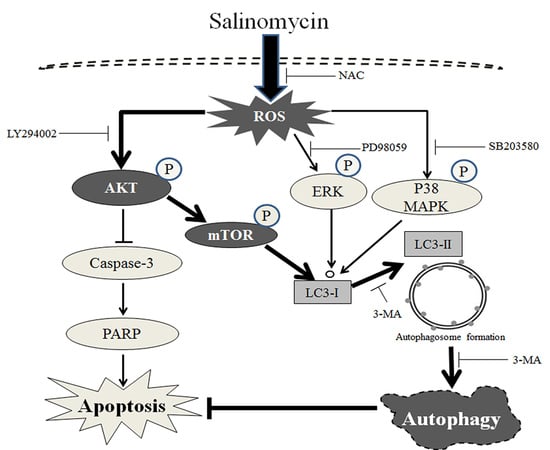
Inhibition of Autophagy Promotes Salinomycin-Induced Apoptosis via Reactive Oxygen Species-Mediated PI3K/AKT/mTOR and ERK/p38 MAPK-Dependent Signaling in Human Prostate Cancer Cells
Abstract
:1. Introduction
2. Results
2.1. Salinomycin Induces Both Apoptosis and Autphagy in Human Prostate Cancer Cells
2.2. Autophagy Inhibition Enhances Salinomycin-Induced Apoptosis in Prostate Cancer Cells
2.3. The PI3K/AKT/mTOR Pathway Is Involved in the Regulation of Both Salinomycin-Induced Autophagy and Apoptosis in Prostate Cancer Cells
2.4. The ERK and p38 MAPK Pathways Are Involved in the Regulation of Salinomycin-Induced Autophagy in Prostate Cancer Cells
2.5. Autophagy Inhibition Stimulates ROS Production in Prostate Cancer Cells
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Cell Lines and Cell Culture
4.3. Annexin V/Propidium Iodide (PI) Assay
4.4. Detection of Acidic Vesicular Organelles
4.5. Immunofluorescence for LC-3
4.6. Measurement of Intracellular ROS Generation
4.7. Western Blot Analysis
4.8. Measurement of Caspase-3 Activity
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| 3-MA | 3-Methyladenine |
| AO | Acridine orange |
| AVOs | Acidic vesicular organelles |
| BSA | Bovine serum albumin |
| DAPI | 4′,6-Diamidino-2-phenylindole dihydrochloride |
| DCF-DA | 2′,7′-Dichlorodihydrofluorescein diacetate |
| ER | Endoplasmic reticulum |
| ERK | Extracellular signal-regulated kinases |
| JNK | c-Jun N-terminal kinase |
| MAPK | Mitogen-activated protein kinases |
| MTT | 3-(4,5-Dimethyl-thiazol-2-yl)-2,5-diphenyl-etrazolium bromide |
| NAC | N-acetyl-l-cystein |
| PARP | Poly (ADP-ribose) polymerase |
| PI | Propidium iodide |
| PVDF | Polyvinylidenefluoride |
| ROS | Reactive oxygen species |
References
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2013. CA Cancer J. Clin. 2013, 63, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, D.; Jones, D.; Wade, M.; Grey, J.; Nakjang, S.; Guo, W.; Cork, D.; Davies, B.R.; Wedge, S.R.; Robson, C.N.; et al. Development and exploitation of a novel mutant androgen receptor modelling strategy to identify new targets for advanced prostate cancer therapy. Oncotarget 2015, 6, 26029–26040. [Google Scholar] [CrossRef] [PubMed]
- Mimeault, M.; Batra, S.K. New promising drug targets in cancer- and metastasis-initiating cells. Drug Discov. Today 2010, 15, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Chen, R.; Wang, Z.; Huang, Z.; Kong, N.; Zhang, M.; Han, W.; Lou, F.; Yang, J.; Zhang, Q.; et al. Autophagy and chemotherapy resistance: A promising therapeutic target for cancer treatment. Cell Death Dis. 2013, 4, e838. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Klionsky, D.J. Eaten alive: A history of macroautophagy. Nat. Cell Biol. 2010, 12, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Glick, D.; Barth, S.; Macleod, K.F. Autophagy: Cellular and molecular mechanisms. J. Pathol. 2010, 221, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Levine, B. Autophagic cell death: The story of a misnomer. Nat. Rev. Mol. Cell Biol. 2008, 9, 1004–1010. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; Yu, S.N.; Lee, S.Y.; Chun, S.S.; Choi, Y.L.; Park, Y.M.; Song, C.S.; Chatterjee, B.; Ahn, S.C. Salinomycin-induced apoptosis of human prostate cancer cells due to accumulated reactive oxygen species and mitochondrial membrane depolarization. Biochem. Biophys. Res. Commun. 2011, 413, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Kongara, S.; Karantza, V. The interplay between autophagy and ROS in tumorigenesis. Front. Oncol. 