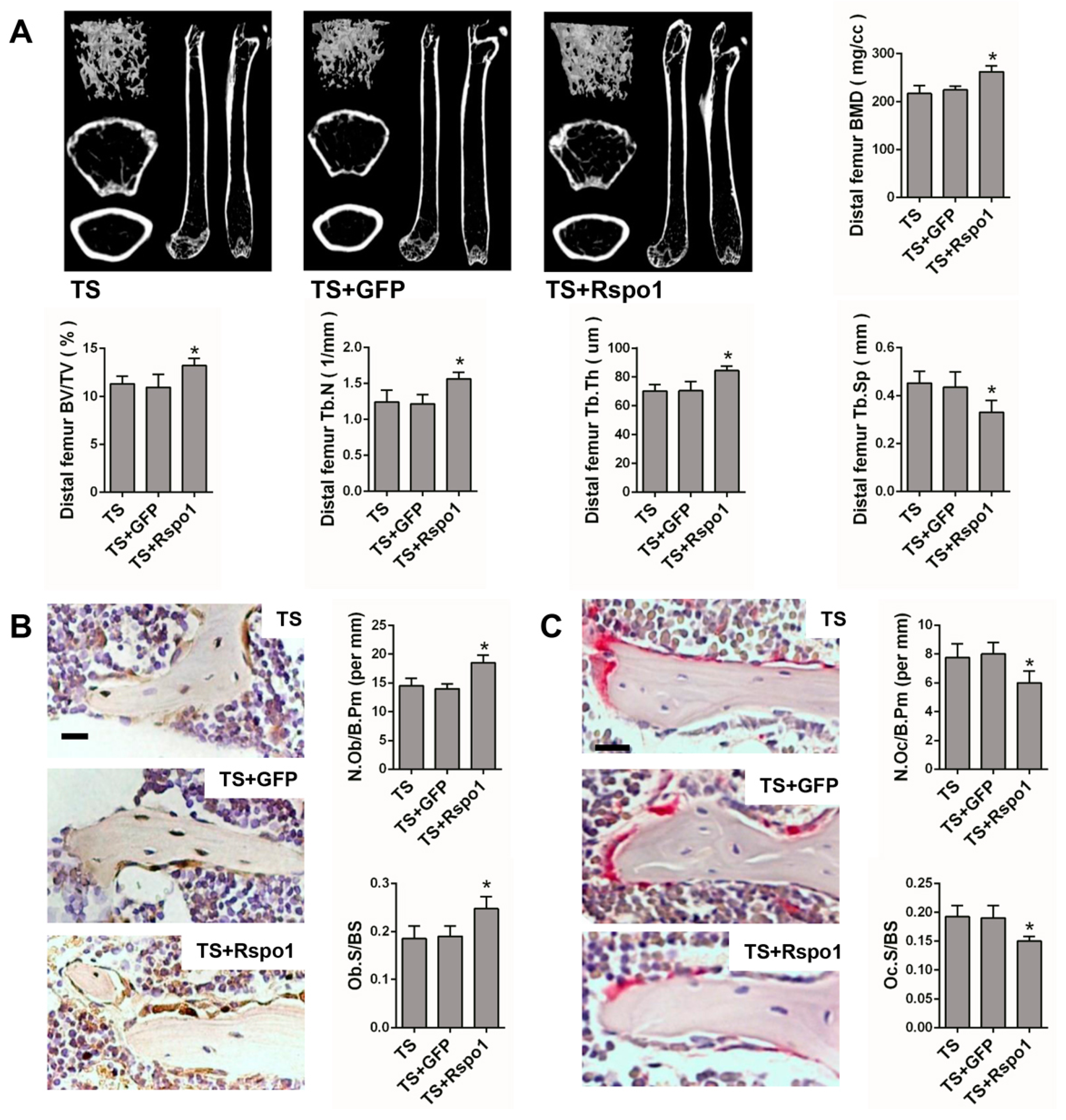
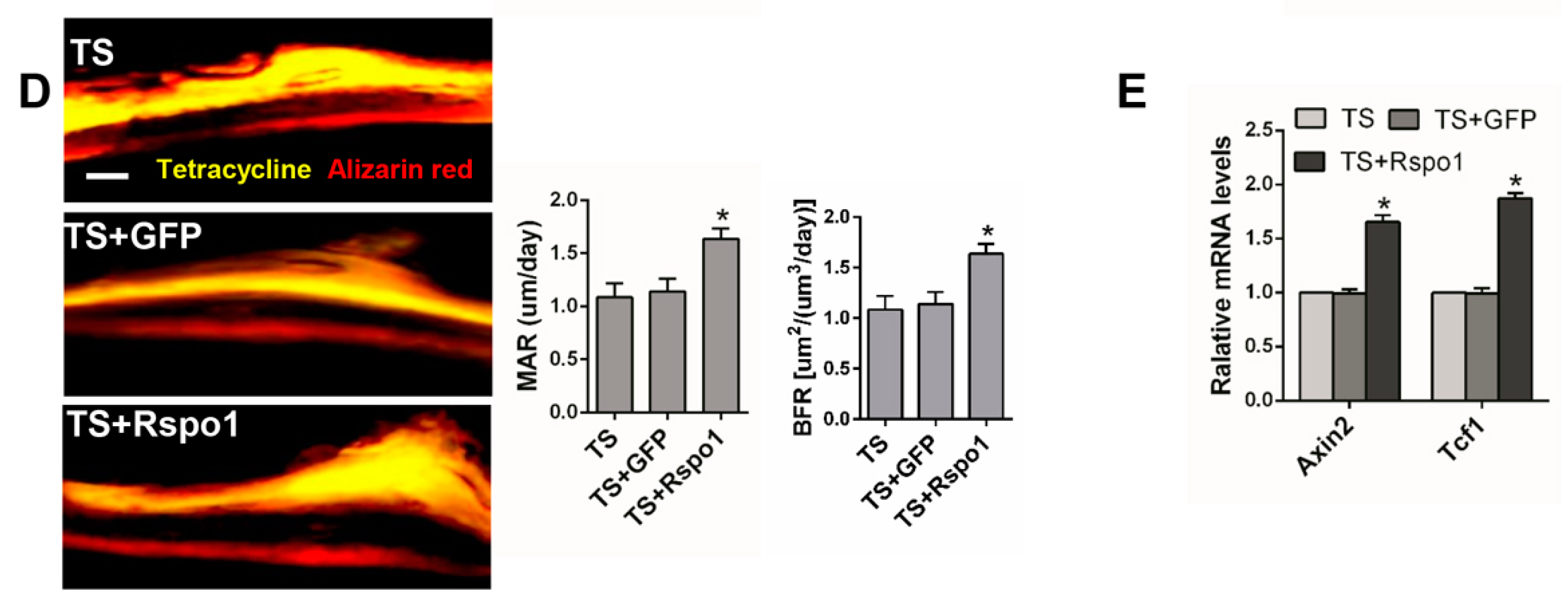
2.5. Adenovirus-Mediated Delivery of Rspo1 Increased the Bone Mass of TS Mice
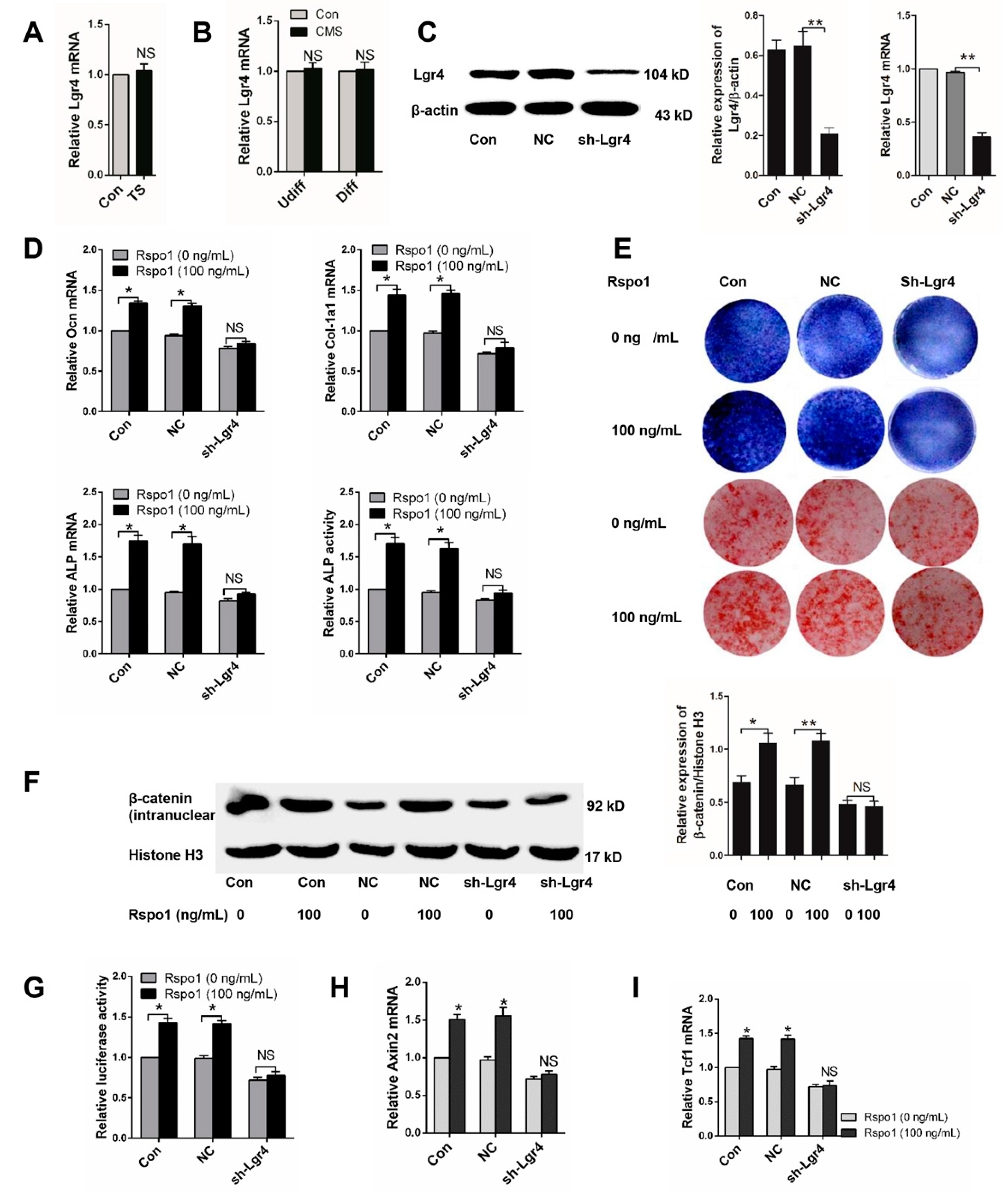
2.6. Lgr4 Was Essential for Rspo1 to Enhance the Osteogenic Differentiation and Wnt/β-Catenin Signaling of BMSCs
3. Discussion
4. Materials and Methods
4.1. Animals and Grouping
4.2. Hindlimb Unloading and Adenovirus Injection of Mice
4.3. OVX Mice Model
4.4. Cell Culture and Induction of Osteogenic Differentiation
4.5. Adenovirus or Lentivirus Transfection
4.6. CMS Stimulation for BMSCs and MC3T3-E1 Cells
4.7. TOPflash Dual-Luciferase Reporter Assays
4.8. Protein Extraction and Western Blot Analysis
4.9. RNA Isolation and Quantitative Real-Time PCR
4.10. ALP Activity Assay
4.11. ALP and Alizarin Red Staining
4.12. ELISA
4.13. Micro-Computed Tomography Scanning and Quantitative Analysis
4.14. Bone Immunohistochemistry
4.15. Skeleton Histomorphometric Analysis
4.16. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Robling, A.G.; Turner, C.H. Mechanical signaling for bone modeling and remodeling. Crit. Rev. Eukaryot. Gene Expr. 2009, 19, 319–338. [Google Scholar] [CrossRef] [PubMed]
- Akhter, M.P.; Alvarez, G.K.; Cullen, D.M.; Recker, R.R. Disuse-related decline in trabecular bone structure. Biomech. Model. Mechanobiol. 2011, 10, 423–429. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, A.D.; Spector, E.R.; Evans, H.J.; Sibonga, J.D. Skeletal responses to space flight and the bed rest analog: A review. J. Musculoskelet. Neuronal Interact. 2007, 7, 33–47. [Google Scholar] [PubMed]
- Ohshima, H.; Mukai, C. Bone metabolism in human space flight and bed rest study. Clin. Calcium 2008, 18, 1245–1253. [Google Scholar] [PubMed]
- Lau, R.Y.; Guo, X. A review on current osteoporosis research: With special focus on disuse bone loss. J. Osteoporos. 2011, 2011, 293808. [Google Scholar] [CrossRef] [PubMed]
- Spector, E.R.; Smith, S.M.; Sibonga, J.D. Skeletal effects of long-duration head-down bed rest. Aviat. Space Environ. Med. 2009, 80 (Suppl. S5), A23–A28. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.J.; Diko, S.; Baehr, L.M.; Baar, K.; Bodine, S.C.; Christiansen, B.A. Contribution of mechanical unloading to trabecular bone loss following non-invasive knee injury in mice. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2016, 34, 1680–1687. [Google Scholar] [CrossRef] [PubMed]
