Hypaphorine Attenuates Lipopolysaccharide-Induced Endothelial Inflammation via Regulation of TLR4 and PPAR-γ Dependent on PI3K/Akt/mTOR Signal Pathway
Abstract
:1. Introduction
2. Results
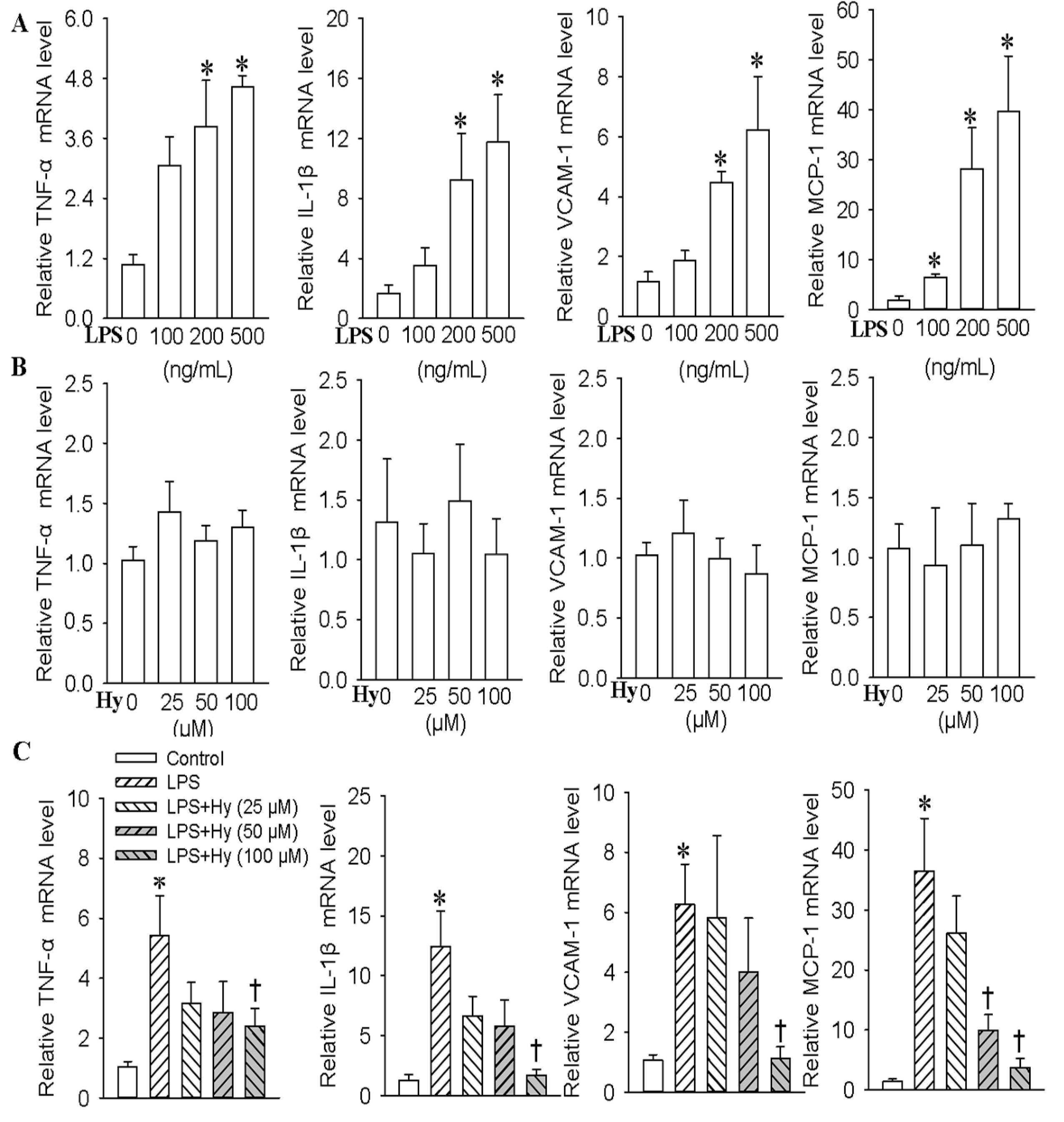
2.1. Effects of Hy on the Expressions of TNF-α, IL-1β, VCAM-1 and MCP-1 in HMEC-1 Cells Response to LPS
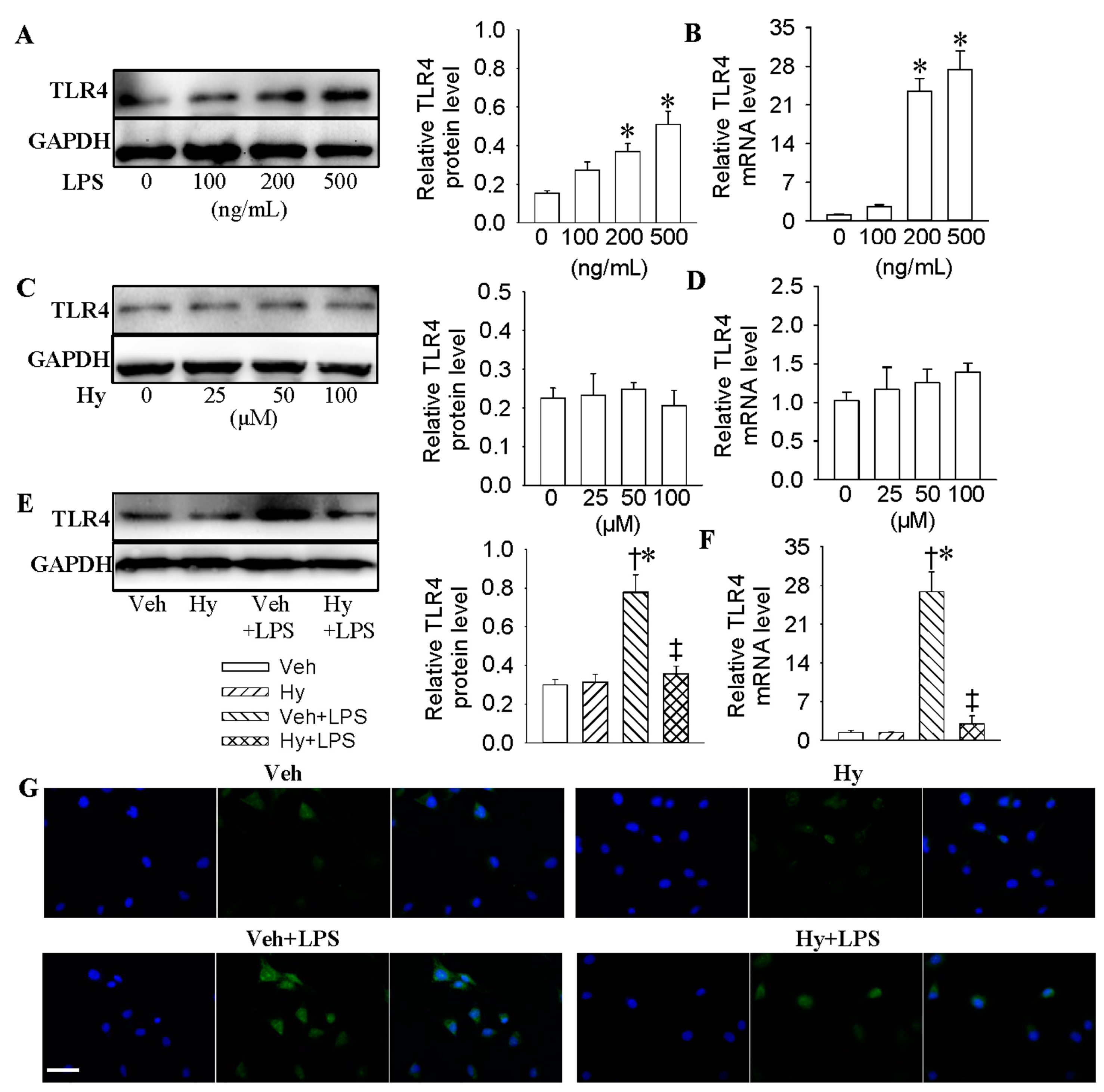
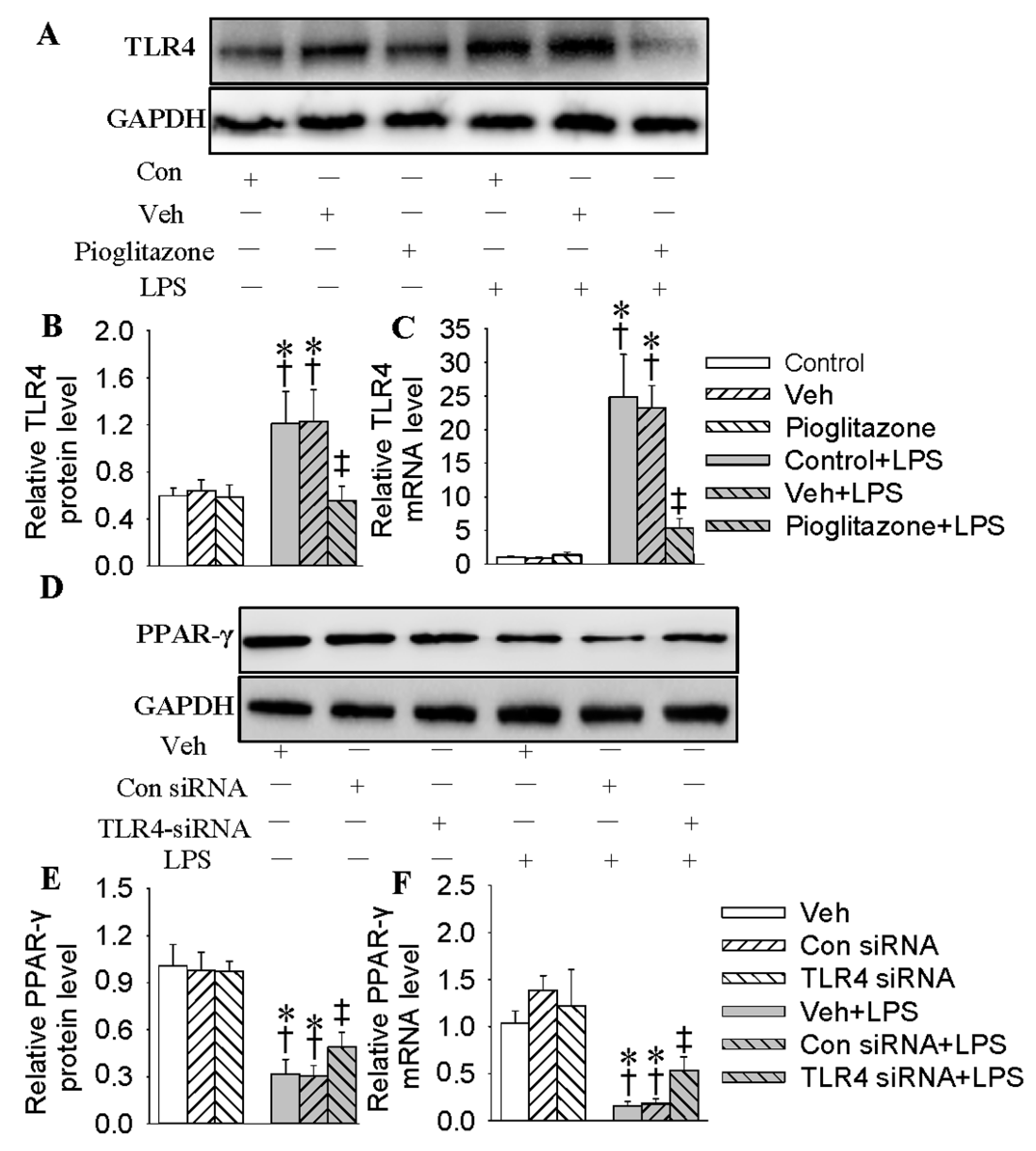
2.2. Inhibition of TLR4 Was Involved in the Anti-Inflammatory Action of Hy on HMEC-1 Cells Response to LPS
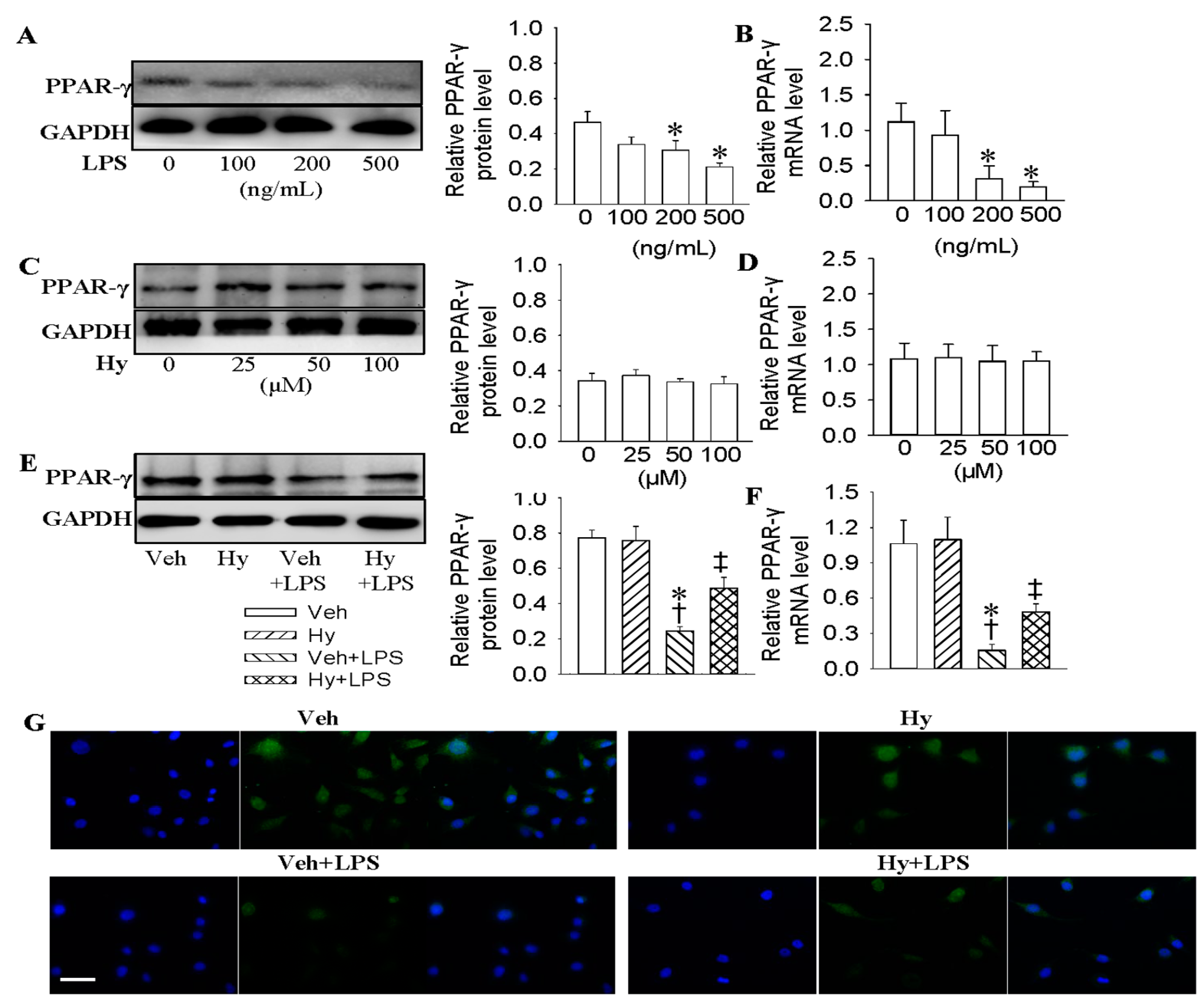
2.3. Activation of PPAR-γ Mediated the Protective Role of Hy against LPS-Evoked Inflammation in HMEC-1 Cells
2.4. Interaction of PPAR-γ with TLR4 in HMEC-1 Cells in Response to LPS
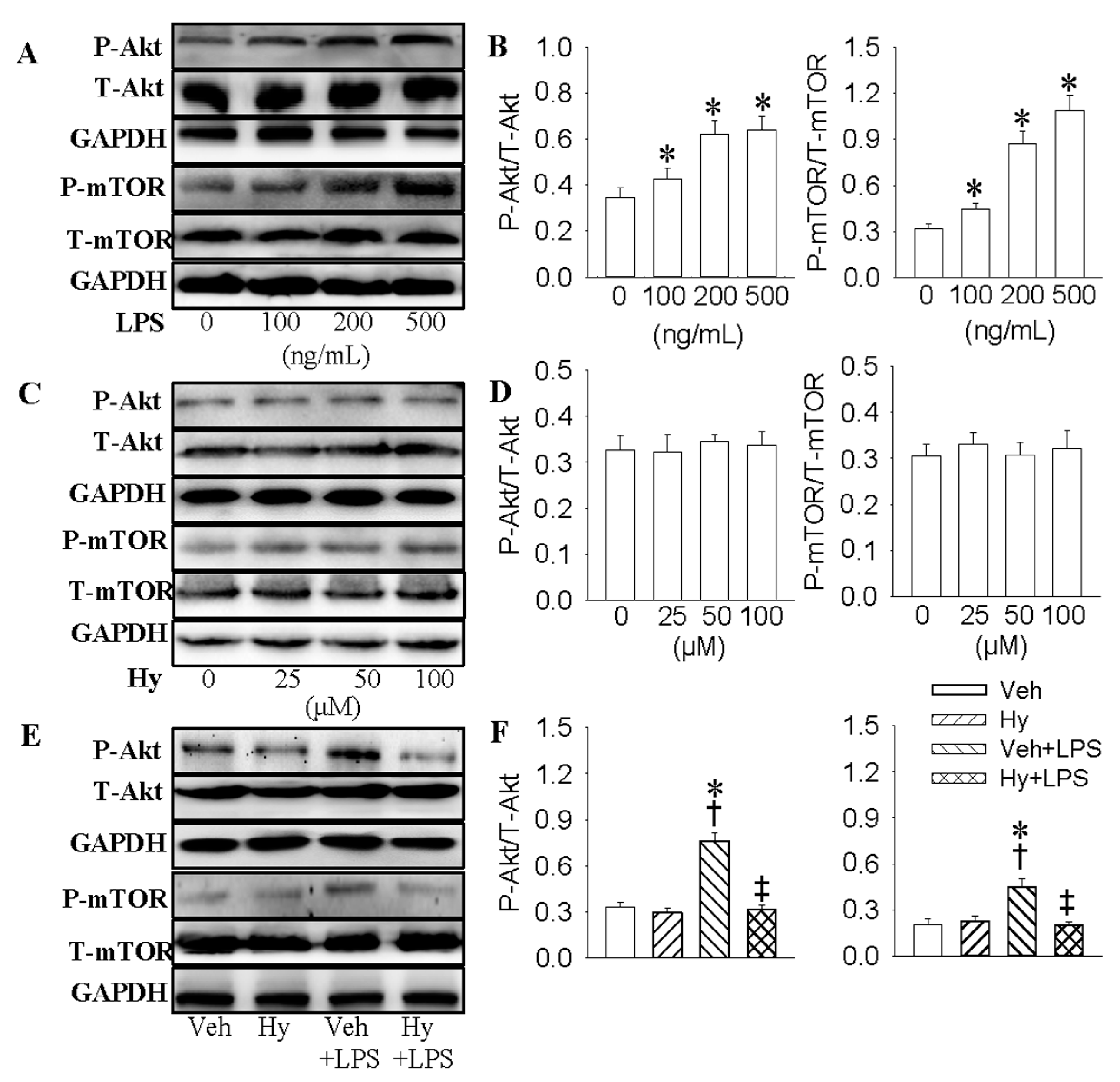
2.5. PI3K/Akt/mTOR Signaling Pathway Was Responsible for the Inhibitory Effect of Hy on LPS-Induced Inflammation Response in HMEC-1 Cells
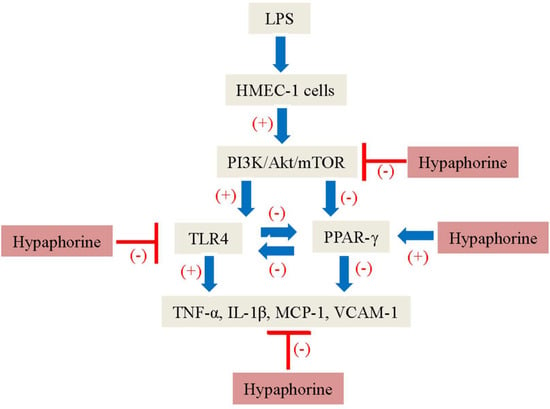
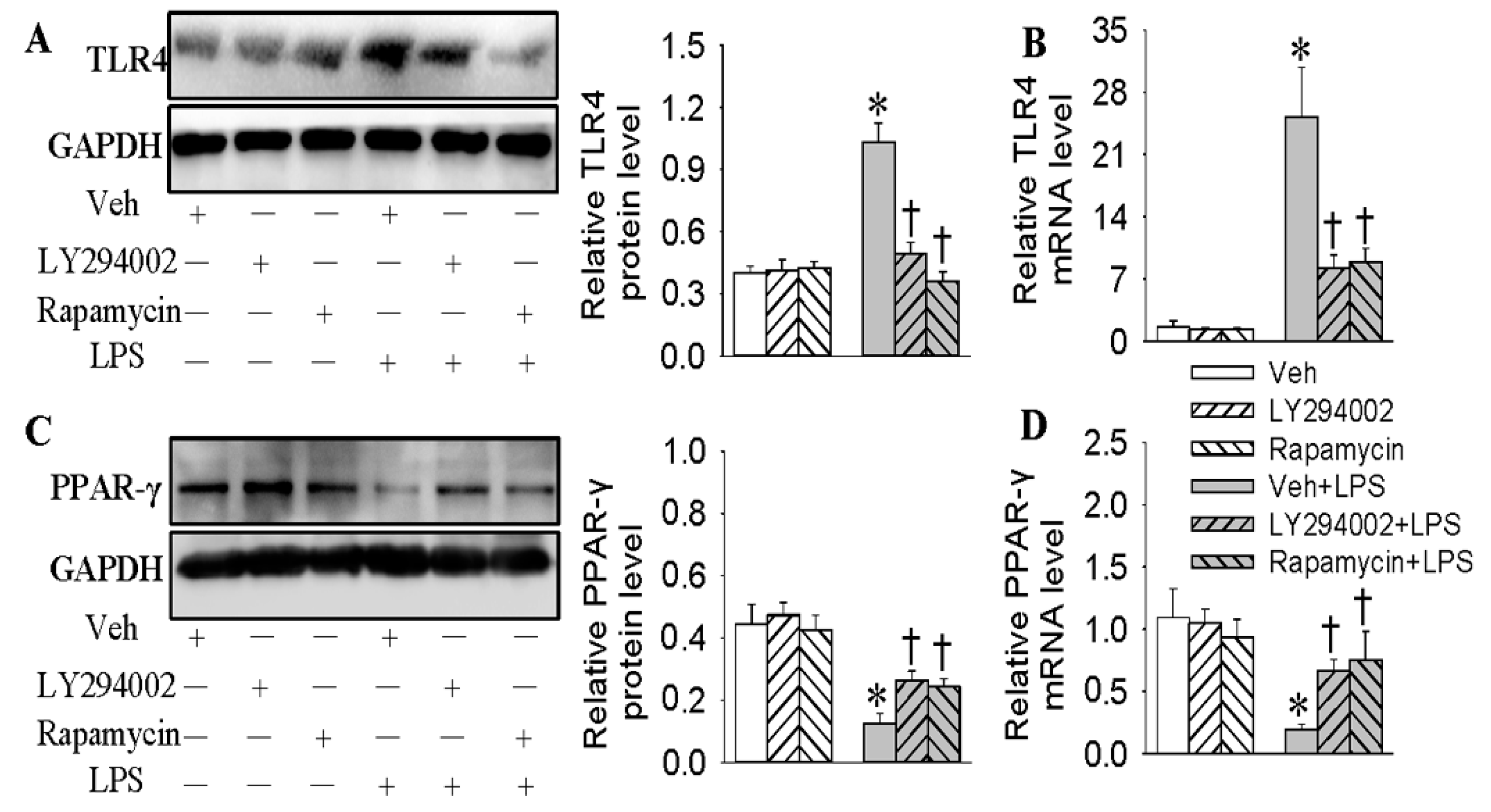
2.6. Negative Correlation of TLR4 and PPAR-γ in LPS-Stimulated HMEC-1 Cells Was Dependent on the PI3K/Akt/mTOR Signaling Pathway
3. Discussion
4. Material and Methods
4.1. Reagents and Antibodies
4.2. Cell Culture
4.3. Real-Time Quantitative PCR Analysis
4.4. Western Blot Analysis
4.5. Immunofluorescence Microscopy
4.6. siRNA Transfections
4.7. Enzyme-Linked Immunosorbent Assay (ELISA)
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wiedemair, W.; Tukovic, Z.; Jasak, H.; Poulikakos, D.; Kurtcuoglu, V. The breakup of intravascular microbubbles and its impact on the endothelium. Biomech. Model. Mechanobiol. 2017, 16, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Cines, D.B.; Pollak, E.S.; Buck, C.A.; Loscalzo, J.; Zimmerman, G.A.; McEver, R.P.; Pober, J.S.; Wick, T.M.; Konkle, B.A.; Schwartz, B.S.; et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood 1998, 91, 3527–3561. [Google Scholar] [PubMed]