2012, 2, 171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Li, Y.; Park, K.A.; Byun, H.S.; Won, M.; Jeon, J.; Lee, Y.; Seok, J.H.; Choi, S.W.; Lee, S.H.; et al. Cucurbitacin induces autophagy through mitochondrial ROS production which counteracts to limit caspase-dependent apoptosis. Autophagy 2012, 8, 559–576. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.T.; Qin, Y.; Zhou, Z.W.; He, Z.X.; Zhang, X.; Yang, T.; Yang, Y.X.; Wang, D.; Qiu, J.X.; Zhou, S.F. Plumbagin induces G2/M arrest, apoptosis, and autophagy via p38 MAPK- and PI3K/Akt/mTOR-mediated pathways in human tongue squamous cell carcinoma cells. Drug Des. Dev. Ther. 2015, 9, 1601–1626. [Google Scholar]
- Niu, N.K.; Wang, Z.L.; Pan, S.T.; Ding, H.Q.; Au, G.H.; He, Z.X.; Zhou, Z.W.; Xiao, G.; Yang, Y.X.; Zhang, X.; et al. Pro-apoptotic and pro-autophagic effects of the Aurora kinase A inhibitor alisertib (MLN8237) on human osteosarcoma U-2 OS and MG-63 cells through the activation of mitochondria-mediated pathway and inhibition of p38 MAPK/PI3K/Akt/mTOR signaling pathway. Drug Des. Dev. Ther. 2015, 9, 1555–1584. [Google Scholar]
- Naujokat, C.; Fuchs, D.; Opelz, G. Salinomycin in cancer: A new mission for an old agent. Mol. Med. Rep. 2010, 3, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Pressman, B.C. Antibiotic models for carrier-mediated transport through membranes. Antimicrob. Agents Chemother. 1969, 9, 28–34. [Google Scholar] [PubMed]
- Gupta, P.B.; Onder, T.T.; Jiang, G.; Tao, K.; Kuperwasser, C.; Weinberg, R.A.; Lander, E.S. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell 2009, 138, 645–659. [Google Scholar] [CrossRef] [PubMed]
- Al Dhaheri, Y.; Attoub, S.; Arafat, K.; Abuqamar, S.; Eid, A.; Al Faresi, N.; Iratni, R. Salinomycin induces apoptosis and senescence in breast cancer: Upregulation of p21, downregulation of survivin and histone H3 and H4 hyperacetylation. Biochim. Biophys. Acta 2013, 1830, 3121–3135. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; Kim, S.H.; Yu, S.N.; Park, S.K.; Choi, H.D.; Yu, H.S.; Ji, J.H.; Seo, Y.K.; Ahn, S.C. Salinomycin enhances doxorubicin-induced cytotoxicity in multidrug resistant MCF-7/MDR human breast cancer cells via decreased efflux of doxorubicin. Mol. Med. Rep. 2015, 12, 1898–1904. [Google Scholar] [CrossRef] [PubMed]
- Jangamreddy, J.R.; Ghavami, S.; Grabarek, J.; Kratz, G.; Wiechec, E.; Fredriksson, B.A.; Rao Pariti, R.K.; Cieslar-Pobuda, A.; Panigrahi, S.; Los, M.J. Salinomycin induces activation of autophagy, mitophagy and affects mitochondrial polarity: Differences between primary and cancer cells. Biochim. Biophys. Acta 2013, 1833, 2057–2069. [Google Scholar] [CrossRef] [PubMed]
- Boehmerle, W.; Endres, M. Salinomycin induces calpain and cytochrome c-mediated neuronal cell death. Cell Death Dis. 2011, 2, e168. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liang, C.; Xue, F.; Chen, W.; Zhi, X.; Feng, X.; Bai, X.; Liang, T. Salinomycin decreases doxorubicin resistance in hepatocellular carcinoma cells by inhibiting the β-catenin/TCF complex association via FOXO3a activation. Oncotarget 2015, 6, 10350–10365. [Google Scholar] [CrossRef] [PubMed]
- Yao, T.P. The role of ubiquitin in autophagy-dependent protein aggregate processing. Genes Cancer 2010, 1, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.; Kondo, S.; Le, W.; Jankovic, J. The role of autophagy-lysosome pathway in neurodegeneration associated with Parkinson's disease. Brain 2008, 131, 1969–1978. [Google Scholar] [CrossRef] [PubMed]
