Oral Mucositis: Melatonin Gel an Effective New Treatment
Abstract
:1. Overview of Mucositis Pathobiology
2. Pathophysiology of Mucositis
3. Mucositis Management
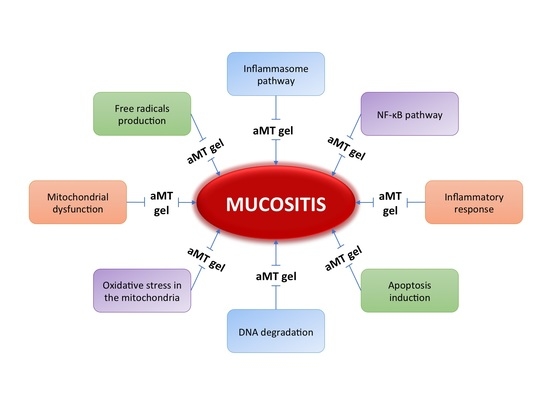
4. Melatonin: A New Treatment for Mucositis
4.1. Melatonin in the Oral Cavity
4.2. Melatonin as a Radio-Protective Agent
4.3. The Use of Melatonin in the Prevention of Radiation-Induced Mucositis
5. Conclusions
Acknowledgments
Conflicts of Interest
Abbreviations
| ApoE | Apolipoprotein E |
| COX-2 | Cyclooxygenase-2 |
| CTCAE | Common terminology criteria for adverse events |
| EGF | Epidermal growth factor |
| G-CSF | Granulocyte colony-stimulating factor |
| GIT | Gastrointestinal tract |
| GM-CSF | Granulocyte-macrophage colony stimulating factor |
| IL-1 | Interleukin-1 |
| MAPK | Mitogen-activated protein kinase |
| MMP | Matrix metalloproteinase |
| mTOR | Mammalian target of rapamycin |
| NAC | N-acetyl cysteine |
| NCI | National Cancer Institute |
| NF-κB | Nuclear factor-κB |
| NLRP3 | NACHT, LRR, and PYD domains-containing protein 3 |
| NSAID | Non-steroidal anti-inflammatory drug |
| PARs | Protease-activated receptors |
| PGE | Prostaglandin E |
| PLAG | 1-palmitoyl-2-linoleoyl-3-acetyl-rac-glycerol |
| rhEGF | Recombinant human EGF |
| ROS | Reactive oxygen species |
| SAP | Serum amyloid-P |
| Smad7 | Mothers against decapentaplegic homolog 7 |
| TCM | Traditional Chinese medicine |
| TGF-β | Transforming growth factor-β |
| TNF-α | Tumor necrosis factor-α |
References
- Trotti, A.; Bellm, L.A.; Epstein, J.B.; Frame, D.; Fuchs, H.J.; Gwede, C.K.; Komaroff, E.; Nalysnyk, L.; Zilberberg, M.D. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: A systematic literature review. Radiother. Oncol. 2003, 66, 253–262. [Google Scholar] [CrossRef]
- Itoh, Y.; Kubota, S.; Kawamura, M.; Nomoto, Y.; Murao, T.; Yamakawa, K.; Ishihara, S.; Hirasawa, N.; Asano, A.; Yanagawa, S.; et al. A multicenter survey of stage T1 glottic cancer treated with radiotherapy delivered in 2.25-Gy fractions in clinical practice: An initial 5-year analysis. Nagoya J. Med. Sci. 2016, 78, 399–406. [Google Scholar] [PubMed]
- Epstein, J.B.; Schubert, M.M. Managing pain in mucositis. Semin. Oncol. Nurs. 2004, 20, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Sonis, S.T. Oral mucositis in head and neck cancer: Risk, biology, and management. Am. Soc. Clin. Oncol. Educ. Book 2013. [Google Scholar] [CrossRef] [PubMed]
- Campos, M.I.; Campos, C.N.; Aarestrup, F.M.; Aarestrup, B.J. Oral mucositis in cancer treatment: Natural history, prevention and treatment. Mol. Clin. Oncol. 2014, 2, 337–340. [Google Scholar] [PubMed]
- Kyriakopoulos, C.E.; Braden, A.M.; Kolesar, J.M.; Eickhoff, J.C.; Bailey, H.H.; Heideman, J.; Liu, G.; Wisinski, K.B. A phase I study of tivantinib in combination with temsirolimus in patients with advanced solid tumors. Investig. New Drugs 2016. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.J. Cancer treatment-induced mucositis pain: Strategies for assessment and management. Ther. Clin. Risk Manag. 2006, 2, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Dorr, W. Modulation of repopulation processes in oral mucosa: Experimental results. Int. J. Radiat. Biol. 2003, 79, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Sonis, S.T. Oral mucositis in cancer therapy. J. Support. Oncol. 2004, 2, 3–8. [Google Scholar] [PubMed]
- Lee, C.S.; Ryan, E.J.; Doherty, G.A. Gastro-intestinal toxicity of chemotherapeutics in colorectal cancer: The role of inflammation. World J. Gastroenterol. 2014, 20, 3751–3761. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, F.; Acuna-Castroviejo, D.; Doerrier, C.; Dayoub, J.C.; Lopez, L.C.; Venegas, C.; Garcia, J.A.; Lopez, A.; Volt, H.; Luna-Sanchez, M.; et al. Melatonin blunts the mitochondrial/NLRP3 connection and protects against radiation-induced oral mucositis. J. Pineal Res. 2015, 58, 34–49. [Google Scholar] [CrossRef] [PubMed]
- Escames, G.; Lopez, L.C.; Garcia, J.A.; Garcia-Corzo, L.; Ortiz, F.; Acuna-Castroviejo, D. Mitochondrial DNA and inflammatory diseases. Hum. Genet. 2012, 131, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Villa, A.; Sonis, S.T. Mucositis: Pathobiology and management. Curr. Opin. Oncol. 2015, 27, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Volpato, L.E.R.; Silva, T.C.; Oliveira, T.M.; Sakai, V.T.; Machado, M.A.A.M. Radiation therapy and chemotherapy-induced oral mucositis. Rev. Bras. Otorrinolaringol. 2007, 73, 562–568. [Google Scholar] [CrossRef]
- Khaw, A.; Logan, R.; Keefe, D.; Bartold, M. Radiation-induced oral mucositis and periodontitis—Proposal for an inter-relationship. Oral Dis. 2014, 20, e7–e18. [Google Scholar] [CrossRef] [PubMed]
- Keefe, D.M.; Brealey, J.; Goland, G.J.; Cummins, A.G. Chemotherapy for cancer causes apoptosis that precedes hypoplasia in crypts of the small intestine in humans. Gut 2000, 47, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Bowen, J.M.; Keefe, D.M. New pathways for alimentary mucositis. J. Oncol. 2008, 2008, 907892. [Google Scholar] [CrossRef] [PubMed]
- Cameron, S.; Schwartz, A.; Sultan, S.; Schaefer, I.M.; Hermann, R.; Rave-Frank, M.; Hess, C.F.; Christiansen, H.; Ramadori, G. Radiation-induced damage in different segments of the rat intestine after external beam irradiation of the liver. Exp. Mol. Pathol. 2012, 92, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Gil, B.; Abdel Moneim, A.E.; Ortiz, F.; Shen, Y.-Q.; Soto-Mercado, V.; Mendivil-Perez, M.; Librero, A.G.; Acuña-Castroviejo, D.; Molina-Navarro, M.M.; Garcia-Verdugo, J.M.; et al. Melatonin protects rats from radiotherapy-induced gut toxicity. PLoS ONE 2017, 12, e0174474. [Google Scholar] [CrossRef] [PubMed]
- Scully, C.; Epstein, J.; Sonis, S. Oral mucositis: A challenging complication of radiotherapy, chemotherapy, and radiochemotherapy: Part 1, pathogenesis and prophylaxis of mucositis. Head Neck 2003, 25, 1057–1070. [Google Scholar] [CrossRef] [PubMed]