- Nomura, J.; Busso, N.; Ives, A.; Matsui, C.; Tsujimoto, S.; Shirakura, T.; Tamura, M.; Kobayashi, T.; So, A.; Yamanaka, Y. Xanthine oxidase inhibition by febuxostat attenuates experimental atherosclerosis in mice. Sci. Rep. 2014, 4, 4554. [Google Scholar] [CrossRef] [PubMed]
- Gray, S.P.; Jandeleit-Dahm, K.A. The role of NADPH oxidase in vascular disease—Hypertension, atherosclerosis & stroke. Curr. Pharm. Des. 2015, 21, 5933–5944. [Google Scholar] [PubMed]
- Han, J.M.; Li, H.; Cho, M.H.; Baek, S.H.; Lee, C.H.; Park, H.Y.; Jeong, T.S. Soy-Leaf Extract Exerts Atheroprotective Effects via Modulation of Kruppel-Like Factor 2 and Adhesion Molecules. Int. J. Mol. Sci. 2017, 18, 373. [Google Scholar] [CrossRef] [PubMed]
- Hertle, E.; Arts, I.C.; van der Kallen, C.J.; Feskens, E.J.; Schalkwijk, C.G.; Stehouwer, C.D.; van Greevenbroek, M.M. The alternative complement pathway is longitudinally associated with adverse cardiovascular outcomes. The CODAM study. Thromb. Haemost. 2016, 115, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Steven, S.; Munzel, T.; Daiber, A. Exploiting the Pleiotropic Antioxidant Effects of Established Drugs in Cardiovascular Disease. Int. J. Mol. Sci. 2015, 16, 18185–18223. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Srivastava, M.; Saqib, U.; Liu, D.; Faisal, S.M.; Sugathan, S.; Bishnoi, S.; Baig, M.S. Potential therapeutic targets for inflammation in toll-like receptor 4 (TLR4)-mediated signaling pathways. Int. Immunopharmacol. 2016, 40, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Hu, X.; Cao, Y.; Zhang, Z.; Zhang, N. Saikosaponin a inhibits lipopolysaccharide-oxidative stress and inflammation in Human umbilical vein endothelial cells via preventing TLR4 translocation into lipid rafts. Free Radic. Biol. Med. 2015, 89, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Roshan, M.H.; Tambo, A. The Role of TLR2, TLR4, and TLR9 in the Pathogenesis of Atherosclerosis. Int. J. Inflamm. 2016, 2016, 1532832. [Google Scholar] [CrossRef] [PubMed]
