Real-World Experiences with the Combination Treatment of Ledipasvir plus Sofosbuvir for 12 Weeks in HCV Genotype 1-Infected Japanese Patients: Achievement of a Sustained Virological Response in Previous Users of Peginterferon plus Ribavirin with HCV NS3/4A Inhibitors
Abstract
:1. Introduction
2. Patients and Methods
2.1. Patients
2.2. Clinical and Laboratory Assessments
2.3. DNA Extraction and IL28B Genotyping
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Treatment Response and Efficacy of Combination Treatment with Ledipasvir plus Sofosbuvir
3.3. Analysis of Resistance-Associated Variants (RAVs) in Relapsers to Ledipasvir plus Sofosbuvir
3.4. Safety in Ledipasvir plus Sofosbuvir Treatment
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Survival Statistics of Japanese Association of Clinical Cancer Centers. Cancer Survival Rates at Japanese Association of Clinical Cancer Centers. Available online: https://kapweb.chiba-cancer-registry.org/?lang=en (accessed on 28 March 2017).
- Tateishi, R.; Okanoue, T.; Fujiwara, N.; Okita, K.; Kiyosawa, K.; Omata, M.; Kumada, H.; Hayashi, N.; Koike, K. Clinical characteristics, treatment, and prognosis of non-B, non-C hepatocellular carcinoma: A large retrospective multicenter cohort study. J. Gastroenterol. 2015, 50, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Tada, T.; Kumada, T.; Toyoda, H.; Kiriyama, S.; Tanikawa, M.; Hisanaga, Y.; Kanamori, A.; Kitabatake, S.; Yama, T.; Tanaka, J. Viral eradication reduces all-cause mortality in patients with chronic hepatitis C virus infection: A propensity score analysis. Liver Int. 2016, 36, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Noda, I.; Kitamoto, M.; Nakahara, H.; Hayashi, R.; Okimoto, T.; Monzen, Y.; Yamada, H.; Imagawa, M.; Hiraga, N.; Tanaka, J.; et al. Regular surveillance by imaging for early detection and better prognosis of hepatocellular carcinoma in patients infected with hepatitis C virus. J. Gastroenterol. 2010, 45, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Omata, M.; Kanda, T.; Yu, M.L.; Yokosuka, O.; Lim, S.G.; Jafri, W.; Tateishi, R.; Hamid, S.S.; Chuang, W.L.; Chutaputti, A.; et al. APASL consensus statements and management algorithms for hepatitis C virus infection. Hepatol. Int. 2012, 6, 409–435. [Google Scholar] [CrossRef] [PubMed]
- Maruoka, D.; Imazeki, F.; Arai, M.; Kanda, T.; Fujiwara, K.; Yokosuka, O. Longitudinal changes of the laboratory data of chronic hepatitis C patients with sustained virological response on long-term follow-up. J. Viral Hepat. 2012, 19, e97–e104. [Google Scholar] [CrossRef] [PubMed]
- Chayama, K.; Takahashi, S.; Toyota, J.; Karino, Y.; Ikeda, K.; Ishikawa, H.; Watanabe, H.; McPhee, F.; Hughes, E.; Kumada, H. Dual therapy with the nonstructural protein 5A inhibitor, daclatasvir, and the nonstructural protein 3 protease inhibitor, asunaprevir, in hepatitis C virus genotype 1b-infected null responders. Hepatology 2012, 55, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Kanda, T.; Nakamoto, S.; Jiang, X.; Miyamura, T.; Nakatani, S.M.; Ono, S.K.; Takahashi-Nakaguchi, A.; Gonoi, T.; Yokosuka, O. Prevalence of hepatitis C virus subgenotypes 1a and 1b in Japanese patients: Ultra-deep sequencing analysis of HCV NS5B genotype-specific region. PLoS ONE 2013, 8, e73615. [Google Scholar] [CrossRef] [PubMed]
- Iio, E.; Shimada, N.; Abe, H.; Atsukawa, M.; Yoshizawa, K.; Takaguchi, K.; Eguchi, Y.; Nomura, H.; Kuramitsu, T.; Kang, J.H.; et al. Efficacy of daclatasvir/asunaprevir according to resistance-associated variants in chronic hepatitis C with genotype 1. J. Gastroenterol. 2017, 52, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Omata, M.; Kanda, T.; Wei, L.; Yu, M.L.; Chuang, W.L.; Ibrahim, A.; Lesmana, C.R.; Sollano, J.; Kumar, M.; Jindal, A.; et al. APASL consensus statements and recommendation on treatment of hepatitis C. Hepatol. Int. 2016, 10, 702–726. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Ikeda, K.; Suzuki, F.; Toyota, J.; Karino, Y.; Chayama, K.; Kawakami, Y.; Ishikawa, H.; Watanabe, H.; Hu, W.; et al. Dual oral therapy with daclatasvir and asunaprevir for patients with HCV genotype 1b infection and limited treatment options. J. Hepatol. 2013, 58, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Yasui, S.; Nakamura, M.; Suzuki, E.; Arai, M.; Haga, Y.; Sasaki, R.; Wu, S.; Nakamoto, S.; Imazeki, F.; et al. Daclatasvir plus Asunaprevir treatment for real-world HCV genotype 1-infected patients in Japan. Int. J. Med. Sci. 2016, 13, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Kowdley, K.V.; Gordon, S.C.; Reddy, K.R.; Rossaro, L.; Bernstein, D.E.; Lawitz, E.; Shiffman, M.L.; Schiff, E.; Ghalib, R.; Ryan, M.; et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N. Engl. J. Med. 2014, 370, 1879–1888. [Google Scholar] [CrossRef] [PubMed]