- Allison, R.R.; Ambrad, A.A.; Arshoun, Y.; Carmel, R.J.; Ciuba, D.F.; Feldman, E.; Finkelstein, S.E.; Gandhavadi, R.; Heron, D.E.; Lane, S.C.; et al. Multi-institutional, randomized, double-blind, placebo-controlled trial to assess the efficacy of a mucoadhesive hydrogel (MuGard) in mitigating oral mucositis symptoms in patients being treated with chemoradiation therapy for cancers of the head and neck. Cancer 2014, 120, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Quinn, B. Efficacy of a supersaturated calcium phosphate oral rinse for the prevention and treatment of oral mucositis in patients receiving high-dose cancer therapy: A review of current data. Eur. J. Cancer Care 2013, 22, 564–579. [Google Scholar] [CrossRef] [PubMed]
- Buchsel, P.C. Polyvinylpyrrolidone-sodium hyaluronate gel (Gelclair®): A bioadherent oral gel for the treatment of oral mucositis and other painful oral lesions. Expert Opin. Drug Metab. Toxicol. 2008, 4, 1449–1454. [Google Scholar] [CrossRef] [PubMed]
- Raphael, M.F.; den Boer, A.M.; Kollen, W.J.; Mekelenkamp, H.; Abbink, F.C.; Kaspers, G.J.; Zomer-Kooijker, K.; Molmans, B.H.; Tissing, W.J. Caphosol, a therapeutic option in case of cancer therapy-induced oral mucositis in children? Results from a prospective multicenter double blind randomized controlled trial. Support. Care Cancer 2014, 22, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Lalla, R.V.; Sonis, S.T.; Peterson, D.E. Management of oral mucositis in patients who have cancer. Dent. Clin. N. Am. 2008, 52, 61–77. [Google Scholar] [CrossRef] [PubMed]
- Leenstra, J.L.; Miller, R.C.; Qin, R.; Martenson, J.A.; Dornfeld, K.J.; Bearden, J.D.; Puri, D.R.; Stella, P.J.; Mazurczak, M.A.; Klish, M.D.; et al. Doxepin rinse versus placebo in the treatment of acute oral mucositis pain in patients receiving head and neck radiotherapy with or without chemotherapy: A phase III, randomized, double-blind trial (NCCTG-N09C6 [Alliance]). J. Clin. Oncol. 2014, 32, 1571–1577. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, E.B.; Peterson, D.E.; Schubert, M.; Keefe, D.; McGuire, D.; Epstein, J.; Elting, L.S.; Fox, P.C.; Cooksley, C.; Sonis, S.T. Clinical practice guidelines for the prevention and treatment of cancer therapy-induced oral and gastrointestinal mucositis. Cancer 2004, 100, 2026–2046. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.Z.; Zhang, Y. Efficacy and safety of transdermal fentanyl for the treatment of oral mucositis pain caused by chemoradiotherapy in patients with esophageal squamous cell carcinoma. Support. Care Cancer 2014, 23, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Gotzsche, P.C.; Johansen, H.K. Nystatin prophylaxis and treatment in severely immunodepressed patients. Cochrane Database Syst. Rev. 2014, 9, CD002033. [Google Scholar]
- Silva, F.C.; Marto, J.M.; Salgado, A.; Machado, P.; Silva, A.N.; Almeida, A.J. Nystatin and lidocaine pastilles for the local treatment of oral mucositis. Pharm. Dev. Technol. 2017, 22, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Bey, A.; Ahmed, S.S.; Hussain, B.; Devi, S.; Hashmi, S.H. Prevention and management of antineoplastic therapy induced oral mucositis. Natl. J. Maxillofac. Surg. 2010, 1, 127–134. [Google Scholar] [PubMed]
- Epstein, J.B.; Vickars, L.; Spinelli, J.; Reece, D. Efficacy of chlorhexidine and nystatin rinses in prevention of oral complications in leukemia and bone marrow transplantation. Oral Surg. Oral Med. Oral Pathol. 1992, 73, 682–689. [Google Scholar] [CrossRef]
- Cardona, A.; Balouch, A.; Abdul, M.M.; Sedghizadeh, P.P.; Enciso, R. Efficacy of chlorhexidine for the prevention and treatment of oral mucositis in cancer patients: A systematic review with meta-analyses. J. Oral Pathol. Med. 2017, 23, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Migliorati, C.A.; Oberle-Edwards, L.; Schubert, M. The role of alternative and natural agents, cryotherapy, and/or laser for management of alimentary mucositis. Support. Care Cancer 2006, 14, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Peterson, D.E.; Ohrn, K.; Bowen, J.; Fliedner, M.; Lees, J.; Loprinzi, C.; Mori, T.; Osaguona, A.; Weikel, D.S.; Elad, S.; et al. Systematic review of oral cryotherapy for management of oral mucositis caused by cancer therapy. Support. Care Cancer 2013, 21, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Tayyem, A.Q. Cryotherapy effect on oral mucositis severity among recipients of bone marrow transplantation: A literature review. Clin. J. Oncol. Nurs. 2014, 18, E84–E87. [Google Scholar] [CrossRef] [PubMed]
- Riley, P.; McCabe, M.G.; Glenny, A. Oral cryotherapy for preventing oral mucositis in patients receiving cancer treatment. JAMA Oncol. 2016, 2, 1365–1366. [Google Scholar] [CrossRef] [PubMed]
- Kadakia, K.C.; Rozell, S.A.; Butala, A.A.; Loprinzi, C.L. Supportive cryotherapy: A review from head to toe. J. Pain Symptom Manag. 2014, 47, 1100–1115. [Google Scholar] [CrossRef] [PubMed]
- Nicolatou-Galitis, O.; Sarri, T.; Bowen, J.; di Palma, M.; Kouloulias, V.E.; Niscola, P.; Riesenbeck, D.; Stokman, M.; Tissing, W.; Yeoh, E.; et al. Systematic review of amifostine for the management of oral mucositis in cancer patients. Support. Care Cancer 2013, 21, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Moslehi, A.; Taghizadeh-Ghehi, M.; Gholami, K.; Hadjibabaie, M.; Jahangard-Rafsanjani, Z.; Sarayani, A.; Javadi, M.; Esfandbod, M.; Ghavamzadeh, A. N-acetyl cysteine for prevention of oral mucositis in hematopoietic sct: A double-blind, randomized, placebo-controlled trial. Bone Marrow Transplant. 2014, 49, 818–823. [Google Scholar] [CrossRef] [PubMed]
- Spielberger, R.; Stiff, P.; Bensinger, W.; Gentile, T.; Weisdorf, D.; Kewalramani, T.; Shea, T.; Yanovich, S.; Hansen, K.; Noga, S.; et al. Palifermin for oral mucositis after intensive therapy for hematologic cancers. N. Engl. J. Med. 2004, 351, 2590–2598. [Google Scholar] [CrossRef] [PubMed]