- Cabahug-Zuckerman, P.; Frikha-Benayed, D.; Majeska, R.J.; Tuthill, A.; Yakar, S.; Judex, S.; Schaffler, M.B. Osteocyte Apoptosis Caused by Hindlimb Unloading is Required to Trigger Osteocyte RANKL Production and Subsequent Resorption of Cortical and Trabecular Bone in Mice Femurs. J. Bone Miner. Res. 2016, 31, 1356–1365. [Google Scholar] [CrossRef] [PubMed]
- Spatz, J.M.; Wein, M.N.; Gooi, J.H.; Qu, Y.; Garr, J.L.; Liu, S.; Barry, K.J.; Uda, Y.; Lai, F.; Dedic, C.; et al. The Wnt Inhibitor Sclerostin Is Up-regulated by Mechanical Unloading in Osteocytes in Vitro. J. Biol. Chem. 2015, 290, 16744–16758. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Jee, W.S.; Li, X.; Paszty, C.; Ke, H.Z. Sclerostin antibody increases bone mass by stimulating bone formation and inhibiting bone resorption in a hindlimb-immobilization rat model. Bone 2011, 48, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Lara-Castillo, N.; Kim-Weroha, N.A.; Kamel, M.A.; Javaheri, B.; Ellies, D.L.; Krumlauf, R.E.; Thiagarajan, G.; Johnson, M.L. In vivo mechanical loading rapidly activates β-catenin signaling in osteocytes through a prostaglandin mediated mechanism. Bone 2015, 76, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Liang, L.; Dou, Y.; Huang, Z.; Mo, H.; Wang, Y.; Yu, B. Mechanical strain regulates osteogenic and adipogenic differentiation of bone marrow mesenchymal stem cells. BioMed Res. Int. 2015, 2015, 873251. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Zhang, Y.; Jing, D.; Shen, Y.; Tang, G.; Huang, S.; Zhao, Z. Mechanobiology of mesenchymal stem cells: Perspective into mechanical induction of MSC fate. Acta Biomater. 2015, 20, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Nieponice, A.; Maul, T.M.; Cumer, J.M.; Soletti, L.; Vorp, D.A. Mechanical stimulation induces morphological and phenotypic changes in bone marrow-derived progenitor cells within a three-dimensional fibrin matrix. J. Biomed. Mater. Res. A 2007, 81, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Lohberger, B.; Kaltenegger, H.; Stuendl, N.; Payer, M.; Rinner, B.; Leithner, A. Effect of cyclic mechanical stimulation on the expression of osteogenesis genes in human intraoral mesenchymal stromal and progenitor cells. BioMed Res. Int. 2014, 2014, 189516. [Google Scholar] [CrossRef] [PubMed]