- Menendez-Gutierrez, M.P.; Roszer, T.; Ricote, M. Biology and therapeutic applications of peroxisome proliferator-activated receptors. Curr. Top. Med. Chem. 2012, 12, 548–584. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.; Chang, L.; Fan, Y.; Zhang, J.; Chen, Y.E. PPARs and the cardiovascular system. Antioxid. Redox Signal. 2009, 11, 1415–1452. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, W.A.; Law, R. The central role of fat and effect of peroxisome proliferator-activated receptor-γ on progression of insulin resistance and cardiovascular disease. Am. J. Cardiol. 2003, 92, 3j–9j. [Google Scholar] [CrossRef]
- Wu, K.; Yang, Y.; Liu, D.; Qi, Y.; Zhang, C.; Zhao, J.; Zhao, S. Activation of PPARgamma suppresses proliferation and induces apoptosis of esophageal cancer cells by inhibiting TLR4-dependent MAPK pathway. Oncotarget 2016, 7, 44572–44582. [Google Scholar] [PubMed]
- Bae, J.; Chen, J.; Zhao, L. Chronic activation of pattern recognition receptors suppresses brown adipogenesis of multipotent mesodermal stem cells and brown pre-adipocytes. Biochem. Cell Biol. Biochim. Biol. Cell. 2015, 93, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Mateu, A.; Ramudo, L.; Manso, M.A.; de Dios, I. Cross-talk between TLR4 and PPARgamma pathways in the arachidonic acid-induced inflammatory response in pancreatic acini. Int. J. Biochem. Cell Biol. 2015, 69, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, M.; Honda, K.; Nakai, I.; Kishida, A.; Ohsaki, A. Hypaphorine, an indole alkaloid from Erythrina velutina, induced sleep on normal mice. Bioorganic Med. Chem. Lett. 2008, 18, 3992–3994. [Google Scholar] [CrossRef] [PubMed]
- Mollica, A.; Locatelli, M.; Stefanucci, A.; Pinnen, F. Synthesis and bioactivity of secondary metabolites from marine sponges containing dibrominated indolic systems. Molecules 2012, 17, 6083–6099. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Guo, H.; Jing, Z.; Yang, L.; Chen, C.; Peng, L.; Wang, X.; Yan, L.; Ye, R.; Jin, X.; et al. N-Oleoylethanolamine Reduces Inflammatory Cytokines and Adhesion Molecules in TNF-α-induced Human Umbilical Vein Endothelial Cells by Activating CB2 and PPAR-α. J. Cardiovasc. Pharmacol. 2016, 68, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Kong, J.; Jin, J.; Kong, J.; He, Y.; Dong, S.; Ji, L.; Liu, D.; He, D.; Kong, L.; et al. A novel anti-inflammatory mechanism of high density lipoprotein through up-regulating annexin A1 in vascular endothelial cells. Biochim. Biophys. Acta 2016, 1861, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-Diez, R.; Gonzalez-Guerrero, C.; Ocana-Salceda, C.; Rodrigues-Diez, R.R.; Egido, J.; Ortiz, A.; Ruiz-Ortega, M.; Ramos, A.M. Calcineurin inhibitors cyclosporine A and tacrolimus induce vascular inflammation and endothelial activation through TLR4 signaling. Sci. Rep. 2016, 6, 27915. [Google Scholar] [CrossRef] [PubMed]
- Masat, E.; Gasparini, C.; Agostinis, C.; Bossi, F.; Radillo, O.; de Seta, F.; Tamassia, N.; Cassatella, M.A.; Bulla, R. RelB activation in anti-inflammatory decidual endothelial cells: A master plan to avoid pregnancy failure? Sci. Rep. 2015, 5, 14847. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Li, Y.; Jin, J.; Zhang, X.; Lopes-Virella, M.F.; Huang, Y. Toll-like receptor 4 activation in microvascular endothelial cells triggers a robust inflammatory response and cross talk with mononuclear cells via interleukin-6. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1696–1706. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.A.; Yan, C.; Wang, L.; Li, G.; Xu, Y.; Xia, X. Urolithin A attenuates ox-LDL-induced endothelial dysfunction partly by modulating microRNA-27 and ERK/PPAR-γ pathway. Mol. Nutr. Food Res. 2016, 60, 1933–1943. [Google Scholar] [CrossRef] [PubMed]
- Mukohda, M.; Stump, M.; Ketsawatsomkron, P.; Hu, C.; Quelle, F.W.; Sigmund, C.D. Endothelial PPAR-γ provides vascular protection from IL-1β-induced oxidative stress. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H39–H48. [Google Scholar] [CrossRef] [PubMed]