- Vitale, K.M.; Violago, L.; Cofnas, P.; Bishop, J.; Jin, Z.; Bhatia, M.; Kung, A.L.; George, D.; Garvin, J.; Satwani, P. Impact of palifermin on incidence of oral mucositis and healthcare utilization in children undergoing autologous hematopoietic stem cell transplantation for malignant diseases. Pediatr. Transplant. 2014, 18, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Yuan, A.; Sonis, S. Emerging therapies for the prevention and treatment of oral mucositis. Expert Opin. Emerg. Drugs 2014, 19, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Bian, L.; Li, F.; Cotrim, A.; Wang, D.; Lu, J.; Deng, Y.; Bird, G.; Sowers, A.; Mitchell, J.B.; et al. Preventive and therapeutic effects of Smad7 on radiation-induced oral mucositis. Nat. Med. 2013, 19, 421–428. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Li, A.G.; Wang, D.; Han, S.; Zheng, B.; Goumans, M.J.; Ten Dijke, P.; Wang, X.J. Overexpression of Smad7 results in severe pathological alterations in multiple epithelial tissues. EMBO J. 2002, 21, 2580–2590. [Google Scholar] [CrossRef] [PubMed]
- Nicolatou-Galitis, O.; Sarri, T.; Bowen, J.; di Palma, M.; Kouloulias, V.E.; Niscola, P.; Riesenbeck, D.; Stokman, M.; Tissing, W.; Yeoh, E.; et al. Systematic review of anti-inflammatory agents for the management of oral mucositis in cancer patients. Support. Care Cancer 2013, 21, 3179–3189. [Google Scholar] [CrossRef] [PubMed]
- Labar, B.; Mrsic, M.; Pavletic, Z.; Bogdanic, V.; Nemet, D.; Aurer, I.; Radman, I.; Filipovic-Grcic, N.; Sertic, D.; Kalenic, S.; et al. Prostaglandin E2 for prophylaxis of oral mucositis following BMT. Bone Marrow Transplant. 1993, 11, 379–382. [Google Scholar] [PubMed]
- Kostler, W.J.; Hejna, M.; Wenzel, C.; Zielinski, C.C. Oral mucositis complicating chemotherapy and/or radiotherapy: Options for prevention and treatment. CA Cancer J. Clin. 2001, 51, 290–315. [Google Scholar] [CrossRef] [PubMed]
- Coeffier, M.; Marion, R.; Leplingard, A.; Lerebours, E.; Ducrotte, P.; Dechelotte, P. Glutamine decreases interleukin-8 and interleukin-6 but not nitric oxide and prostaglandins E2 production by human gut in vitro. Cytokine 2002, 18, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.E.; Jones, D.P.; Ziegler, T.R. Glutamine prevents cytokine-induced apoptosis in human colonic epithelial cells. J. Nutr. 2003, 133, 3065–3071. [Google Scholar] [PubMed]
- Anderson, P.M.; Ramsay, N.K.; Shu, X.O.; Rydholm, N.; Rogosheske, J.; Nicklow, R.; Weisdorf, D.J.; Skubitz, K.M. Effect of low-dose oral glutamine on painful stomatitis during bone marrow transplantation. Bone Marrow Transplant 1998, 22, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Jebb, S.A.; Osborne, R.J.; Maughan, T.S.; Mohideen, N.; Mack, P.; Mort, D.; Shelley, M.D.; Elia, M. 5-fluorouracil and folinic acid-induced mucositis: No effect of oral glutamine supplementation. Br. J. Cancer 1994, 70, 732–735. [Google Scholar] [CrossRef] [PubMed]
- Touchefeu, Y.; Montassier, E.; Nieman, K.; Gastinne, T.; Potel, G.; Bruley des Varannes, S.; Le Vacon, F.; de la Cochetiere, M.F. Systematic review: The role of the gut microbiota in chemotherapy- or radiation-induced gastrointestinal mucositis—Current evidence and potential clinical applications. Aliment. Pharmacol. Ther. 2014, 40, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Ciais, G.; Namer, M.; Schneider, M.; Demard, F.; Pourreau-Schneider, N.; Martin, P.M.; Soudry, M.; Franquin, J.C.; Zattara, H. Laser therapy in the prevention and treatment of mucositis caused by anticancer chemotherapy. Bull. Cancer 1992, 79, 183–191. [Google Scholar] [PubMed]
- Barasch, A.; Peterson, D.E.; Tanzer, J.M.; D’Ambrosio, J.A.; Nuki, K.; Schubert, M.M.; Franquin, J.C.; Clive, J.; Tutschka, P. Helium-neon laser effects on conditioning-induced oral mucositis in bone marrow transplantation patients. Cancer 1995, 76, 2550–2556. [Google Scholar] [CrossRef]
- Cowen, D.; Tardieu, C.; Schubert, M.; Peterson, D.; Resbeut, M.; Faucher, C.; Franquin, J.C. Low energy helium-neon laser in the prevention of oral mucositis in patients undergoing bone marrow transplant: Results of a double blind randomized trial. Int. J. Radiat. Oncol. Biol. Phys. 1997, 38, 697–703. [Google Scholar] [CrossRef]
- Oberoi, S.; Zamperlini-Netto, G.; Beyene, J.; Treister, N.S.; Sung, L. Effect of prophylactic low level laser therapy on oral mucositis: A systematic review and meta-analysis. PLoS ONE 2014, 9, e107418. [Google Scholar] [CrossRef] [PubMed]
- Alvarino-Martin, C.; Sarrion-Perez, M.G. Prevention and treatment of oral mucositis in patients receiving chemotherapy. J. Clin. Exp. Dent. 2014, 6, e74–e80. [Google Scholar] [CrossRef] [PubMed]
- Allan, E.; Barney, C.; Baum, S.; Kessling, T.; Diavolitsis, V.M.; Blakaj, D.; Grecula, J.C.; Rocco, J.W.; van Putten, M.; Bhatt, A.D. Low-level laser therapy and laser debridement for management of oral mucositis in patients with head and neck cancer receiving chemotherapy and radiation. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 883. [Google Scholar] [CrossRef]
- Sonis, S.T.; Hashemi, S.; Epstein, J.B.; Nair, R.G.; Raber-Durlacher, J.E. Could the biological robustness of low level laser therapy (photobiomodulation) impact its use in the management of mucositis in head and neck cancer patients. Oral Oncol. 2016, 54, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Yeung, C.Y.; Chan, W.T.; Jiang, C.B.; Cheng, M.L.; Liu, C.Y.; Chang, S.W.; Chiang Chiau, J.S.; Lee, H.C. Amelioration of chemotherapy-induced intestinal mucositis by orally administered probiotics in a mouse model. PLoS ONE 2015, 10, e0138746. [Google Scholar] [CrossRef] [PubMed]
- Osterlund, P.; Ruotsalainen, T.; Korpela, R.; Saxelin, M.; Ollus, A.; Valta, P.; Kouri, M.; Elomaa, I.; Joensuu, H. Lactobacillus supplementation for diarrhoea related to chemotherapy of colorectal cancer: A randomised study. Br. J. Cancer 2007, 97, 1028–1034. [Google Scholar] [CrossRef] [PubMed]