- Rubin, J.; Rubin, C.; Jacobs, C.R. Molecular pathways mediating mechanical signaling in bone. Gene 2006, 367, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Sankaran, J.S.; Li, B.; Donahue, L.R.; Judex, S. Modulation of unloading-induced bone loss in mice with altered ERK signaling. Mamm. Genome Off. J. Int. Mamm. Genome Soc. 2016, 27, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.A.; Chatterjee-Kishore, M.; Yaworsky, P.J.; Cullen, D.M.; Zhao, W.; Li, C.; Kharode, Y.; Sauter, L.; Babij, P.; Brown, E.L.; et al. Wnt/β-catenin signaling is a normal physiological response to mechanical loading in bone. J. Biol. Chem. 2006, 281, 31720–31728. [Google Scholar] [CrossRef] [PubMed]
- Ruffner, H.; Sprunger, J.; Charlat, O.; Leighton-Davies, J.; Grosshans, B.; Salathe, A.; Zietzling, S.; Beck, V.; Therier, M.; Isken, A.; et al. R-Spondin potentiates Wnt/β-catenin signaling through orphan receptors LGR4 and LGR5. PLoS ONE 2012, 7, e40976. [Google Scholar] [CrossRef] [PubMed]
- Glinka, A.; Dolde, C.; Kirsch, N.; Huang, Y.L.; Kazanskaya, O.; Ingelfinger, D.; Boutros, M.; Cruciat, C.M.; Niehrs, C. LGR4 and LGR5 are R-spondin receptors mediating Wnt/β-catenin and Wnt/PCP signalling. EMBO Rep. 2011, 12, 1055–1061. [Google Scholar] [CrossRef]
- Carmon, K.S.; Gong, X.; Lin, Q.; Thomas, A.; Liu, Q. R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/β-catenin signaling. Proc. Natl. Acad. Sci. USA 2011, 108, 11452–11457. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.S.; Turcotte, T.J.; Yoon, J.K. Dynamic expression of R-spondin family genes in mouse development. Gene Expr. Patterns GEP 2007, 7, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Knight, M.N.; Hankenson, K.D. R-spondins: Novel matricellular regulators of the skeleton. Matrix Biol. 2014, 37, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.X.; Mao, W.W.; Zheng, X.F.; Jiang, L.S. The role of