Morphofunctional Alterations in Zebrafish (Danio rerio) Gills after Exposure to Mercury Chloride
Abstract
:1. Introduction
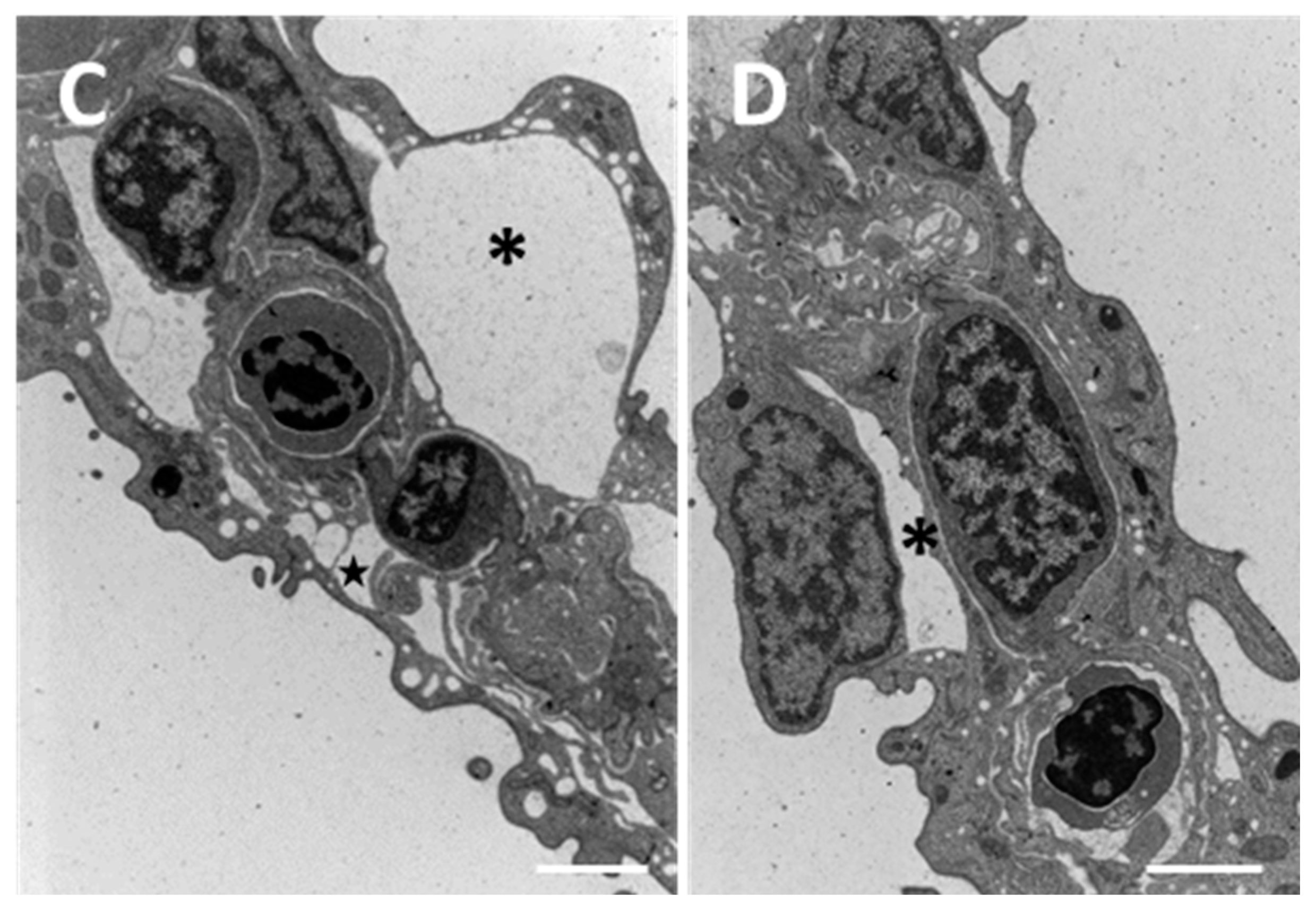
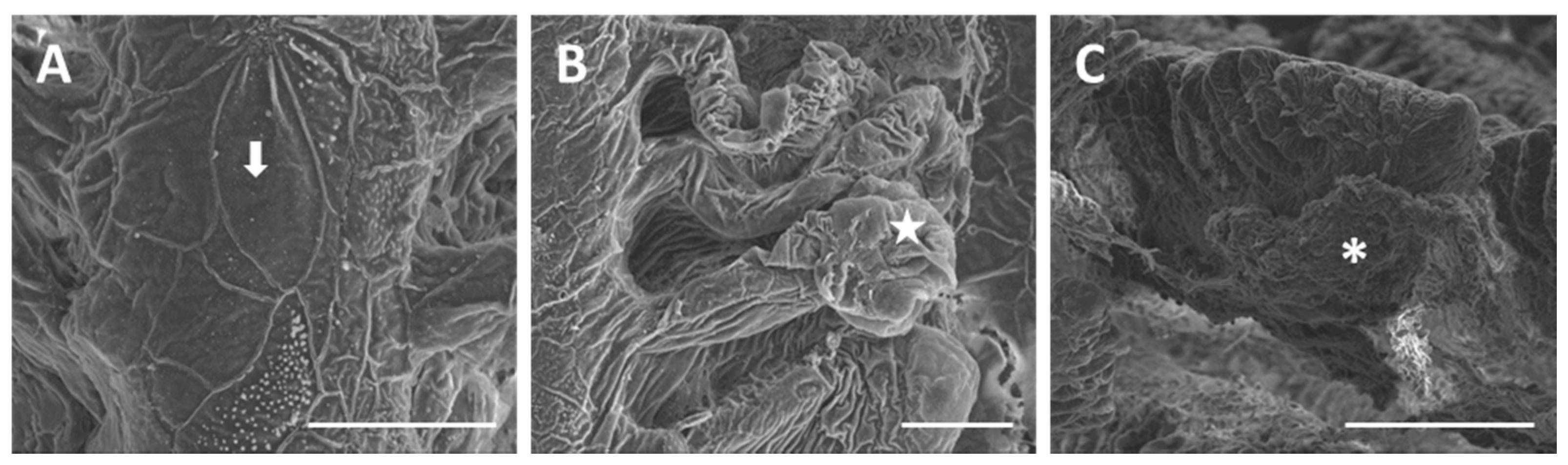
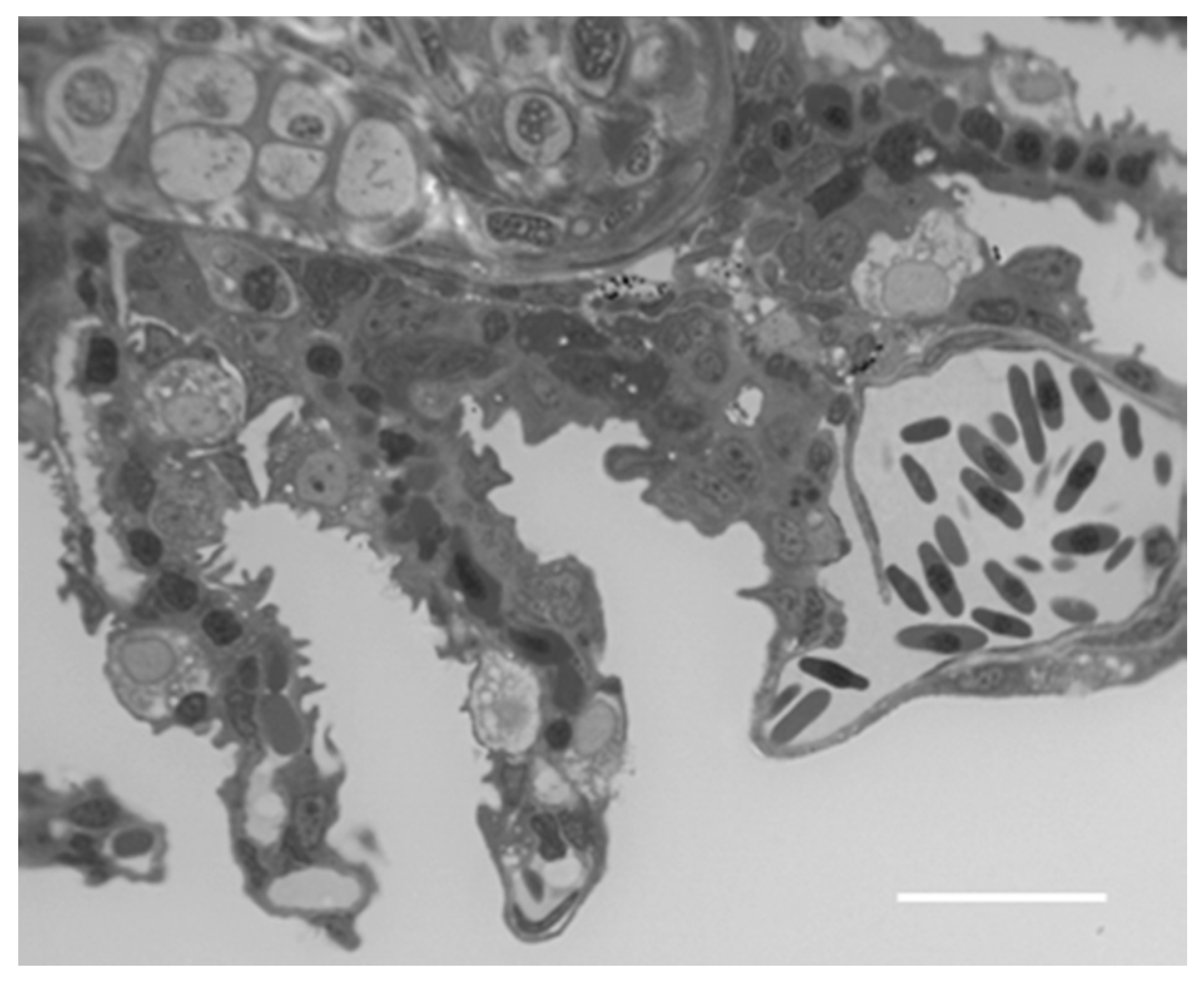
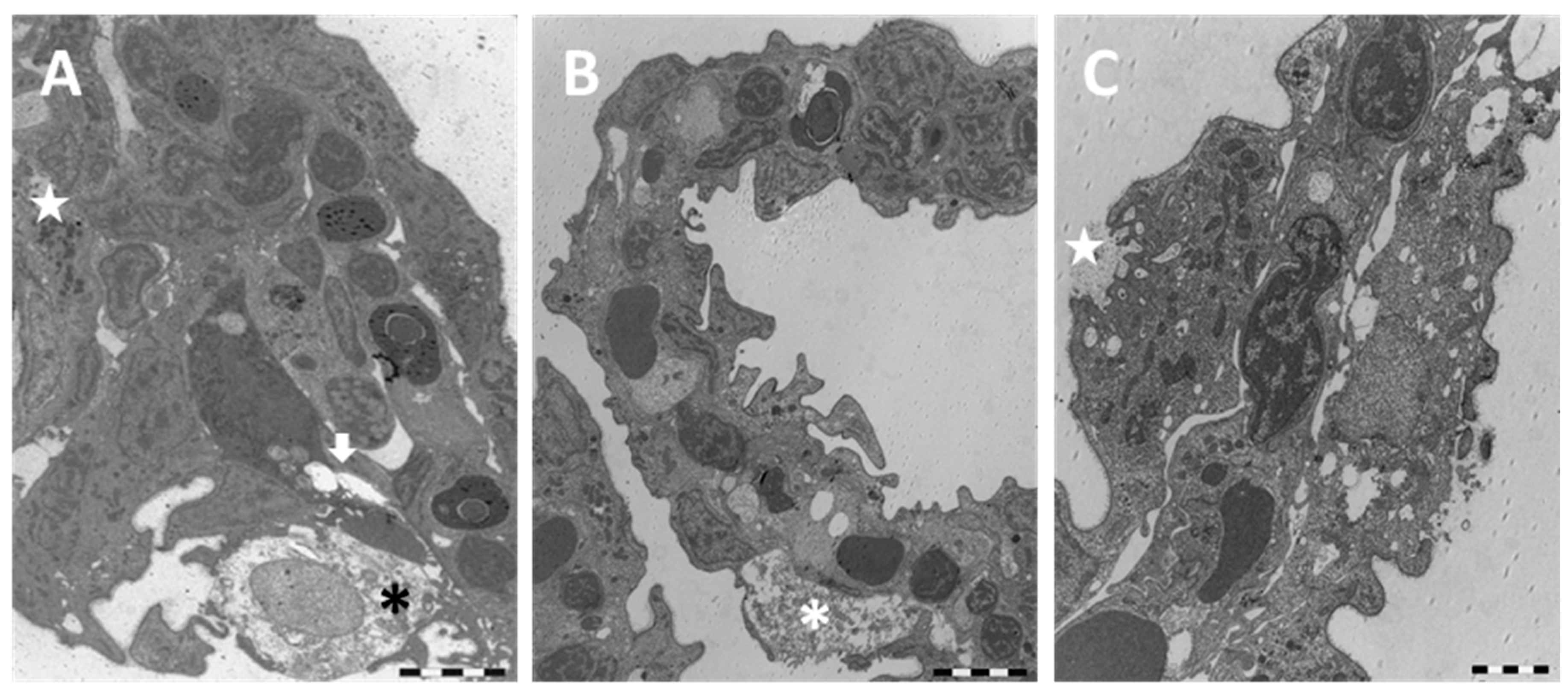
2. Results
2.1. Structure and Ultrastructure
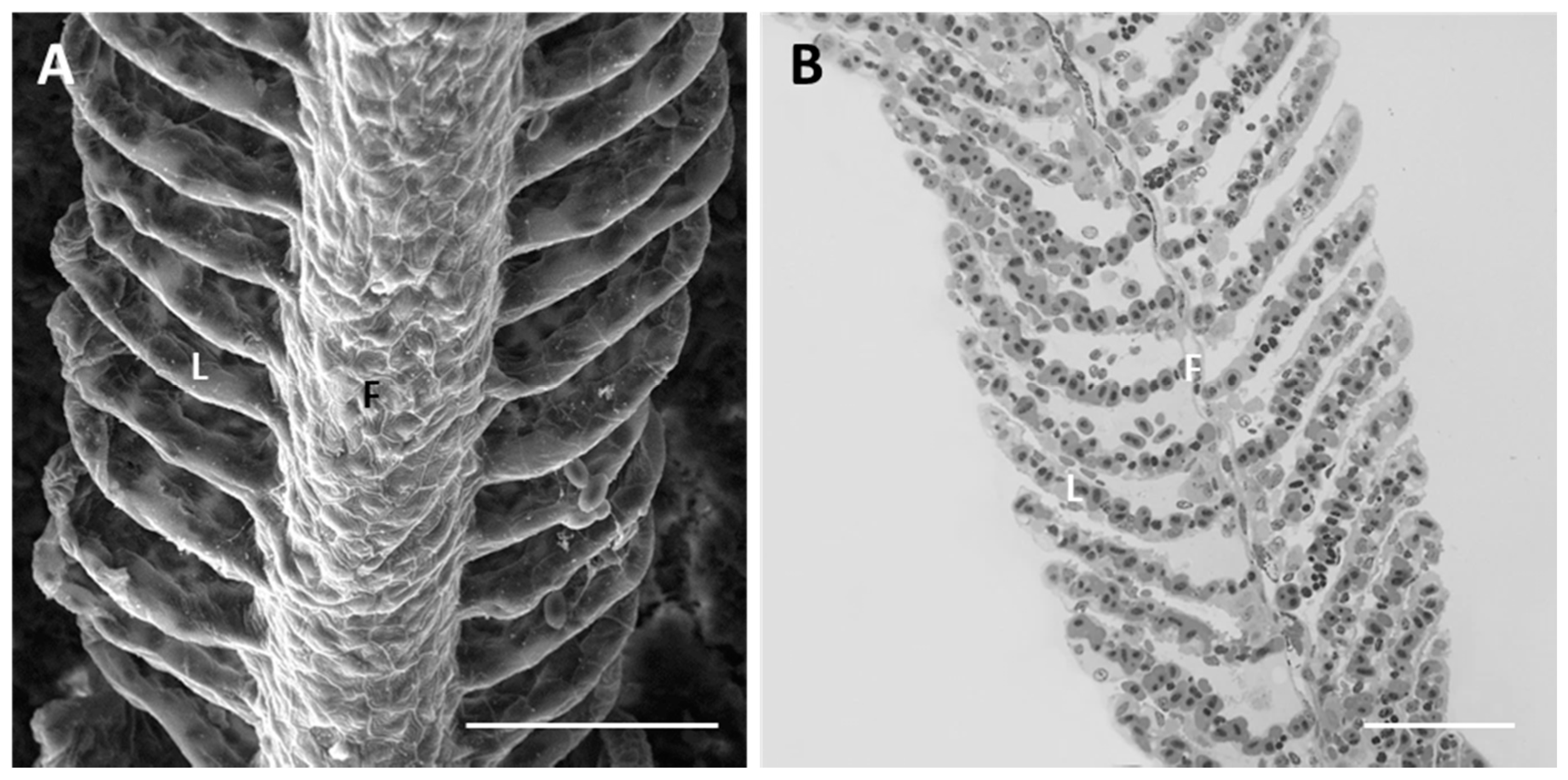
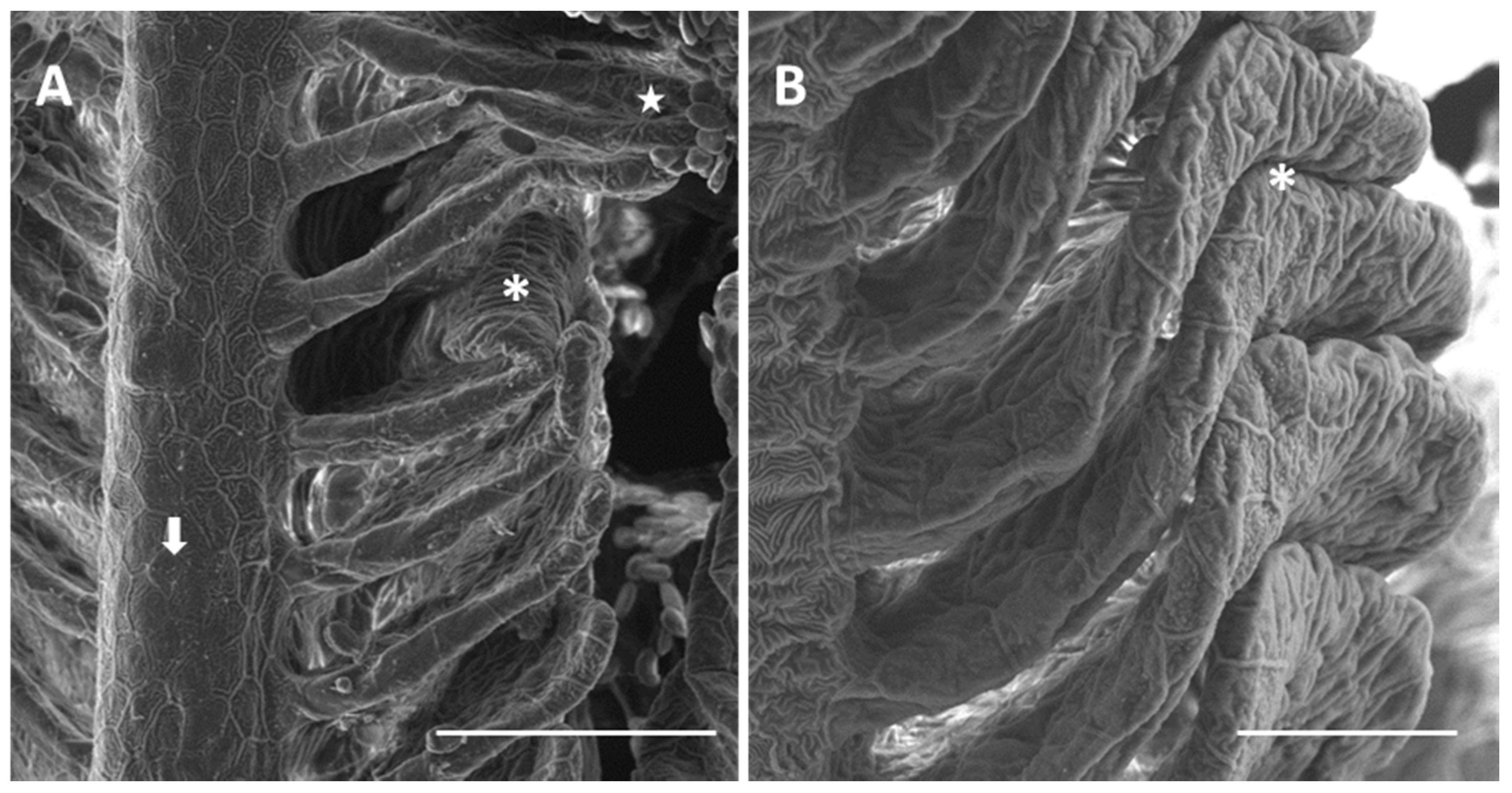
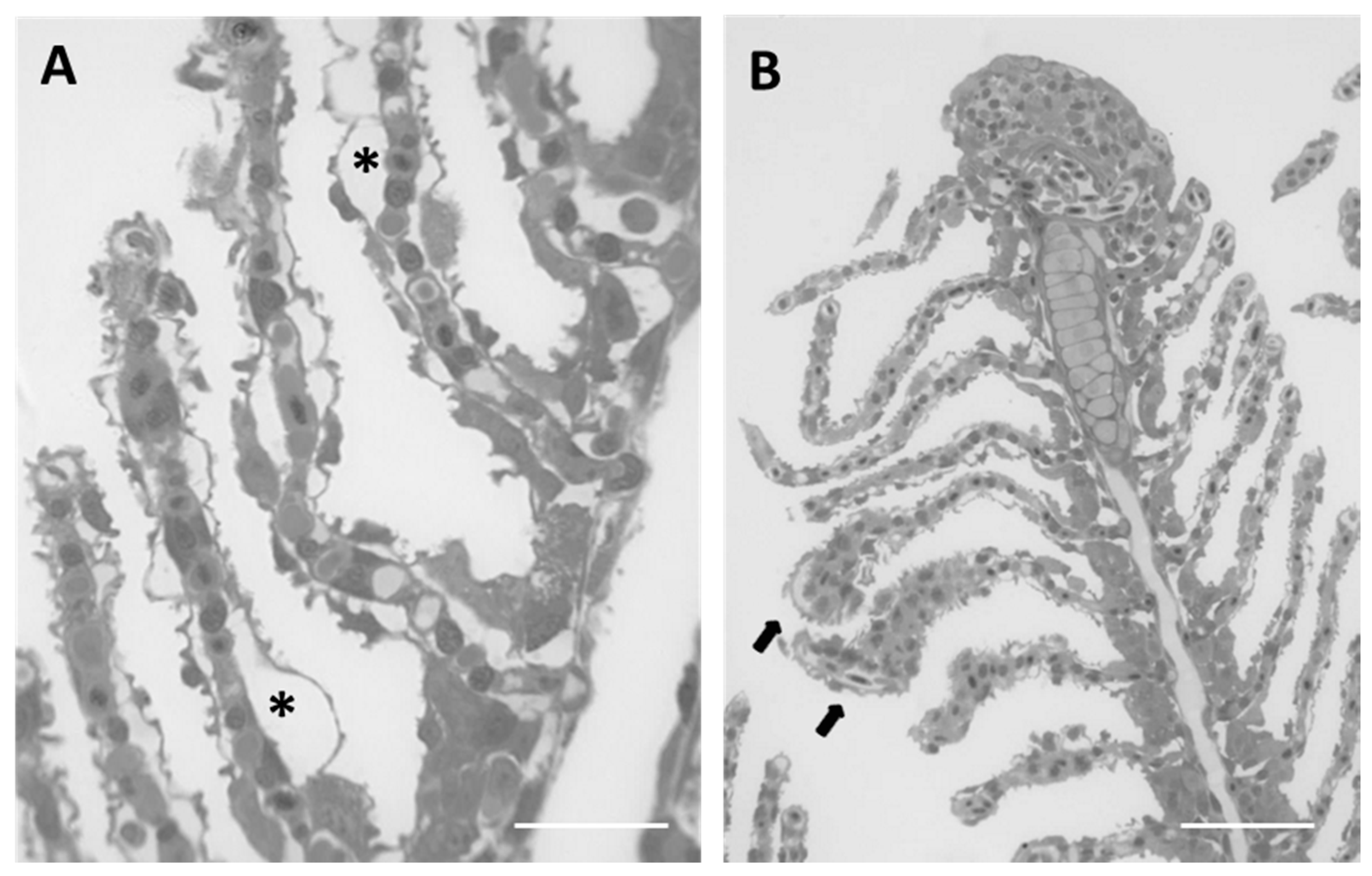
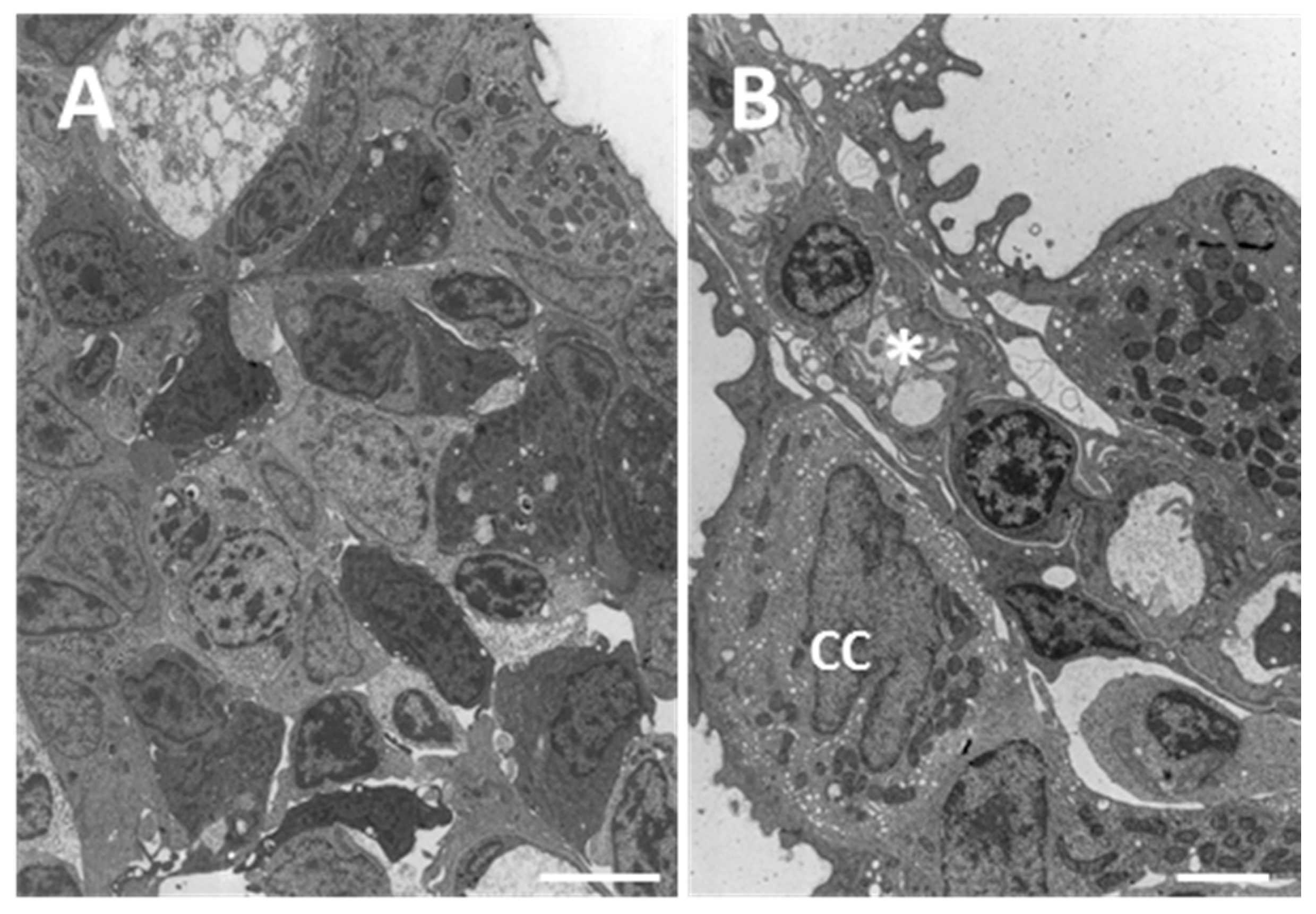
2.1.1. Control
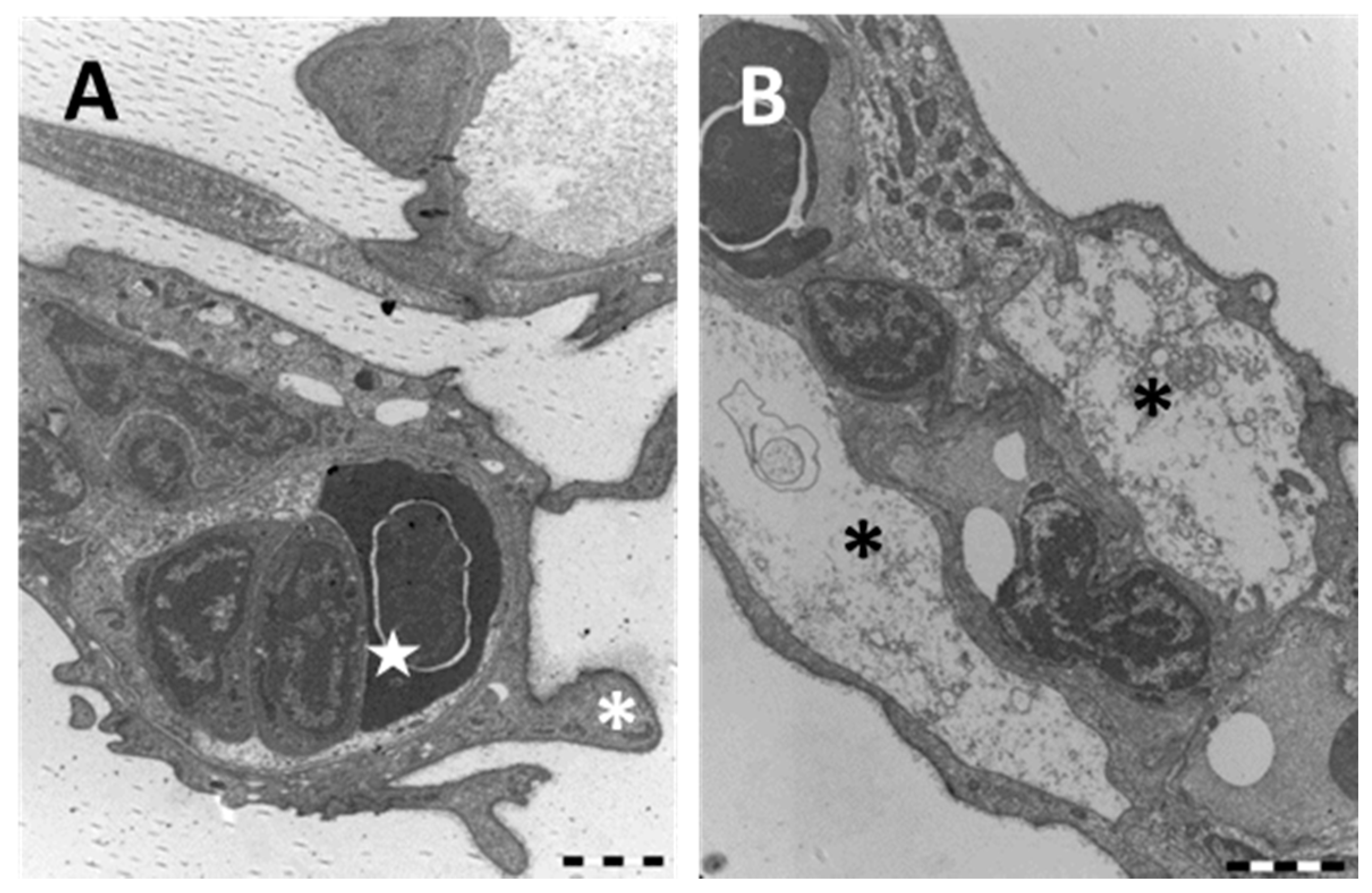
2.1.2. Exposed Fish
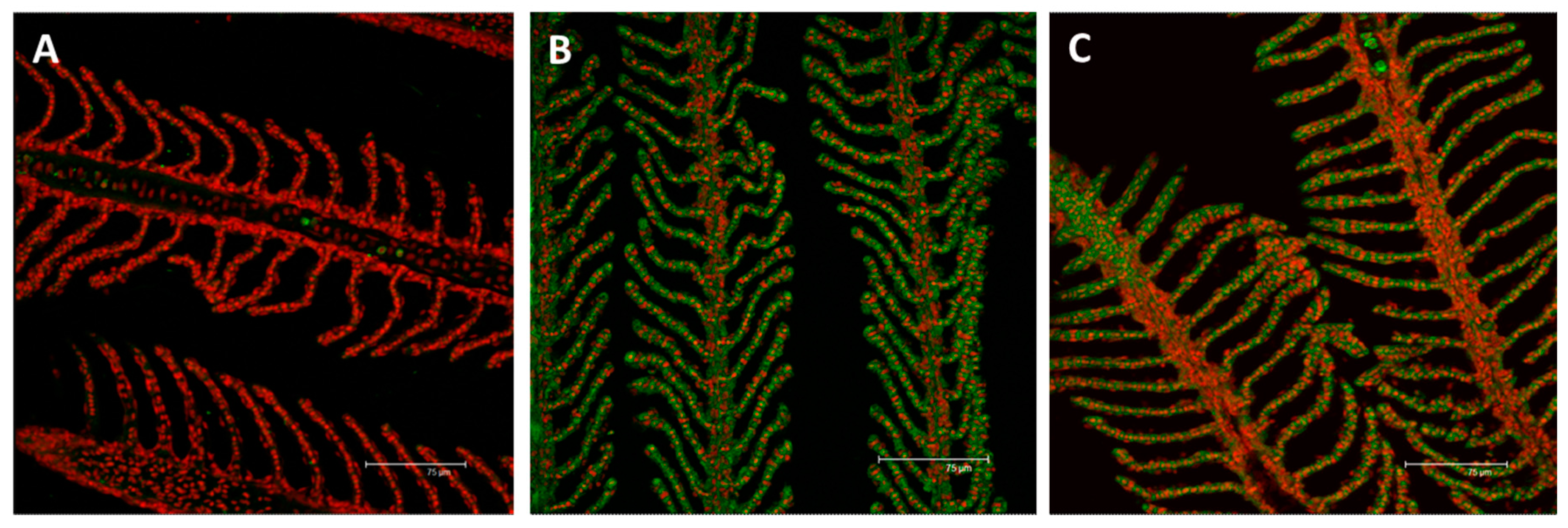
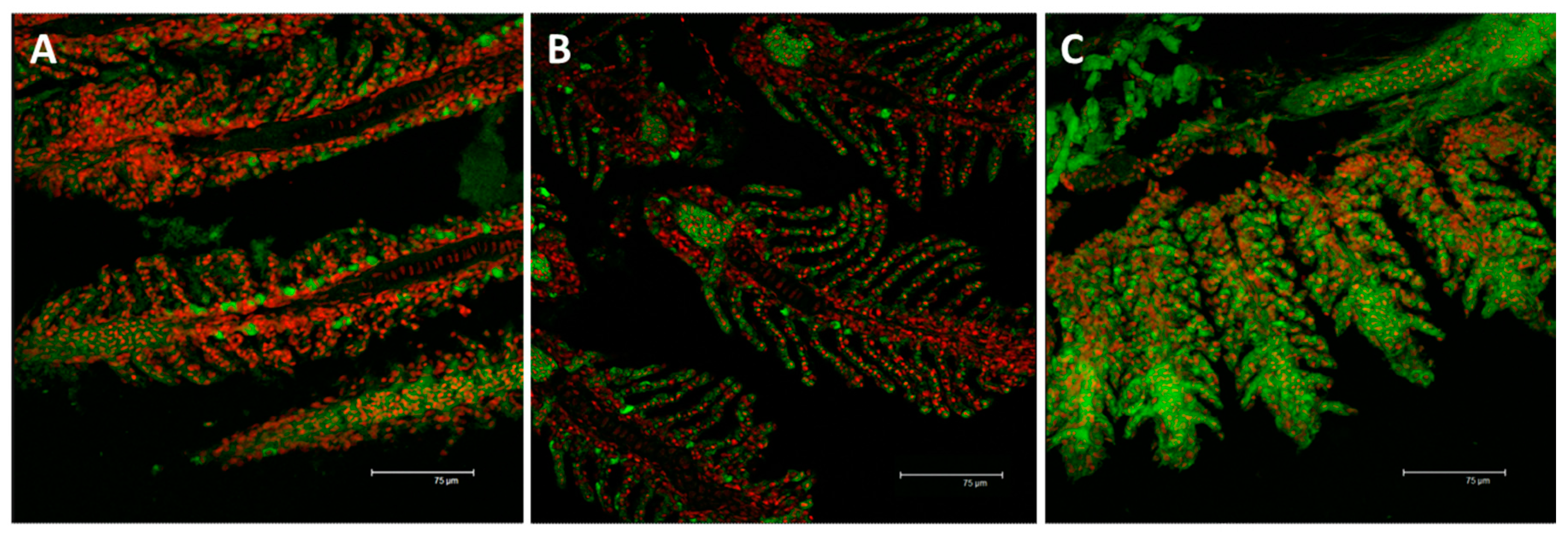
2.2. Immunofluorescence and Real Time PCR
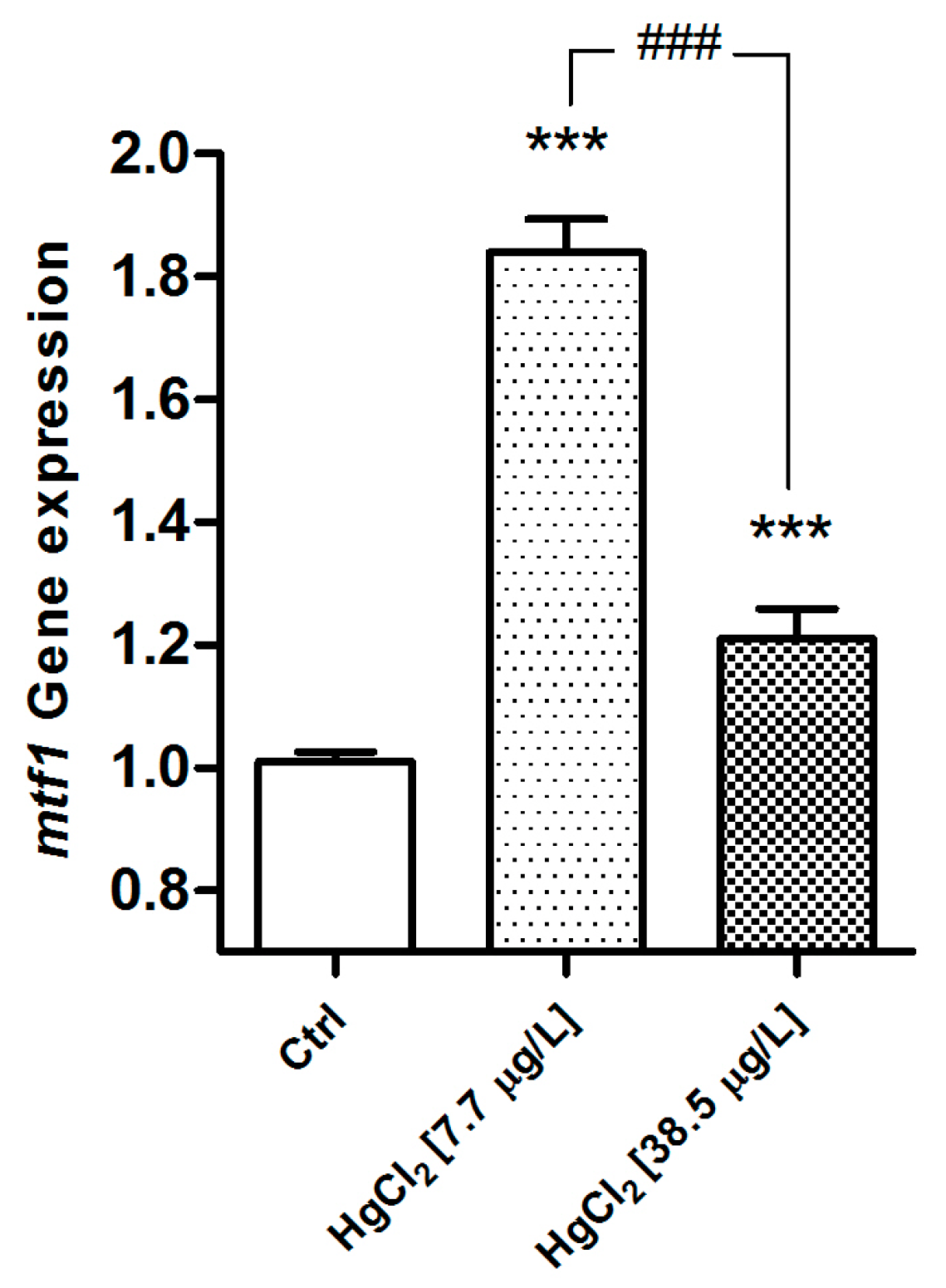
2.2.1. MT
2.2.2. Na+/K+-ATPase
3. Discussion
3.1. Chloride Cells
3.2. Metallothioneins
3.3. Na+/K+-ATPase
4. Materials and Methods
4.1. Fish Maintenance and Experimental Set-Up
4.2. Light Microscopy and Electron Microscopy
4.3. Immunohistochemistry
4.4. Quantitative RT-PCR
4.5. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bebianno, M.J.; Santos, C.; Canário, J.; Gouveia, N.; Sena-Carvalho, D.; Vale, C. Hg and metallothionein-like proteins in the black scabbard fish Aphanopus carbo. Food Chem. Toxicol. 2007, 45, 1443–1452. [Google Scholar] [CrossRef] [PubMed]
- Giari, L.; Simoni, E.; Manera, M.; Dezfuli, B.S. Histo-cytological responses of Dicentrarchus labrax (L.) following mercury exposure. Ecotoxicol. Environ. Saf. 2008, 70, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Wiener, J.G. Mercury exposed: Advances in environmental analysis and ecotoxicology of a highly toxic metal. Environ. Toxicol. Chem. 2013, 32, 2175–2178. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, M.; Kumar, K.P.; Ramesh, M. Haematological and biochemical responses of freshwater teleost fish Cyprinus carpio (Actinopterygii: Cypriniformes) during acute and chronic sublethal exposure to lindane. Pestic. Biochem. Phys. 2011, 100, 206–211. [Google Scholar] [CrossRef]
- Authman, M.M.; Ibrahim, S.A.; El-Kasheif, M.A.; Gaber, H.S. Heavy metals pollution and their effects on gills and liver of the Nile Catfish inhabiting El-Rahawy Drain, Egypt. Glob. Vet. 2013, 10, 103–115. [Google Scholar]
- Afshan, S.; Ali, S.; Ameen, U.S.; Farid, M.; Bharwana, S.A.; Hannan, F.; Ahmad, R. Effect of different heavy metal pollution on fish. Res. J. Chem. Environ. 2014, 2, 74–79. [Google Scholar]
- Brunelli, E.; Talarico, E.; Corapi, B.; Perrotta, I.; Tripepi, S. Effects of a sublethal concentration of sodium lauryl sulphate on the morphology and Na+/K+ ATPase activity in the gill of the ornate wrasse (Thalassoma pavo). Ecotoxicol. Environ. Saf. 2008, 71, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Heath, A.C. Water Pollution and Fish Physiology, 2nd ed.; Lewis: Boca Raton, FL, USA, 1995; pp. 47–62. [Google Scholar]