- Bent, E.H.; Gilbert, L.A.; Hemann, M.T. A senescence secretory switch mediated by PI3K/AKT/mTOR activation controls chemoprotective endothelial secretory responses. Genes Dev. 2016, 30, 1811–1821. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Banerjee, N.; Barnes, R.C.; Pfent, C.M.; Talcott, S.T.; Dashwood, R.H.; Mertens-Talcott, S.U. Mango polyphenolics reduce inflammation in intestinal colitis-involvement of the miR-126/PI3K/AKT/mTOR axis in vitro and in vivo. Mol. Carcinog. 2017, 56, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Abe, J.; Berk, B.C. Novel mechanisms of endothelial mechanotransduction. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2378–2386. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.; Nheu, L.; Komesaroff, P.A. Cell adhesion molecules as pharmaceutical target in atherosclerosis. Mini Rev. Med. Chem. 2012, 12, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Talreja, J.; Kabir, M.H.; M, B.F.; Stechschulte, D.J.; Dileepan, K.N. Histamine induces Toll-like receptor 2 and 4 expression in endothelial cells and enhances sensitivity to Gram-positive and Gram-negative bacterial cell wall components. Immunology 2004, 113, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; You, S.W.; Yang, Y.J.; Wei, X.Y.; Wang, Y.Z.; Wang, X.; Hao, D.J.; Kuang, F.; Shang, L.X. Systemic injection of low-dose lipopolysaccharide fails to break down the blood-brain barrier or activate the TLR4-MyD88 pathway in neonatal rat brain. Int. J. Mol. Sci. 2014, 15, 10101–10115. [Google Scholar] [CrossRef] [PubMed]
- Pasterkamp, G.; Van Keulen, J.K.; de Kleijn, D.P. Role of Toll-like receptor 4 in the initiation and progression of atherosclerotic disease. Eur. J. Clin. Investig. 2004, 34, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Outzen, E.M.; Zaki, M.; Mehryar, R.; Abdolalizadeh, B.; Sajid, W.; Boonen, H.C.; Sams, A.; Sheykhzade, M. LPS, but not Angiotensin ll, lnduces Direct Pro-lnflammatory Effects in Cultured Mouse Arteries and Human Endothelial and Vascular Smooth Muscle Cells. Basic Clin. Pharmacol. Toxicol. 