R-spondins and their receptors in bone metabolism. Prog. Biophys. Mol. Biol. 2016, 122, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Hankenson, K.D.; Sweetwyne, M.T.; Shitaye, H.; Posey, K.L. Thrombospondins and novel TSR-containing proteins, R-spondins, regulate bone formation and remodeling. Curr. Osteoporos. Rep. 2010, 8, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Kim, K.-A.; Liu, J.; Abo, A.; Feng, X.; Cao, X.; Li, Y. R-spondin1 synergizes with Wnt3A in inducing osteoblast differentiation and osteoprotegerin expression. FEBS Lett. 2008, 582, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Kronke, G.; Uderhardt, S.; Kim, K.A.; Stock, M.; Scholtysek, C.; Zaiss, M.M.; Surmann-Schmitt, C.; Luther, J.; Katzenbeisser, J.; David, J.P.; et al. R-spondin 1 protects against inflammatory bone damage during murine arthritis by modulating the Wnt pathway. Arthritis Rheum. 2010, 62, 2303–2312. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Brennan, T.A.; Russell, E.; Kim, J.-H.; Egan, K.P.; Chen, Q.; Israelite, C.; Schultz, D.C.; Johnson, F.B.; Pignolo, R.J. R-spondin 1 promotes vibration-induced bone formation in mouse models of osteoporosis. J. Mol. Med. 2013, 91, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.R.; Choi, B.S.; Park, J.M.; Lee, D.H.; Lee, J.E.; Kim, H.S.; Yoon, J.K.; Song, D.K.; Nam, J.S.; Lee, S.S. Rspo 1 promotes osteoblast differentiation via Wnt signaling pathway. Indian J. Biochem. Biophys. 2013, 50, 19–25. [Google Scholar] [PubMed]
- De Lau, W.B.; Snel, B.; Clevers, H.C. The R-spondin protein family. Genome Biol. 2012, 13, 242. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.R.; Yoon, J.K. The R-spondin family of proteins: Emerging regulators of WNT signaling. Int. J. Biochem. Cell Biol. 2012, 44, 2278–2287. [Google Scholar] [CrossRef] [PubMed]
- Zuo, B.; Zhu, J.; Li, J.; Wang, C.; Zhao, X.; Cai, G.; Li, Z.; Peng, J.; Wang, P.; Shen, C.; et al. MicroRNA-103a Functions as a Mechanosensitive microRNA to Inhibit Bone Formation Through Targeting Runx2. J. Bone Miner. Res. 2015, 30, 330–345. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Zheng, X.F.; Yang, Y.H.; Li, B.; Wang, Y.R.; Jiang, S.D.; Jiang, L.S. LGR4 acts as a key receptor for R-spondin 2 to promote osteogenesis through Wnt signaling pathway. Cell. Signal. 2016, 28, 989–1000. [Google Scholar] [CrossRef] [PubMed]