- Van den Heuvel, M.R.; Power, M.; Richards, J.; MacKinnon, M.; Dixon, D.G. Disease and gill lesions in yellow perch (Perca flavescens) exposed to oil sands mining-associated waters. Ecotoxicol. Environ. Saf. 2000, 46, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Liu, C.; Dawson, R.; Long, A.; Xu, F. Uptake of cadmium adsorbed on particulates by gills of goldfish (Carassius auratus). Ecotoxicol. Environ. Saf. 2000, 47, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.H. The fish gill: Site of action and model for toxic effects of environmental pollutants. Environ. Health Persp. 1987, 71, 47–58. [Google Scholar] [CrossRef]
- Monteiro, S.M.; Oliveira, E.; Fontaínhas-Fernandes, A.; Sousa, M. Effects of sublethal and lethal copper concentrations on the gill epithelium ultrastructure of Nile tilapia, Oreochromis niloticus. Zool. Stud. 2012, 51, 977–987. [Google Scholar]
- De Oliveira Ribeiro, C.A.; Guimaraes, J.R.D.; Pfeiffer, W.C. Accumulation and distribution of inorganic mercury in a tropical fish (Trichomycterus zonatus). Ecotoxicol. Environ. Saf. 1996, 34, 190–195. [Google Scholar] [CrossRef]
- Wang, R.; Wong, M.H.; Wang, W.X. Mercury exposure in the freshwater tilapia Oreochromis niloticus. Environ. Pollut. 2010, 158, 2694–2701. [Google Scholar] [CrossRef] [PubMed]
- Klinck, J.; Dunbar, M.; Brown, S.; Nichols, J.; Winter, A.; Hughes, C.; Playle, R.C. Influence of water chemistry and natural organic matter on active and passive uptake of inorganic mercury by gills of rainbow trout (Oncorhynchus mykiss). Aquat. Toxicol. 2005, 72, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.; Raimundo, J.; Barata, M.; Araújo, O.; Pousão-Ferreira, P.; Canário, J.; Pacheco, M. A new page on the road book of inorganic mercury in fish body–tissue distribution and elimination following waterborne exposure and post-exposure periods. Metallomics 2015, 7, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Niyogi, S.; Wood, C.M. Effects of chronic waterborne and dietary metal exposures on gill metal-binding: Implications for the biotic ligand model. Hum. Ecol. Risk Assess. 2003, 9, 813–846. [Google Scholar] [CrossRef]
- Glynn, A.W.; Norrgren, L.; Müssener, Å. Differences in uptake of inorganic mercury and cadmium in the gills of the zebrafish, Brachydanio rerio. Aquat. Toxicol. 1994, 30, 13–26. [Google Scholar] [CrossRef]
- Andres, S.; Laporte, J.M.; Mason, R.P. Mercury accumulation and flux across the gills and the intestine of the blue crab (Callinectes sapidus). Aquat. Toxicol. 2002, 56, 303–320. [Google Scholar] [CrossRef]