- Maycotte, P.; Thorburn, A. Autophagy and cancer therapy. Cancer Biol. Ther. 2011, 11, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.J.; Chee, C.E.; Huang, S.; Sinicrope, F.A. The role of autophagy in cancer: Therapeutic implications. Mol. Cancer Ther. 2011, 10, 1533–1541. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Zhang, X.; Sun, H.; Zhang, J.; Yan, M.; Zhang, H. Autophagy inhibition promotes 5-fluorouraci-induced apoptosis by stimulating ROS formation in human non-small cell lung cancer A549 cells. PLoS ONE 2013, 8, e56679. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Jing, K.; Jeong, S.; Kim, N.; Song, K.S.; Heo, J.Y.; Park, J.H.; Seo, K.S.; Han, J.; Park, J.I.; et al. The omega-3 polyunsaturated fatty acid DHA induces simultaneous apoptosis and autophagy via mitochondrial ROS-mediated Akt-mTOR signaling in prostate cancer cells expressing mutant p53. BioMed Res. Int. 2013, 2013, 568671. [Google Scholar] [CrossRef] [PubMed]
- Vilquin, P.; Villedieu, M.; Grisard, E.; Ben Larbi, S.; Ghayad, S.E.; Heudel, P.E.; Bachelot, T.; Corbo, L.; Treilleux, I.; Vendrell, J.A.; et al. Molecular characterization of anastrozole resistance in breast cancer: Pivotal role of the Akt/mTOR pathway in the emergence of de novo or acquired resistance and importance of combining the allosteric Akt inhibitor MK-2206 with an aromatase inhibitor. Int. J. Cancer 2013, 133, 1589–1602. [Google Scholar] [CrossRef] [PubMed]
- Milone, M.R.; Pucci, B.; Bruzzese, F.; Carbone, C.; Piro, G.; Costantini, S.; Capone, F.; Leone, A.; Di Gennaro, E.; Caraglia, M.; et al. Acquired resistance to zoledronic acid and the parallel acquisition of an aggressive phenotype are mediated by p38-MAP kinase activation in prostate cancer cells. Cell Death Dis. 2013, 4, e641. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.D.; Oh, J.; Park, H.J.; Bae, K.; Lee, S.K. Magnolol inhibits angiogenesis by regulating ROS-mediated apoptosis and the PI3K/AKT/mTOR signaling pathway in mES/EB-derived endothelial-like cells. Int. J. Oncol. 2013, 43, 600–610. [Google Scholar] [PubMed]
- Bui-Xuan, N.H.; Tang, P.M.; Wong, C.K.; Fung, K.P. Photo-activated pheophorbide-a, an active component of Scutellaria barbata, enhances apoptosis via the suppression of ERK-mediated autophagy in the estrogen receptor-negative human breast adenocarcinoma cells MDA-MB-231. J. Ethnopharmacol. 2010, 131, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Orrenius, S.; Kaminskyy, V.O.; Zhivotovsky, B. Autophagy in toxicology: Cause or consequence? Annu. Rev. Pharmacol. Toxicol. 2013, 53, 275–297. [Google Scholar] [CrossRef] [PubMed]





© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.-Y.; Park, K.-I.; Kim, S.-H.; Yu, S.-N.; Park, S.-G.; Kim, Y.W.; Seo, Y.-K.; Ma, J.-Y.; Ahn, S.-C. Inhibition of Autophagy Promotes Salinomycin-Induced Apoptosis via Reactive Oxygen Species-Mediated PI3K/AKT/mTOR and ERK/p38 MAPK-Dependent Signaling in Human Prostate Cancer Cells. Int. J. Mol. Sci. 2017, 18, 1088. https://doi.org/10.3390/ijms18051088
Kim K-Y, Park K-I, Kim S-H, Yu S-N, Park S-G, Kim YW, Seo Y-K, Ma J-Y, Ahn S-C. Inhibition of Autophagy Promotes Salinomycin-Induced Apoptosis via Reactive Oxygen Species-Mediated PI3K/AKT/mTOR and ERK/p38 MAPK-Dependent Signaling in Human Prostate Cancer Cells. International Journal of Molecular Sciences. 2017; 18(5):1088. https://doi.org/10.3390/ijms18051088
Chicago/Turabian StyleKim, Kwang-Youn, Kwang-Il Park, Sang-Hun Kim, Sun-Nyoung Yu, Sul-Gi Park, Young Woo Kim, Young-Kyo Seo, Jin-Yeul Ma, and Soon-Cheol Ahn. 2017. "Inhibition of Autophagy Promotes Salinomycin-Induced Apoptosis via Reactive Oxygen Species-Mediated PI3K/AKT/mTOR and ERK/p38 MAPK-Dependent Signaling in Human Prostate Cancer Cells" International Journal of Molecular Sciences 18, no. 5: 1088. https://doi.org/10.3390/ijms18051088