- Delia, P.; Sansotta, G.; Donato, V.; Messina, G.; Frosina, P.; Pergolizzi, S.; de Renzis, C.; Famularo, G. Prevention of radiation-induced diarrhea with the use of VSL#3, a new high-potency probiotic preparation. Am. J. Gastroenterol. 2002, 97, 2150–2152. [Google Scholar] [PubMed]
- Pottel, L.; Lycke, M.; Boterberg, T.; Pottel, H.; Goethals, L.; Duprez, F.; Maes, A.; Goemaere, S.; Rottey, S.; Foubert, I.; et al. Echium oil is not protective against weight loss in head and neck cancer patients undergoing curative radio(chemo)therapy: A randomised-controlled trial. BMC Complement. Altern. Med. 2014, 14, 382. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Hamme, G.; Beckmann, K.; Radtke, J.; Efferth, T.; Greten, H.J.; Rostock, M.; Schroder, S. A survey of chinese medicinal herbal treatment for chemotherapy-induced oral mucositis. Evid. Based Complement. Altern. Med. 2013, 2013, 284959. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xi, J.; Schroder, S.; Wang, W.; Xie, T.; Wang, Z.; Bao, S.; Fei, J. Chimonanthus nitens var. Salicifolius aqueous extract protects against 5-fluorouracil induced gastrointestinal mucositis in a mouse model. Evid. Based Complement. Altern. Med. 2013, 2013, 789263. [Google Scholar] [CrossRef] [PubMed]
- Sencer, S.F.; Zhou, T.; Freedman, L.S.; Ives, J.A.; Chen, Z.; Wall, D.; Nieder, M.L.; Grupp, S.A.; Yu, L.C.; Sahdev, I.; et al. Traumeel s in preventing and treating mucositis in young patients undergoing sct: A report of the children’s oncology group. Bone Marrow Transplant 2012, 47, 1409–1414. [Google Scholar] [CrossRef] [PubMed]
- Braga, F.T.; Santos, A.C.; Bueno, P.C.; Silveira, R.C.; Santos, C.B.; Bastos, J.K.; Carvalho, E.C. Use of chamomilla recutita in the prevention and treatment of oral mucositis in patients undergoing hematopoietic stem cell transplantation: A randomized, controlled, phase ii clinical trial. Cancer Nurs. 2014, 38, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Elkerm, Y.; Tawashi, R. Date palm pollen as a preventative intervention in radiation- and chemotherapy-induced oral mucositis: A pilot study. Integr. Cancer Ther. 2014, 13, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Fidler, P.; Loprinzi, C.L.; O’Fallon, J.R.; Leitch, J.M.; Lee, J.K.; Hayes, D.L.; Novotny, P.; Clemens-Schutjer, D.; Bartel, J.; Michalak, J.C. Prospective evaluation of a chamomile mouthwash for prevention of 5-FU-induced oral mucositis. Cancer 1996, 77, 522–525. [Google Scholar] [CrossRef]
- Oberbaum, M.; Yaniv, I.; Ben-Gal, Y.; Stein, J.; Ben-Zvi, N.; Freedman, L.S.; Branski, D. A randomized, controlled clinical trial of the homeopathic medication TRAUMEEL s® in the treatment of chemotherapy-induced stomatitis in children undergoing stem cell transplantation. Cancer 2001, 92, 684–690. [Google Scholar] [CrossRef]
- Saadeh, C.E. Chemotherapy- and radiotherapy-induced oral mucositis: Review of preventive strategies and treatment. Pharmacotherapy 2005, 25, 540–554. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.P.; Lee, S.W.; Song, S.Y.; Ahn, S.D.; Shin, S.S.; Choi, E.K.; Kim, J.H. Recombinant human epidermal growth factor treatment of radiation-induced severe oral mucositis in patients with head and neck malignancies. Eur. J. Cancer Care 2009, 18, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.M.; Schroeder, G.; Skubitz, K.M. Oral glutamine reduces the duration and severity of stomatitis after cytotoxic cancer chemotherapy. Cancer 1998, 83, 1433–1439. [Google Scholar] [CrossRef]
- Huang, E.Y.; Leung, S.W.; Wang, C.J.; Chen, H.C.; Sun, L.M.; Fang, F.M.; Yeh, S.A.; Hsu, H.C.; Hsiung, C.Y. Oral glutamine to alleviate radiation-induced oral mucositis: A pilot randomized trial. Int. J. Radiat. Oncol. Biol. Phys. 2000, 46, 535–539. [Google Scholar] [CrossRef]
- Lopes, N.N.; Plapler, H.; Chavantes, M.C.; Lalla, R.V.; Yoshimura, E.M.; Alves, M.T. Cyclooxygenase-2 and vascular endothelial growth factor expression in 5-fluorouracil-induced oral mucositis in hamsters: Evaluation of two low-intensity laser protocols. Support. Care Cancer 2009, 17, 1409–1415. [Google Scholar] [CrossRef] [PubMed]
- Antunes, H.S.; Herchenhorn, D.; Small, I.A.; Araujo, C.M.; Viegas, C.M.; Cabral, E.; Rampini, M.P.; Rodrigues, P.C.; Silva, T.G.; Ferreira, E.M.; et al. Phase III trial of low-level laser therapy to prevent oral mucositis in head and neck cancer patients treated with concurrent chemoradiation. Radiother. Oncol. 2013, 109, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Acuna-Castroviejo, D.; Escames, G.; Venegas, C.; Diaz-Casado, M.E.; Lima-Cabello, E.; Lopez, L.C.; Rosales-Corral, S.; Tan, D.X.; Reiter, R.J. Extrapineal melatonin: Sources, regulation, and potential functions. Cell. Mol. Life Sci. 2014, 71, 2997–3025. [Google Scholar] [CrossRef] [PubMed]
- Urata, Y.; Honma, S.; Goto, S.; Todoroki, S.; Iida, T.; Cho, S.; Honma, K.; Kondo, T. Melatonin induces γ-glutamylcysteine synthetase mediated by activator protein-1 in human vascular endothelial cells. Free Radic. Biol. Med. 1999, 27, 838–847. [Google Scholar] [CrossRef]
- Manchester, L.C.; Coto-Montes, A.; Boga, J.A.; Andersen, L.P.H.; Zhou, Z.; Galano, A.; Vriend, J.; Tan, D.-X.; Reiter, R.J. Melatonin: An ancient molecule that makes oxygen metabolically tolerable. J. Pineal Res. 2015, 59, 403–419. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-M.; Zhang, Y. Melatonin: A well-documented antioxidant with conditional pro-oxidant actions. J. Pineal Res. 2014, 57, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Mayo, J.C.; Tan, D.-X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Abdel Moneim, A.E.; Ortiz, F.; Leonardo-Mendonca, R.C.; Vergano-Villodres, R.; Guerrero-Martinez, J.A.; Lopez, L.C.; Acuna-Castroviejo, D.; Escames, G. Protective effects of melatonin against oxidative damage induced by Egyptian cobra (Naja haje) crude venom in rats. Acta Trop. 2014, 143, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; Macias, M.; Escames, G.; Leon, J.; Acuna-Castroviejo, D. Melatonin but not vitamins C and E maintains glutathione homeostasis in t-butyl hydroperoxide-induced mitochondrial oxidative stress. FASEB J. 2000, 14, 1677–1679. [Google Scholar] [CrossRef] [PubMed]