- Wiener, J.G.; Spry, D.J. Toxicological significance of mercury in freshwater fish. In Environmental Contaminants in Wildlife—Interpreting Tissue Concentrations; Beyer, W.N., Heinz, G.H., Redmon-Norwood, A.W., Eds.; Lewis Publishers: Boca Raton, FL, USA, 1996; pp. 297–339. [Google Scholar]
- Watras, C.J.; Back, R.C.; Halvorsen, S.; Hudson, R.J.M.; Morrison, K.A.; Wente, S.P. Bioaccumulation of mercury in pelagic freshwater food webs. Sci. Total Environ. 1998, 219, 183–208. [Google Scholar] [CrossRef]
- Allen, J.I.; Moore, M.N. Environmental prognostics: Is the current use of biomarkers appropriate for environmental risk evaluation? Mar. Environ. Res. 2004, 58, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Au, D.W.T. The application of histo-cytopathological biomarkers in marine pollution monitoring: A review. Mar. Poll. Bull. 2004, 48, 817–834. [Google Scholar] [CrossRef] [PubMed]
- Jagoe, C.H.; Faivre, A.; Newman, M.C. Morphological and morphometric changes in the gills of mosquitofish (Gambusia holbrooki) after exposure to mercury (II). Aquat. Toxicol. 1996, 34, 163–183. [Google Scholar] [CrossRef]
- Canli, M.; Stagg, R.M. The effects of in vivo exposure to cadmium, copper and zinc on the activities of gill ATPases in the Norway lobster, Nephrops norvegicus. Arch. Environ. Contam. Toxicol. 1996, 31, 494–501. [Google Scholar] [CrossRef]
- de Oliveira Ribeiro, C.A.; Belger, L.; Pelletier, E.; Rouleau, C. Histopathological evidence of inorganic mercury and methyl mercury toxicity in the arctic charr (Salvelinus alpinus). Environ. Res. 2002, 90, 217–225. [Google Scholar] [CrossRef]
- De Oliveira Ribeiro, C.O.; Pelletier, E.; Pfeiffer, W.C.; Rouleau, C. Comparative uptake, bioaccumulation, and gill damages of inorganic mercury in tropical and nordic freshwater fish. Environ. Res. 2000, 83, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Jagoe, C.H.; Shaw-Allen, P.L.; Brundage, S. Gill Na+, K+-ATPase activity in largemouth bass (Micropterus salmoides) from three reservoirs with different levels of mercury contamination. Aquat. Toxicol. 1996, 36, 161–176. [Google Scholar] [CrossRef]
- Poopal, R.K.; Ramesh, M.; Dinesh, B. Short-term mercury exposure on Na+/K+-ATPase activity and ion regulation in gill and brain of an Indian major carp, Cirrhinus mrigala. J. Trace Elem. Med. Biol. 2013, 27, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lim, B.; Kim, B.D.; Lee, Y.M. Effects of heavy metals on transcription and enzyme activity of Na+. J. Toxicol. Environ. Health Sci. 2016, 8, 128–134. [Google Scholar] [CrossRef]
- Stagg, R.M.; Rusin, J.; Brown, F. Na+, K+-ATPase activity in the gills of the flounder (Platichthys flesus) in relation to mercury contamination in the Firth of Forth. Mar. Environ. Res. 1992, 33, 255–266. [Google Scholar] [CrossRef]