- Morey-Holton, E.R.; Globus, R.K. Hindlimb unloading rodent model: Technical aspects. J. Appl. Physiol. 2002, 92, 1367–1377. [Google Scholar] [CrossRef]
- Milstead, J.R.; Simske, S.J.; Bateman, T.A. Spaceflight and hindlimb suspension disuse models in mice. Biomed. Sci. Instrum. 2004, 40, 105–110. [Google Scholar] [PubMed]
- Shen, T.; Qiu, L.; Chang, H.; Yang, Y.; Jian, C.; Xiong, J.; Zhou, J.; Dong, S. Cyclic tension promotes osteogenic differentiation in human periodontal ligament stem cells. Int. J. Clin. Exp. Pathol. 2014, 7, 7872–7880. [Google Scholar] [PubMed]
- Javaheri, B.; Stern, A.R.; Lara, N.; Dallas, M.; Zhao, H.; Liu, Y.; Bonewald, L.F.; Johnson, M.L. Deletion of a single β-catenin allele in osteocytes abolishes the bone anabolic response to loading. J. Bone Miner. Res. 2014, 29, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Tu, X.; Rhee, Y.; Condon, K.W.; Bivi, N.; Allen, M.R.; Dwyer, D.; Stolina, M.; Turner, C.H.; Robling, A.G.; Plotkin, L.I.; et al. Sost downregulation and local Wnt signaling are required for the osteogenic response to mechanical loading. Bone 2012, 50, 209–217. [Google Scholar] [CrossRef] [PubMed]
- De Lau, W.; Barker, N.; Low, T.Y.; Koo, B.K.; Li, V.S.; Teunissen, H.; Kujala, P.; Haegebarth, A.; Peters, P.J.; van de Wetering, M.; et al. Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature 2011, 476, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Zhou, W.; Zhou, X.; Li, D.; Weng, J.; Yi, Z.; Cho, S.G.; Li, C.; Yi, T.; Wu, X. Regulation of bone formation and remodeling by G-protein-coupled receptor 48. Development 2009, 136, 2747–2756. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Yang, Z.; Ma, Y.; Yue, Z.; Lin, H.; Qu, G.; Huang, J.; Dai, W.; Li, C.; Zheng, C.; et al. LGR4 is a receptor for RANKL and negatively regulates osteoclast differentiation and bone resorption. Nat. Med. 2016, 22, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Spyropoulou, A.; Karamesinis, K.; Basdra, E.K. Mechanotransduction pathways in bone pathobiology. Biochim. Biophys. Acta 2015, 1852, 1700–1708. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.Y.; Li, F.; Geng, N.; Gong, P.; Huang, S.J.; Meng, L.X.; Lan, J.; Ban, Y. Fluid flow modulates the expression of genes involved in the Wnt signaling pathway in osteoblasts in 3D culture conditions. Int. J. Mol. Med. 2014, 33, 1282–1288. [Google Scholar] [CrossRef] [PubMed]
- Sawakami, K.; Robling, A.G.; Ai, M.; Pitner, N.D.; Liu, D.; Warden, S.J.; Li, J.; Maye, P.; Rowe, D.W.; Duncan, R.L.; et al. The Wnt co-receptor LRP5 is essential for skeletal mechanotransduction but not for the anabolic bone response to parathyroid hormone treatment. J. Biol. Chem. 2006, 281, 23698–23711. [Google Scholar] [CrossRef] [PubMed]
- Robling, A.G.; Niziolek, P.J.; Baldridge, L.A.; Condon, K.W.; Allen, M.R.; Alam, I.; Mantila, S.M.; Gluhak-Heinrich, J.; Bellido, T.M.; Harris, S.E.; et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J. Biol. Chem. 2008, 283, 5866–5875. [Google Scholar] [CrossRef] [PubMed]
- Gaudio, A.; Pennisi, P.; Bratengeier, C.; Torrisi, V.; Lindner, B.; Mangiafico, R.A.; Pulvirenti, I.; Hawa, G.; Tringali, G.; Fiore, C.E. Increased sclerostin serum levels associated with bone formation and resorption markers in patients with immobilization-induced bone loss. J. Clin. Endocrinol. Metab. 2010, 95, 2248–2253. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.S.; Robling, A.G. New Insights into Wnt-Lrp5/6-β-Catenin Signaling in Mechanotransduction. Front. Endocrinol. 2014, 5, 246. [Google Scholar]
- Morse, A.; McDonald, M.M.; Kelly, N.H.; Melville, K.M.; Schindeler, A.; Kramer, I.; Kneissel, M.; van der Meulen, M.C.; Little, D.G. Mechanical load increases in bone formation via a sclerostin-independent pathway. J. Bone Miner. Res. 2014, 29, 2456–2467. [Google Scholar] [CrossRef] [PubMed]
- Vico, L.; Collet, P.; Guignandon, A.; Lafage-Proust, M.H.; Thomas, T.; Rehaillia, M.; Alexandre, C. Effects of long-term microgravity exposure on cancellous and cortical weight-bearing bones of cosmonauts. Lancet 2000, 355, 1607–1611. [Google Scholar] [CrossRef]