- Acuna Castroviejo, D.; Lopez, L.C.; Escames, G.; Lopez, A.; Garcia, J.A.; Reiter, R.J. Melatonin-mitochondria interplay in health and disease. Curr. Top. Med. Chem. 2011, 11, 221–240. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Casado, M.E.; Lima, E.; Garcia, J.A.; Doerrier, C.; Aranda, P.; Sayed, R.K.; Guerra-Librero, A.; Escames, G.; Lopez, L.C.; Acuna-Castroviejo, D. Melatonin rescues zebrafish embryos from the parkinsonian phenotype restoring the parkin/PINK1/DJ-1/MUL1 network. J. Pineal Res. 2016, 61, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Doerrier, C.; Garcia, J.A.; Volt, H.; Diaz-Casado, M.E.; Luna-Sanchez, M.; Fernandez-Gil, B.; Escames, G.; Lopez, L.C.; Acuna-Castroviejo, D. Permeabilized myocardial fibers as model to detect mitochondrial dysfunction during sepsis and melatonin effects without disruption of mitochondrial network. Mitochondrion 2016, 27, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Acuna-Castroviejo, D.; Escames, G.; Rodriguez, M.I.; Lopez, L.C. Melatonin role in the mitochondrial function. Front. Biosci. 2007, 12, 947–963. [Google Scholar] [CrossRef] [PubMed]
- Escames, G.; Leon, J.; Macias, M.; Khaldy, H.; Acuna-Castroviejo, D. Melatonin counteracts lipopolysaccharide-induced expression and activity of mitochondrial nitric oxide synthase in rats. FASEB J. 2003, 17, 932–934. [Google Scholar] [CrossRef] [PubMed]
- Escames, G.; López, L.C.; Tapias, V.; Utrilla, P.; Reiter, R.J.; Hitos, A.B.; León, J.; Rodríguez, M.I.; Acuña-Castroviejo, D. Melatonin counteracts inducible mitochondrial nitric oxide synthase-dependent mitochondrial dysfunction in skeletal muscle of septic mice. J. Pineal Res. 2006, 40, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.A.; Volt, H.; Venegas, C.; Doerrier, C.; Escames, G.; Lopez, L.C.; Acuna-Castroviejo, D. Disruption of the NF-κb/NLRP3 connection by melatonin requires retinoid-related orphan receptor-α and blocks the septic response in mice. FASEB J. 2015, 29, 3863–3875. [Google Scholar] [CrossRef] [PubMed]
- Volt, H.; Garcia, J.A.; Doerrier, C.; Diaz-Casado, M.E.; Guerra-Librero, A.; Lopez, L.C.; Escames, G.; Tresguerres, J.A.; Acuna-Castroviejo, D. Same molecule but different expression: Aging and sepsis trigger NLRP3 inflammasome activation, a target of melatonin. J. Pineal Res. 2016, 60, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Stehle, J.H.; Saade, A.; Rawashdeh, O.; Ackermann, K.; Jilg, A.; Sebesteny, T.; Maronde, E. A survey of molecular details in the human pineal gland in the light of phylogeny, structure, function and chronobiological diseases. J. Pineal Res. 2011, 51, 17–43. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Rosales-Corral, S.A.; Liu, X.Y.; Acuna-Castroviejo, D.; Escames, G.; Tan, D.X. Melatonin in the oral cavity: Physiological and pathological implications. J. Periodontal Res. 2015, 50, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Almughrabi, O.M.; Marzouk, K.M.; Hasanato, R.M.; Shafik, S.S. Melatonin levels in periodontal health and disease. J. Periodontal Res. 2013, 48, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Moreno, G.; Guardia, J.; Ferrera, M.J.; Cutando, A.; Reiter, R.J. Melatonin in diseases of the oral cavity. Oral Dis. 2010, 16, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Kara, A.; Akman, S.; Ozkanlar, S.; Tozoglu, U.; Kalkan, Y.; Canakci, C.F.; Tozoglu, S. Immune modulatory and antioxidant effects of melatonin in experimental periodontitis in rats. Free Radic. Biol. Med. 2013, 55, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Cutando, A.; Lopez-Valverde, A.; Gomez-de-Diego, R.; Arias-Santiago, S.; de Vicente-Jimenez, J. Effect of gingival application of melatonin on alkaline and acid phosphatase, osteopontin and osteocalcin in patients with diabetes and periodontal disease. Med. Oral Patol. Oral Cir. Bucal 2013, 18, e657–e663. [Google Scholar] [CrossRef] [PubMed]
- Cutando, A.; Arana, C.; Gomez-Moreno, G.; Escames, G.; Lopez, A.; Ferrera, M.J.; Reiter, R.J.; Acuna-Castroviejo, D. Local application of melatonin into alveolar sockets of beagle dogs reduces tooth removal-induced oxidative stress. J. Periodontol. 2007, 78, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Poeggeler, B.; Thuermann, S.; Dose, A.; Schoenke, M.; Burkhardt, S.; Hardeland, R. Melatonin’s unique radical scavenging properties—Roles of its functional substituents as revealed by a comparison with its structural analogs. J. Pineal Res. 2002, 33, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Zavodnik, I.B.; Domanski, A.V.; Lapshina, E.A.; Bryszewska, M.; Reiter, R.J. Melatonin directly scavenges free radicals generated in red blood cells and a cell-free system: Chemiluminescence measurements and theoretical calculations. Life Sci. 2006, 79, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, B.; Pompon, D.; Ducrocq, C. Nitrosation of melatonin by nitric oxide and peroxynitrite. J. Pineal Res. 2000, 29, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Reiter, R.J.; Tan, D.X.; Garcia, J.J.; Manchester, L.C.; Karbownik, M.; Calvo, J.R. Chromium(III)-induced 8-hydroxydeoxyguanosine in DNA and its reduction by antioxidants: Comparative effects of melatonin, ascorbate, and vitamin E. Environ. Health Perspect. 2000, 108, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Davanipour, Z.; Poulsen, H.E.; Weimann, A.; Sobel, E. Endogenous melatonin and oxidatively damaged guanine in DNA. BMC Endocr. Disord. 2009, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.M.; Min, K.J.; Kwon, T.K. Melatonin-mediated Bim up-regulation and cyclooxygenase-2 (COX-2) down-regulation enhances tunicamycin-induced apoptosis in MDA-MB-231 cells. J. Pineal Res. 2015, 58, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Cutando, A.; Montero, J.; Gomez-de Diego, R.; Ferrera, M.J.; Lopez-Valverde, A. Effect of topical application of melatonin on serum levels of C-reactive protein (CRP), interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) in patients with type 1 or type 2 diabetes and periodontal disease. J. Clin. Exp. Dent. 2015, 7, e628–e633. [Google Scholar] [CrossRef] [PubMed]