- De Boeck, G.; Ngo, T.T.H.; van Campenhout, K.; Blust, R. Differential metallothionein induction patterns in three freshwater fish during sublethal copper exposure. Aquat. Toxicol. 2003, 65, 413–424. [Google Scholar] [CrossRef]
- Hamza-Chaffai, A.; Amiard, J.C.; Pellerin, J.; Joux, L.; Berthet, B. The potential use of metallothionein in the clam Ruditapes decussatus as a biomarker of in situ metal exposure. Comp. Biochem. Physiol. C 2000, 127, 185–197. [Google Scholar] [CrossRef]
- Roesijadi, G. Metallothionein induction as a measure of response to metal exposure in aquatic animals. Environ. Health Perspect. 1994, 102, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Montaser, M.; Mahfouz, M.E.; El-Shazly, S.A.; Abdel-Rahman, G.H.; Bakry, S. Toxicity of heavy metals on fish at Jeddah coast KSA: Metallothionein expression as a biomarker and histopathological study on liver and gills. World J. Fish Mar. Sci. 2010, 2, 174–185. [Google Scholar]
- Alvarado, N.E.; Quesada, I.; Hylland, K.; Marigómez, I.; Soto, M. Quantitative changes in metallothionein expression in target cell-types in the gills of turbot (Scophthalmus maximus) exposed to Cd, Cu, Zn and after a depuration treatment. Aquat. Toxicol. 2006, 77, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Brunelli, E.; Mauceri, A.; Maisano, M.; Bernabò, I.; Giannetto, A.; de Domenico, E.; Fasulo, S. Ultrastructural and immunohistochemical investigation on the gills of the teleost, Thalassoma pavo L., exposed to cadmium. Acta Histochem. 2011, 113, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.J.; Teraoka, H.; Heideman, W.; Peterson, R.E. Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol. Sci. 2005, 86, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Craig, P.M.; Hogstrand, C.; Wood, C.M.; McClelland, G.B. Gene expression endpoints following chronic waterborne copper exposure in a genomic model organism, the zebrafish, Danio rerio. Physiol. Genom. 2009, 40, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.T.; Baatrup, E. Ultrastructural localization of mercury accumulations in the gills, hepatopancreas, midgut, and antennal glands of the brown shrimp, Crangon crangon. Aquat. Toxicol. 1988, 13, 309–324. [Google Scholar] [CrossRef]
- Bianchini, A.; Gilles, R. Toxicity and accumulation of mercury in three species of crabs with different osmoregulatory capacities. Bull. Environ. Contam. Toxicol. 1996, 57, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, L. Gill morphology in the zebrafish, Brachydanio rerio (Hamilton-Buchanan). J. Fish Biol. 1983, 23, 511–524. [Google Scholar] [CrossRef]
- Mallatt, J. Fish gill structural changes induced by toxicants and other irritants: A statistical review. Can. J. Fish Aquat. Sci. 1985, 42, 630–648. [Google Scholar] [CrossRef]