2017, 120, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Kim, J.M.; Baik, E.J.; Ryu, J.H.; Lee, S.H. Isobavachalcone attenuates lipopolysaccharide-induced ICAM-1 expression in brain endothelial cells through blockade of toll-like receptor 4 signaling pathways. Eur. J. Pharmacol. 2015, 754, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Balakumar, P.; Kathuria, S. Submaximal PPARgamma activation and endothelial dysfunction: New perspectives for the management of cardiovascular disorders. Br. J. Pharmacol. 2012, 166, 1981–1992. [Google Scholar] [CrossRef] [PubMed]
- Lagana, A.S.; Vitale, S.G.; Nigro, A.; Sofo, V.; Salmeri, F.M.; Rossetti, P.; Rapisarda, A.M.; La Vignera, S.; Condorelli, R.A.; Rizzo, G.; et al. Pleiotropic Actions of Peroxisome Proliferator-Activated Receptors (PPARs) in Dysregulated Metabolic Homeostasis, Inflammation and Cancer: Current Evidence and Future Perspectives. Int. J. Mol. Sci. 2016, 17, 999. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.T.; Lakshmi, S.P.; Kleinhenz, J.M.; Sutliff, R.L.; Hart, C.M.; Reddy, R.C. Endothelial cell peroxisome proliferator-activated receptor gamma reduces endotoxemic pulmonary inflammation and injury. J. Immunol. 2012, 189, 5411–5420. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhan, R.X.; Chen, J.Q.; Gao, Y.; Chen, L.; Kong, Y.; Zhong, X.J.; Liu, M.Q.; Chu, J.J.; Yan, G.Q.; et al. Pharmacological activation of PPAR γ ameliorates vascular endothelial insulin resistance via a non-canonical PPAR γ-dependent nuclear factor-κ B trans-repression pathway. Eur. J. Pharmacol. 