- Brazao, V.; Colato, R.P.; Santello, F.H.; Filipin Mdel, V.; Toldo, M.P.; do Vale, G.T.; Tirapelli, C.R.; do Prado Junior, J.C. Interleukin-17, oxidative stress, and inflammation: Role of melatonin during Trypanosoma cruzi infection. J. Pineal Res. 2015, 59, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Shaeib, F.; Khan, S.N.; Ali, I.; Najafi, T.; Maitra, D.; Abdulhamid, I.; Saed, G.M.; Pennathur, S.; Abu-Soud, H.M. Melatonin prevents myeloperoxidase heme destruction and the generation of free iron mediated by self-generated hypochlorous acid. PLoS ONE 2015, 10, e0120737. [Google Scholar] [CrossRef] [PubMed]
- Molpeceres, V.; Mauriz, J.L.; García-Mediavilla, M.V.; González, P.; Barrio, J.P.; González-Gallego, J. Melatonin is able to reduce the apoptotic liver changes induced by aging via inhibition of the intrinsic pathway of apoptosis. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.W.; Cho, H.I.; Lee, S.M. Melatonin inhibits mTOR-dependent autophagy during liver ischemia/reperfusion. Cell. Physiol. Biochem. 2014, 33, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Yoo, Y.M.; Han, T.Y.; Kim, H.S. Melatonin suppresses autophagy induced by clinostat in preosteoblast MC3T3-E1 cells. Int. J. Mol. Sci. 2016, 17, 526. [Google Scholar] [CrossRef] [PubMed]
- Limon-Pacheco, J.H.; Gonsebatt, M.E. The glutathione system and its regulation by neurohormone melatonin in the central nervous system. Cent. Nerv. Syst. Agents Med. Chem. 2010, 10, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Chen, L.D.; Poeggeler, B.; Manchester, L.C.; Reiter, R.J. Melatonin: A potent, endogenous hydroxyl radical scavenge. Endocr. J. 1993, 1, 57–60. [Google Scholar]
- Vijayalaxmi; Reiter, R.J.; Meltz, M.L. Melatonin protects human blood lymphocytes from radiation-induced chromo some damage. Mutat. Res. 1995, 346, 23–31. [Google Scholar] [CrossRef]
- Vijayalaxmi; Reiter, R.J.; Sewerynek, E.; Poeggeler, B.; Leal, B.Z.; Meltz, M.L. Marked reduction of radiation-induced micronuclei in human blood lymphocytes pretreated with melatonin. Radiat. Res. 1995, 143, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Vijayalaxmi; Reiter, R.J.; Leal, B.Z.; Meltz, M.L. Effect of melatonin on mitotic and proliferation indices, and sister chromatid exchange in human blood lymphocytes. Mutat. Res. 1996, 351, 187–192. [Google Scholar] [CrossRef]
- Vijayalaxmi; Reiter, R.J.; Herman, T.S.; Meltz, M.L. Melatonin reduces γ radiation-induced primary DNA damage in human blood lymphocytes. Mutat. Res. 1998, 397, 203–208. [Google Scholar] [CrossRef]
- Tesoriere, L.; D’Arpa, D.; Conti, S.; Giaccone, V.; Pintaudi, A.M.; Livrea, M.A. Melatonin protects human red blood cells from oxidative hemolysis: New insights into the radical-scavenging activity. J. Pineal Res. 1999, 27, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Adhikari, J.S.; Rizvi, M.A.; Chaudhury, N.K. Melatonin attenuates 60Co γ-ray-induced hematopoietic, immunological and gastrointestinal injuries in C57BL/6 male mice. Environ. Toxicol. 2017, 32, 501–518. [Google Scholar] [CrossRef] [PubMed]
- Siu, A.W.; Reiter, R.J.; To, C.H. Pineal indoleamines and vitamin E reduce nitric oxide-induced lipid peroxidation in rat retinal homogenates. J. Pineal Res. 1999, 27, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Sainz, R.M.; Mayo, J.C.; Uria, H.; Kotler, M.; Antolin, I.; Rodriguez, C.; Menendez-Pelaez, A. The pineal neurohormone melatonin prevents in vivo and in vitro apoptosis in thymocytes. J. Pineal Res. 1995, 19, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Maestroni, G.J.; Covacci, V.; Conti, A. Hematopoietic rescue via T-cell-dependent, endogenous granulocyte-macrophage colony-stimulating factor induced by the pineal neurohormone melatonin in tumor-bearing mice. Cancer Res. 1994, 54, 2429–2432. [Google Scholar] [PubMed]
- Blickenstaff, R.T.; Brandstadter, S.M.; Reddy, S.; Witt, R. Potential radioprotective agents. 1. Homologs of melatonin. J. Pharm. Sci. 1994, 83, 216–218. [Google Scholar] [CrossRef] [PubMed]
- Vijayalaxmi; Meltz, M.L.; Reiter, R.J.; Herman, T.S.; Kumar, K.S. Melatonin and protection from whole-body irradiation: Survival studies in mice. Mutat. Res. 1999, 425, 21–27. [Google Scholar] [CrossRef]
- Iwata, M.; Iwakawa, M.; Noda, S.; Ohta, T.; Minfu, Y.; Kimura, T.; Shibuya, H.; Imai, T. Correlation between single nucleotide polymorphisms and jejunal crypt cell apoptosis after whole body irradiation. Int. J. Radiat. Biol. 2007, 83, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.S.; Kim, W.D.; Park, W.Y. Melatonin exerts differential actions on X-ray radiation-induced apoptosis in normal mice splenocytes and Jurkat leukemia cells. J. Pineal Res. 2009, 47, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Badr, F.M.; El Habit, O.H.; Harraz, M.M. Radioprotective effect of melatonin assessed by measuring chromosomal damage in mitotic and meiotic cells. Mutat. Res. 1999, 444, 367–372. [Google Scholar] [CrossRef]
- Mornjakovic, Z.; Alicelebic, S.; Bilalovic, N.; Susko, I. Morphometric characteristics of leydig cells after total irradiation of rats treated with melatonin. Med. Arh. 1998, 52, 183–184. [Google Scholar] [PubMed]
- Kim, J.K.; Lee, C.J. Effect of exogenous melatonin on the ovarian follicles in γ-irradiated mouse. Mutat. Res. 2000, 449, 33–39. [Google Scholar] [CrossRef]
- Kim, J.K.; Lee, C.J.; Song, K.W.; Do, B.R.; Yoon, Y.D. γ-radiation accelerates ovarian follicular atresia in immature mice. In Vivo 1999, 13, 21–24. [Google Scholar] [PubMed]