- Varzi, D.; Coupaud, S.A.; Purcell, M.; Allan, D.B.; Gregory, J.S.; Barr, R.J. Bone morphology of the femur and tibia captured by statistical shape modelling predicts rapid bone loss in acute spinal cord injury patients. Bone 2015, 81, 495–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bach-Gansmo, F.L.; Wittig, N.K.; Bruel, A.; Thomsen, J.S.; Birkedal, H. Immobilization and long-term recovery results in large changes in bone structure and strength but no corresponding alterations of osteocyte lacunar properties. Bone 2016, 91, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Price, C.; Delisser, K.; Nasser, P.; Laudier, D.; Clement, M.; Jepsen, K.J.; Schaffler, M.B. Long-term disuse osteoporosis seems less sensitive to bisphosphonate treatment than other osteoporosis. J. Bone Miner. Res. 2005, 20, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Su, J.; Wu, S.; Teng, Y.; Yin, Z.; Guo, Y.; Li, J.; Li, K.; Yao, L.; Li, X. DDR2 (discoidin domain receptor 2) suppresses osteoclastogenesis and is a potential therapeutic target in osteoporosis. Sci. Signal. 2015, 8, ra31. [Google Scholar] [CrossRef] [PubMed]
- Xian, L.; Wu, X.; Pang, L.; Lou, M.; Rosen, C.J.; Qiu, T.; Crane, J.; Frassica, F.; Zhang, L.; Rodriguez, J.P.; et al. Matrix IGF-1 maintains bone mass by activation of mTOR in mesenchymal stem cells. Nat. Med. 2012, 18, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, G.-X.; Zheng, X.-F.; Zhu, C.; Li, B.; Wang, Y.-R.; Jiang, S.-D.; Jiang, L.-S. Evidence of the Role of R-Spondin 1 and Its Receptor Lgr4 in the Transmission of Mechanical Stimuli to Biological Signals for Bone Formation. Int. J. Mol. Sci. 2017, 18, 564. https://doi.org/10.3390/ijms18030564
Shi G-X, Zheng X-F, Zhu C, Li B, Wang Y-R, Jiang S-D, Jiang L-S. Evidence of the Role of R-Spondin 1 and Its Receptor Lgr4 in the Transmission of Mechanical Stimuli to Biological Signals for Bone Formation. International Journal of Molecular Sciences. 2017; 18(3):564. https://doi.org/10.3390/ijms18030564
Chicago/Turabian StyleShi, Gui-Xun, Xin-Feng Zheng, Chao Zhu, Bo Li, Yu-Ren Wang, Sheng-Dan Jiang, and Lei-Sheng Jiang. 2017. "Evidence of the Role of R-Spondin 1 and Its Receptor Lgr4 in the Transmission of Mechanical Stimuli to Biological Signals for Bone Formation" International Journal of Molecular Sciences 18, no. 3: 564. https://doi.org/10.3390/ijms18030564