2015, 754, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.L.; Gao, C.Y.; Fang, C.Q.; Wang, Y.J.; Gao, D.; Yao, G.E.; Xiang, J.; Wang, J.Z.; Li, J.C. PPARγ attenuates intimal hyperplasia by inhibiting TLR4-mediated inflammation in vascular smooth muscle cells. Cardiovasc. Res. 2011, 92, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Liu, J.; Wang, Z.; Li, Z. PPARgamma agonist rosiglitazone ameliorates LPS-induced inflammation in vascular smooth muscle cells via the TLR4/TRIF/IRF3/IP-10 signaling pathway. Cytokine 2011, 55, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Jin, G.Y.; Li, L.C.; Yan, G.H. Inhibition of protein kinase C δ attenuates allergic airway inflammation through suppression of PI3K/Akt/mTOR/HIF-1 α/VEGF pathway. PLoS ONE 2013, 8, e81773. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Yu, Y.; Yu, Y.; Li, Y.; Wang, J.; Geng, W.; Jiang, L.; Li, Q.; Zhou, X.; Sun, Z. Silica nanoparticles induce autophagy and endothelial dysfunction via the PI3K/Akt/mTOR signaling pathway. Int. J. Nanomed. 2014, 9, 5131–5141. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Hu Ch, F.; Li, J.; You, X.; Gao, F.G. Increased translocation of antigens to endosomes and TLR4 mediated endosomal recruitment of TAP contribute to nicotine augmented cross-presentation. Oncotarget 2016, 7, 38451–38466. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Cho, H.J.; Jeong, Y.J.; Chung, I.K.; Magae, J.; Chang, Y.C. 4-O-methylascochlorin suppresses differentiation of 3T3-L1 preadipocytes by inhibiting PPARgamma expression through regulation of AMPK/mTOR signaling pathways. Arch. Biochem. Biophys. 2015, 583, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Jeong, E.; Joung, S.M.; Lee, J.Y. PI3K/Akt contributes to increased expression of Toll-like receptor 4 in macrophages exposed to hypoxic stress. Biochem. Biophys. Res. Commun. 2012, 419, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Li, D.; Cao, L.; Wang, L.; Zhu, S.; Xu, T.; Wang, C.; Pan, D. Positive feedback regulation of proliferation in vascular smooth muscle cells stimulated by lipopolysaccharide is mediated through the TLR 4/Rac1/Akt pathway. PLoS ONE 2014, 9, e92398. [Google Scholar] [CrossRef] [PubMed]