- Kundurovic, Z.; Mornjakovic, Z. Morphometric characteristics of thyroid cells in irradiation-stressed rats treated with pinealectomy and melatonin. Med. Arh. 1992, 46, 9–10. [Google Scholar] [PubMed]
- Ruifrok, A.C.; Weil, M.M.; Thames, H.D.; Mason, K.A. Diurnal variations in the expression of radiation-induced apoptosis. Radiat. Res. 1998, 149, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.R.; Abu-Dief, E.E.; Kamel, E.; Abou El-Ghait, A.T.; Abdulwahed, S.R.; Ahmad, M.H. Melatonin and roentgen irradiation-induced acute radiation enteritis in albino rats: An animal model. Cell. Biol. Int. 2008, 32, 1353–1361. [Google Scholar] [CrossRef] [PubMed]
- Onal, C.; Kayaselcuk, F.; Topkan, E.; Yavuz, M.; Bacanli, D.; Yavuz, A. Protective effects of melatonin and octreotide against radiation-induced intestinal injury. Dig. Dis. Sci. 2011, 56, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Adhikari, J.S.; Rizvi, M.A.; Chaudhury, N.K. Radioprotective potential of melatonin against 60Co γ-ray-induced testicular injury in male C57BL/6 mice. J. Biomed. Sci. 2015, 22, 61. [Google Scholar] [CrossRef] [PubMed]
- Vijayalaxmi; Reiter, R.J.; Tan, D.X.; Herman, T.S.; Thomas, C.R., Jr. Melatonin as a radioprotective agent: A review. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, 639–653. [Google Scholar] [CrossRef] [PubMed]
- Vijayalaxmi; Thomas, C.R., Jr.; Reiter, R.J.; Herman, T.S. Melatonin: From basic research to cancer treatment clinics. J. Clin. Oncol. 2002, 20, 2575–2601. [Google Scholar] [CrossRef] [PubMed]
- Kobashigawa, S.; Kashino, G.; Suzuki, K.; Yamashita, S.; Mori, H. Ionizing radiation-induced cell death is partly caused by increase of mitochondrial reactive oxygen species in normal human fibroblast cells. Radiat. Res. 2015, 183, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Yazdi, A.S.; Menu, P.; Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 2011, 469, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Potten, C.S. A comprehensive study of the radiobiological response of the murine (BDF1) small intestine. Int. J. Radiat. Biol. 1990, 58, 925–973. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, A.; Madesh, M.; Balasubramanian, K.A. Apoptosis in the intestinal epithelium: Its relevance in normal and pathophysiological conditions. J. Gastroenterol. Hepatol. 2000, 15, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Lissoni, P.; Bolis, S.; Brivio, F.; Fumagalli, L. A phase II study of neuroimmunotherapy with subcutaneous low-dose IL-2 plus the pineal hormone melatonin in untreatable advanced hematologic malignancies. Anticancer Res. 2000, 20, 2103–2105. [Google Scholar] [PubMed]
- Hong, Y.; Won, J.; Lee, Y.; Lee, S.; Park, K.; Chang, K.T. Melatonin treatment induces interplay of apoptosis, autophagy, and senescence in human colorectal cancer cells. J. Pineal Res. 2014, 56, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Yang, Y.; Fan, C.; Han, J.; Wang, D.; di, S.; Hu, W.; Liu, D.; Li, X.; Reiter, R.J.; et al. Melatonin as a potential anticarcinogen for non-small-cell lung cancer. Oncotarget 2016, 7, 46768–46784. [Google Scholar] [CrossRef] [PubMed]
- Trubiani, O.; Recchioni, R.; Moroni, F.; Pizzicannella, J.; Caputi, S.; Di Primio, R. Melatonin provokes cell death in human B-lymphoma cells by mitochondrial-dependent apoptotic pathway activation. J. Pineal Res. 2005, 39, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.C.; Pandi-Perumal, S.R.; Esquifino, A.I.; Cardinali, D.P.; Maestroni, G.J. The role of melatonin in immuno-enhancement: Potential application in cancer. Int. J. Exp. Pathol. 2006, 87, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Hevia, D.; Gonzalez-Menendez, P.; Quiros-Gonzalez, I.; Miar, A.; Rodriguez-Garcia, A.; Tan, D.X.; Reiter, R.J.; Mayo, J.C.; Sainz, R.M. Melatonin uptake through glucose transporters: A new target for melatonin inhibition of cancer. J. Pineal Res. 2015, 58, 234–250. [Google Scholar] [CrossRef] [PubMed]
- Blask, D.E.; Sauer, L.A.; Dauchy, R.T.; Holowachuk, E.W.; Ruhoff, M.S.; Kopff, H.S. Melatonin inhibition of cancer growth in vivo involves suppression of tumor fatty acid metabolism via melatonin receptor-mediated signal transduction events. Cancer Res. 1999, 59, 4693–4701. [Google Scholar] [PubMed]
- Tischer, E.; Mitchell, R.; Hartman, T.; Silva, M.; Gospodarowicz, D.; Fiddes, J.C.; Abraham, J.A. The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J. Biol. Chem. 1991, 266, 11947–11954. [Google Scholar] [PubMed]
- Park, S.Y.; Jang, W.J.; Yi, E.Y.; Jang, J.Y.; Jung, Y.; Jeong, J.W.; Kim, Y.J. Melatonin suppresses tumor angiogenesis by inhibiting HIF-1α stabilization under hypoxia. J. Pineal Res. 2010, 48, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Su, S.C.; Hsieh, M.J.; Yang, W.E.; Chung, W.H.; Reiter, R.J.; Yang, S.F. Cancer metastasis: Mechanisms of inhibition by melatonin. J. Pineal Res. 2017, 62. [Google Scholar] [CrossRef] [PubMed]


| Agent | Experiment | Subject | Outcomes | Reference |
|---|---|---|---|---|
| Mouth rinse | ||||
| Traumeel S | A randomized, controlled clinical trial of the homeopathic medication Traumeel S in the treatment of chemotherapy-induced stomatitis in children undergoing stem cell transplantation | Human | The severity and duration of chemotherapy-induced stomatitis were reduced | [71] |
| Topical agents | ||||
| MuGard | Multi-institutional, randomized, double-blind, placebo-controlled trial to assess the efficacy of a mucoadhesive hydrogel (MuGard) in mitigating oral mucositis symptoms in patients being treated with chemoradiation therapy for cancers of the head and neck | Human | MuGard lessened the severity of the developing mucositis and pain | [21] |
| Fentanyl | Efficacy and safety of transdermal fentanyl for the treatment of oral mucositis pain caused by chemoradiotherapy in patients with esophageal squamous cell carcinoma | Human | Fentanyl was effective in treating pain from oral mucositis caused by chemoradiotherapy | [28] |