- Martinez, C.B.R.; Nagae, M.Y.; Zaia, C.T.B.V.; Zaia, D.A.M. Acute morphological and physiological effects of lead in the neotropical fish Prochilodus lineatus. Braz. J. Biol. 2004, 64, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Daoust, P.Y.; Wobeser, G.; Newstead, J.D. Acute pathological effects of inorganic mercury and copper in gills of rainbow trout. Vet. Pathol. 1984, 21, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Kim, J.W.; de Riu, N.; Moniello, G.; Hung, S.S. Histopathological alterations of juvenile green (Acipenser medirostris) and white sturgeon (Acipenser transmontanus) exposed to graded levels of dietary methylmercury. Aquat. Toxicol. 2012, 109, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Perry, S.F. The chloride cell: Structure and function in the gills of freshwater fishes. Annu. Rev. Physiol. 1997, 59, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Parvez, S.; Ansari, R.A.; Ali, M.; Kaur, M.; Hayat, F.; Raisuddin, S. Effects of exposure to multiple trace metals on biochemical, histological and ultrastructural features of gills of a freshwater fish, Channa punctata Bloch. Chem. Biol. Interact. 2008, 174, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Franklin, G.E. Surface ultrastructure changes in the gills of sockeye salmon (Teleostei: Oncorhynchus nerka) during seawater transfer: Comparison of successful and unsuccessful seawater adaptation. J. Morphol. 1990, 206, 13–23. [Google Scholar] [CrossRef]
- Shepherd, K.L. Functions for fish mucus. Rev. Fish Biol. Fish. 1994, 4, 401–429. [Google Scholar] [CrossRef]
- Liao, C.Y.; Fu, J.J.; Shi, J.B.; Zhou, Q.F.; Yuan, C.G.; Jiang, G.B. Methylmercury accumulation, histopathology effects, and cholinesterase activity alterations in medaka (Oryzias latipes) following sublethal exposure to methylmercury chloride. Environ. Toxicol. Pharmacol. 2006, 22, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Mazon, A.F.; Monteiro, E.A.S.; Pinheiro, G.H.D.; Fernadez, M.N. Hematological and physiological changes induced by short-term exposure to copper in the freshwater fish, Prochilodus scrofa. Braz. J. Biol. 2002, 62, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Olojo, E.A.A.; Olurin, K.B.; Oluwemimo, A.D. Histopathology of the gill and liver tissues of the African catfish Clarias gariepinus exposed to lead. Afr. J. Biotechnol. 2005, 4, 117–122. [Google Scholar]
- Parvathi, K.; Sivakumar, P.; Sarasu, C. Effects of chromium on histological alterations of gill, liver and kidney of fresh water teleost, Cyprinus carpio (L.). J. Fish. Int. 2011, 6, 1–5. [Google Scholar] [CrossRef]