- Hoque, M.T.; Robillard, K.R.; Bendayan, R. Regulation of breast cancer resistant protein by peroxisome proliferator-activated receptor α in human brain microvessel endothelial cells. Mol. Pharmacol. 2012, 81, 598–609. [Google Scholar] [CrossRef] [PubMed]
- Antonova, L.V.; Seifalian, A.M.; Kutikhin, A.G.; Sevostyanova, V.V.; Matveeva, V.G.; Velikanova, E.A.; Mironov, A.V.; Shabaev, A.R.; Glushkova, T.V.; Senokosova, E.A.; et al. Conjugation with RGD Peptides and Incorporation of Vascular Endothelial Growth Factor Are Equally Efficient for Biofunctionalization of Tissue-Engineered Vascular Grafts. Int. J. Mol. Sci. 2016, 17, 1920. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, T.; Xu, Y.; Xu, E.; Zhou, M.; Wang, B.; Shen, J. Effects of TLR4 gene silencing on the proliferation and apotosis of hepatocarcinoma HEPG2 cells. Oncol. Lett. 2016, 11, 3054–3060. [Google Scholar] [CrossRef] [PubMed]
- Bao, M.H.; Li, J.M.; Luo, H.Q.; Tang, L.; Lv, Q.L.; Li, G.Y.; Zhou, H.H. NF-κB-Regulated miR-99a Modulates Endothelial Cell Inflammation. Mediat. Inflamm. 2016, 2016, 5308170. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, H.; Zhu, X.; Cai, W.; Qiu, L. Hypaphorine Attenuates Lipopolysaccharide-Induced Endothelial Inflammation via Regulation of TLR4 and PPAR-γ Dependent on PI3K/Akt/mTOR Signal Pathway. Int. J. Mol. Sci. 2017, 18, 844. https://doi.org/10.3390/ijms18040844
Sun H, Zhu X, Cai W, Qiu L. Hypaphorine Attenuates Lipopolysaccharide-Induced Endothelial Inflammation via Regulation of TLR4 and PPAR-γ Dependent on PI3K/Akt/mTOR Signal Pathway. International Journal of Molecular Sciences. 2017; 18(4):844. https://doi.org/10.3390/ijms18040844
Chicago/Turabian StyleSun, Haijian, Xuexue Zhu, Weiwei Cai, and Liying Qiu. 2017. "Hypaphorine Attenuates Lipopolysaccharide-Induced Endothelial Inflammation via Regulation of TLR4 and PPAR-γ Dependent on PI3K/Akt/mTOR Signal Pathway" International Journal of Molecular Sciences 18, no. 4: 844. https://doi.org/10.3390/ijms18040844