| Prophylaxis and decontamination | ||||
| Nystatin | Efficacy of chlorhexidine and nystatin rinses in the prevention of oral complications in leukemia and bone marrow transplantation | Human | Nystatin rinse has not been found to be effective in reducing the severity of chemotherapy-induced mucositis | [32] |
| Antioxidants | ||||
| Amifostine | Chemotherapy- and radiotherapy-induced oral mucositis: Review of preventive strategies and treatment | Human | Amifostine may reduce the frequency of severe esophagitis in patients undergoing concomitant chemotherapy and radiotherapy for non–small cell lung cancer | [72] |
| N-acetyl cysteine (NAC) | N-acetyl cysteine for the prevention of oral mucositis in hematopoietic sct: A double-blind, randomized, placebo-controlled trial | Human | NAC significantly reduced the incidence of severe oral mucositis (grades 3–4) after high-dose chemotherapy and no patient in the NAC group developed grade 4 mucositis | [40] |
| Matricaria chamomilla | Prospective evaluation of a chamomile mouthwash for the prevention of 5-FU-induced oral mucositis | Human | Later phase III trials of Matricaria chamomilla have failed to conclude that the chamomile given in mouthwash formulations is effective in patients with chemotherapy-induced mucositis | [70] |
| Growth factors | ||||
| Keratinocyte growth factor (KGF) | Palifermin for oral mucositis after intensive therapy for hematologic cancers | Human | KGF significantly reduced the incidence of grade 3 and 4 oral mucositis in patients with hematologic malignancies | [41] |
| Recombinant human epidermal growth factor (rhEGF) | Recombinant human epidermal growth factor treatment of radiation-induced severe oral mucositis in patients with head and neck malignancies | Human | rhEGF has been shown to enhance the mucosal wound healing process and has a therapeutic effect on radiation-induced oral mucositis | [73] |
| Anti-inflammatory agents | ||||
| Glutamine | Oral glutamine reduces the duration and severity of stomatitis after cytotoxic cancer chemotherapy. Oral glutamine to alleviate radiation-induced oral mucositis: A pilot randomized trial | Human | In two small, randomized studies prophylactic glutamine mouthwashes significantly reduced the incidence, severity, and duration of oral mucositis in patients undergoing radiotherapy or chemotherapy, respectively | [74,75] |
| Locally applied nonpharmacological agents | ||||
| Low level laser therapy (LLLT) | Cyclooxygenase-2 and vascular endothelial growth factor expression in 5-fluorouracil-induced oral mucositis in hamsters: evaluation of two low-intensity laser protocols | Hamster | LLLT promotes wound healing and appears to have an anti-inflammatory effect, as evidenced by the reduction in neutrophil infiltrate | [76] |
| Phase III trial of low-level laser therapy to prevent oral mucositis in head and neck cancer patients treated with concurrent chemoradiation | Human | Preventive LLLT in HNSCC (head and neck squamous cell carcinoma) patients receiving chemoradiotherapy is an effective tool for reducing the incidence of grade 3-4 oral mucositis | [77] | |
| Molecule, Activity, or Process | Biological Effect of Melatonin | References |
|---|---|---|
| Reactive oxygen species | ||
| OH• − (hydroxyl radical) | ↓ | [100] |
| O2• − (oxygen free radical) | ↓ | [100] |
| H2O2 (hydrogen peroxide) | ↓ | [83] |
| LO• −, LOO• − (alkoxyl, peroxyl radicals) | ↓ | [101] |
| NO (nitric oxide) | ↓ | [83] |
| ONOO• − (peroxynitrite) | ↓ | [102] |
| DNA lesions | ||
| 8-hydroxyguanine | ↓ | [103,104] |
| 8-oxo-2′-deoxyguanosine | ↓ | [103,104] |
| Inflammation | ||
| NF-κB (nuclear factor-κB) | ↓ | [11,91,92] |
| COX-2 (cyclooxygenase-2) | ↓ | [105] |
| Interleukins | ↓ | [106,107] |
| NLRP3 | ↓ | [11,91,92] |
| TNF- α (tumor necrosis factor-α) | ↓ | [106,107] |
| iNOS (inducible nitric oxide synthase) | ↓ | [106,107] |
| MPO (myeloperoxidase) | ↓ | [108] |
| Cell death | ||
| p53 | ↓ | [109] |
| Caspases (Cas-3, 8, 9, …) | ↓ | [11,83] |
| cytochrome c (in cytosol) | ↓ | [109] |
| Bcl-2, Bcl-xL (anti-apoptosis) | ↓ | [11,83] |
| Bax, Bak (pro-apoptosis) | ↓ | [11,83] |
| Autophagy | ||
| Beclin-1, Atg3, Atg12, …. (pro-autophagy) | ↓ | [110,111] |
| mTOR (pro-autophagy) | ↓ | [110,111] |
| PI3K/Akt (anti-autophagy) | ↑ | [110,111] |
| Antioxidative defense system | ||
| GSH (glutathione) | ↑ | [11,83] |
| SOD (superoxide dismutase) | ↑ | [11,83] |
| CAT (catalase) | ↑ | [11,83] |
| GPx (glutathione peroxidase) | ↑ | [11,83] |
| GRd (glutathione reducatse) | ↑ | [11,83] |
| glutathione synthetase | ↑ | [112] |
| γ-glutamyl-cysteinyl synthetase | ↑ | [112] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel Moneim, A.E.; Guerra-Librero, A.; Florido, J.; Shen, Y.-Q.; Fernández-Gil, B.; Acuña-Castroviejo, D.; Escames, G. Oral Mucositis: Melatonin Gel an Effective New Treatment. Int. J. Mol. Sci. 2017, 18, 1003. https://doi.org/10.3390/ijms18051003
Abdel Moneim AE, Guerra-Librero A, Florido J, Shen Y-Q, Fernández-Gil B, Acuña-Castroviejo D, Escames G. Oral Mucositis: Melatonin Gel an Effective New Treatment. International Journal of Molecular Sciences. 2017; 18(5):1003. https://doi.org/10.3390/ijms18051003
Chicago/Turabian StyleAbdel Moneim, Ahmed Esmat, Ana Guerra-Librero, Javier Florido, Ying-Qiang Shen, Beatriz Fernández-Gil, Darío Acuña-Castroviejo, and Germaine Escames. 2017. "Oral Mucositis: Melatonin Gel an Effective New Treatment" International Journal of Molecular Sciences 18, no. 5: 1003. https://doi.org/10.3390/ijms18051003
APA StyleAbdel Moneim, A. E., Guerra-Librero, A., Florido, J., Shen, Y. -Q., Fernández-Gil, B., Acuña-Castroviejo, D., & Escames, G. (2017). Oral Mucositis: Melatonin Gel an Effective New Treatment. International Journal of Molecular Sciences, 18(5), 1003. https://doi.org/10.3390/ijms18051003