- Haaparanta, A.; Valtonen, E.T.; Hoffmann, R.W. Gill anomalies of perch and roach from four lakes differing in water quality. J. Fish Biol. 1997, 50, 575–591. [Google Scholar] [CrossRef]
- Vutukuru, S.S.; Basani, K. Acute effects of mercuric chloride on glycogen and protein content of Zebra fish, Danio rerio. J. Environ. Biol. 2013, 34, 277–281. [Google Scholar] [PubMed]
- Evans, D.H.; Piermarini, P.M.; Choe, K.P. The multifunctional fish gill: Dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol. Rev. 2005, 85, 97–177. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.M.; Laurent, P. Fish gill morphology: Inside out. J. Exp. Zool. 2002, 293, 192–213. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.H.; Piermarini, P.M.; Potts, W.T.W. Ionic transport in the fish gill epithelium. J. Exp. Zool. 1999, 283, 641–652. [Google Scholar] [CrossRef]
- Carmona, R.; García-Gallego, M.; Sanz, A.; Domezain, A.; Ostos-Garrido, M.V. Chloride cells and pavement cells in gill epithelia of Acipenser naccarii: Ultrastructural modifications in seawater-acclimated specimens. J. Fish Biol. 2004, 64, 553–566. [Google Scholar] [CrossRef]
- Sinha, A.K.; Matey, V.; Giblen, T.; Blust, R.; De Boeck, G. Gill remodeling in three freshwater teleosts in response to high environmental ammonia. Aquat. Toxicol. 2014, 155, 166–180. [Google Scholar] [CrossRef] [PubMed]
- Powell, M.D.; Speare, D.J.; Wrigth, G.M. Comparative ultrastructural morphology of lamellar epithelial, chloride and mucuscell glicocalyx of the rainbow trout (Oncorhynchus mykiss) gill. J. Fish Biol. 1994, 44, 725–730. [Google Scholar]
- Amiard, J.C.; Amiard-Triquet, C.; Barka, S.; Pellerin, J.; Rainbow, P.S. Metallothioneins in aquatic invertebrates: Their role in metal detoxification and their use as biomarkers. Aquat. Toxicol. 2006, 76, 160–202. [Google Scholar] [CrossRef] [PubMed]
- Roméo, M.; Cosson, R.P.; Gnassia-Barelli, M.; Risso, C.; Stien, X.; Lafaurie, M. Metallothionein determination in the liver of the sea bass Dicentrarchus labrax treated with copper and B(a)P. Mar. Environ. Res. 1997, 44, 275–284. [Google Scholar] [CrossRef]
- Ecker, D.J.; Butt, T.R.; Sternberg, E.J.; Neeper, M.P.; Debouck, C.; Gorman, J.A.; Crooke, S.T. Yeast metallothionein function in metal ion detoxification. J. Biol. Chem. 1986, 261, 16895–16900. [Google Scholar] [PubMed]
- Huang, P.C.; Morris, S.; Dinman, J.; Pine, R.; Smith, B. Role of metallothionein in detoxification and tolerance to transition metals. Exp. Suppl. 1987, 52, 439–446. [Google Scholar]
- Mieiro, C.L.; Bervoets, L.; Joosen, S.; Blust, R.; Duarte, A.C.; Pereira, M.E.; Pacheco, M. Metallothioneins failed to reflect mercury external levels of exposure and bioaccumulation in marine fish–Considerations on tissue and species specific responses. Chemosphere 2011, 85, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Dang, Z.C.; Berntssen, M.H.G.; Lundebye, A.K.; Flik, G.; Bonga, S.W.; Lock, R.A.C. Metallothionein and cortisol receptor expression in gills of Atlantic salmon, Salmo salar, exposed to dietary cadmium. Aquat. Toxicol. 2001, 53, 91–101. [Google Scholar] [CrossRef]
- Lam, K.L.; Ko, P.W.; Wong, J.K.Y.; Chan, K.M. Metal toxicity and metallothionein gene expression studies in common carp and tilapia. Mar. Environ. Res. 1998, 46, 563–566. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, F.; Cao, W.; Wang, J. The identification of metallothionein in rare minnow (Gobiocypris rarus) and its expression following heavy metal exposure. Environ. Toxicol. Phar. 2014, 37, 1283–1291. [Google Scholar] [CrossRef] [PubMed]
- Rose, S.; Vincent, S.; Meena, B.; Suresh, A.; Mani, R. Metallothionein induction in fresh water catfish Clarias gariepinus on exposure to cadmium. Int. J. Pharm. Pharm. Sci. 2014, 6, 377–383. [Google Scholar]
- Woo, S.; Yum, S.; Jung, J.H.; Shim, W.J.; Lee, C.H.; Lee, T.K. Heavy metal-induced differential gene expression of metallothionein in Javanese medaka, Oryzias javanicus. Mar. Biotechnol. 2006, 8, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Li, H.; Jin, Y.; Yu, H. Cadmium exposure affects on the expression of metallothionein 2 gene in grass carp (Ctenopharyngodon idellus). Genes Genom. 2016, 38, 127–135. [Google Scholar] [CrossRef]
- Olsvik, P.A.; Hindar, K.; Zachariassen, K.E.; Andersen, R.A. Brown trout (Salmo trutta) metallothioneins as biomarkers for metal exposure in two Norwegian rivers. Biomarkers 2001, 6, 274–288. [Google Scholar]
- Nikpour, Y.; Zolgharnein, H.; Sinaei, M.; Najafzadeh, H.; Ghavasi, M. Evaluation of metallothionein expression as a biomarker of mercury exposure in Scatophagus argus. Pak. J. Biol. Sci. 2008, 11, 2269–2273. [Google Scholar] [CrossRef] [PubMed]
- McCormick, S.D.; Regish, A.M.; Christensen, A.K. Distinct freshwater and seawater isoforms of Na+/K+-ATPase in gill chloride cells of Atlantic salmon. J. Exp. Biol. 2009, 212, 3994–4001. [Google Scholar] [CrossRef] [PubMed]
- Foskett, J.K.; Scheffey, C. The chloride cell: Definitive identification as the salt-secretory cell in teleosts. Science 1982, 215, 164–166. [Google Scholar] [CrossRef] [PubMed]
- Hirose, S.; Kaneko, T.; Naito, N.; Takei, Y. Molecular biology of major components of chloride cells. Comp. Biochem. Phys. B 2003, 136, 593–620. [Google Scholar] [CrossRef]
- De la Torre, F.R.; Salibián, A.; Ferrari, L. Assessment of the pollution impact on biomarkers of effect of a freshwater fish. Chemosphere 2007, 68, 1582–1590. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.F.; Beamish, F.W.H. Effects of zinc on branchial ATPase activity in vivo in rainbow trout (Salmo gairdnieri). Compos. Biochem. Physiol. 1980, 66, 77–82. [Google Scholar]
- Watson, C.F.; Benson, W.H. Comparative activity of gill ATPase in three freshwater teleosts exposed to cadmium. Ecotoxicol. Environ. Saf. 1987, 14, 252–259. [Google Scholar] [CrossRef]
- Wendelaar Bonga, S.E. The stress response in fish. Physiol. Revs. 1997, 77, 591–625. [Google Scholar]
- Ay, Ö.; Kalay, M.; Tamer, L.; Canli, M. Copper and lead accumulation in tissues of a freshwater fish Tilapia zillii and its effects on the branchial Na, K-ATPase activity. Bull. Environ. Contam. Toxicol. 1999, 62, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Griffitt, R.J.; Weil, R.; Hyndman, K.A.; Denslow, N.D.; Powers, K.; Taylor, D.; Barber, D.S. Exposure to copper nanoparticles causes gill injury and acute lethality in zebrafish (Danio rerio). Environ. Sci. Technol. 2007, 41, 8178–8186. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, S.M.; Mancera, J.M.; Fontaínhas-Fernandes, A.; Sousa, M. Copper induced alterations of biochemical parameters in the gill and plasma of Oreochromis niloticus. Comp. Biochem. Phys. C 2005, 141, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Atli, G.; Canli, M. Enzymatic responses to metal exposures in a freshwater fish Oreochromis niloticus. Comp. Biochem. Physiol. C 2007, 145, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Macirella, R.; Guardia, A.; Pellegrino, D.; Bernabò, I.; Tronci, V.; Ebbesson, L.O.; Sesti, S.; Tripepi, S.; Brunelli, E. Effects of two sublethal concentrations of mercury chloride on the morphology and metallothionein activity in the liver of Zebrafish (Danio rerio). Int. J. Mol. Sci. 2016, 17, 361. [Google Scholar] [CrossRef] [PubMed]
- Coons, A.H.; Leduc, E.H.; Connolly, J.M. Studies on antibody. I. A method for the histochemical demonstration of specific antibody and its application to a study of the hyperimmune rabbit. J. Exp. Med. 1955, 102, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macirella, R.; Brunelli, E. Morphofunctional Alterations in Zebrafish (Danio rerio) Gills after Exposure to Mercury Chloride. Int. J. Mol. Sci. 2017, 18, 824. https://doi.org/10.3390/ijms18040824
Macirella R, Brunelli E. Morphofunctional Alterations in Zebrafish (Danio rerio) Gills after Exposure to Mercury Chloride. International Journal of Molecular Sciences. 2017; 18(4):824. https://doi.org/10.3390/ijms18040824
Chicago/Turabian StyleMacirella, Rachele, and Elvira Brunelli. 2017. "Morphofunctional Alterations in Zebrafish (Danio rerio) Gills after Exposure to Mercury Chloride" International Journal of Molecular Sciences 18, no. 4: 824. https://doi.org/10.3390/ijms18040824