- Afdhal, N.; Reddy, K.R.; Nelson, D.R.; Lawitz, E.; Gordon, S.C.; Schiff, E.; Nahass, R.; Ghalib, R.; Gitlin, N.; Herring, R.; et al. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N. Engl. J. Med. 2014, 370, 1483–1493. [Google Scholar] [CrossRef] [PubMed]
- Afdhal, N.; Zeuzem, S.; Kwo, P.; Chojkier, M.; Gitlin, N.; Puoti, M.; Romero-Gomez, M.; Zarski, J.P.; Agarwal, K.; Buggisch, P.; et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N. Engl. J. Med. 2014, 370, 1889–1898. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.R.; Bourlière, M.; Sulkowski, M.; Omata, M.; Zeuzem, S.; Feld, J.J.; Lawitz, E.; Marcellin, P.; Welzel, T.M.; Hyland, R.; et al. Ledipasvir and sofosbuvir in patients with genotype 1 hepatitis C virus infection and compensated cirrhosis: An integrated safety and efficacy analysis. Hepatology 2015, 62, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Mizokami, M.; Yokosuka, O.; Takehara, T.; Sakamoto, N.; Korenaga, M.; Mochizuki, H.; Nakane, K.; Enomoto, H.; Ikeda, F.; Yanase, M.; et al. Ledipasvir and sofosbuvir fixed-dose combination with and without ribavirin for 12 weeks in treatment-naive and previously treated Japanese patients with genotype 1 hepatitis C: An open-label, randomised, phase 3 trial. Lancet Infect. Dis. 2015, 15, 645–653. [Google Scholar] [CrossRef]
- Miyamura, T.; Kanda, T.; Nakamoto, S.; Wu, S.; Jiang, X.; Arai, M.; Fujiwara, K.; Imazeki, F.; Yokosuka, O. Roles of ITPA and IL28B genotypes in chronic hepatitis C patients treated with peginterferon plus ribavirin. Viruses 2012, 4, 1264–1278. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, T.; Kanda, T.; Imazeki, F.; Mikata, R.; Tawada, A.; Arai, M.; Fujiwara, K.; Nakamoto, S.; Wu, S.; Tanaka, T.; et al. Response to peginterferon-alpha 2b and ribavirin in Japanese patients with chronic hepatitis C genotype 1. Hepatol. Int. 2013, 7, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Hirotsu, Y.; Kanda, T.; Matsumura, H.; Moriyama, M.; Yokosuka, O.; Omata, M. HCV NS5A resistance-associated variants in a group of real-world Japanese patients chronically infected with HCV genotype 1b. Hepatol. Int. 2015, 9, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Mizokami, M.; Dvory-Sobol, H.; Izumi, N.; Nishiguchi, S.; Doehle, B.; Svarovskaia, E.S.; de-Oertel, S.; Knox, S.; Brainard, D.M.; Miller, M.D.; et al. Resistance analyses of Japanese hepatitis C-Infected patients receiving sofosbuvir or ledipasvir/sofosbuvir containing regimens in phase 3 studies. J. Viral Hepat. 2016, 23, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Zeuzem, S.; Mizokami, M.; Pianko, S.; Mangia, A.; Han, K.H.; Martin, R.; Svarovskaia, E.; Dvory-Sobol, H.; Doehle, B.; Hedskog, C.; et al. NS5A resistance-associated substitutions in patients with genotype 1 hepatitis C virus: Prevalence and effect on treatment outcome. J. Hepatol. 2017, 66, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, E.; Furusyo, N.; Nomura, H.; Dohmen, K.; Higashi, N.; Takahashi, K.; Kawano, A.; Azuma, K.; Satoh, T.; Nakamuta, M.; et al. NS5A resistance-associated variants undermine the effectiveness of ledipasvir and sofosbuvir for cirrhotic patients infected with HCV genotype 1b. J. Gastroenterol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Iio, E.; Shimada, N.; Takaguchi, K.; Senoh, T.; Eguchi, Y.; Atsukawa, M.; Tsubota, A.; Abe, H.; Kato, K.; Kusakabe, A.; et al. Clinical evaluation of sofosbuvir/ledipasvir in chronic hepatitis C genotype 1 with and without prior daclatasvir/asnaprevir therapy. Hepatol. Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Akuta, N.; Sezaki, H.; Suzuki, F.; Fujiyama, S.; Kawamura, Y.; Hosaka, T.; Kobayashi, M.; Kobayashi, M.; Saitoh, S.; Suzuki, Y.; et al. Ledipasvir plus sofosbuvir as salvage therapy for HCV genotype 1 failures to prior NS5A inhibitors regimens. J. Med. Virol. 2017. [Google Scholar] [CrossRef] [PubMed]
- AASLD/IDSA HCV Guidance Panel. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology 2015, 62, 932–954. [Google Scholar]
- European Association for the Study of the Liver. Electronic address: [email protected]. EASL Recommendations on Treatment of Hepatitis C 2016. J. Hepatol. 2017, 66, 153–194. [Google Scholar]
- Lagging, M.; Wejstål, R.; Norkrans, G.; Karlström, O.; Aleman, S.; Weiland, O.; Castedal, M.; Westin, J.; Swedish Consensus Group. Treatment of hepatitis C virus infection: Updated Swedish Guidelines 2016. Infect. Dis. 2017, 15, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, S.; Kanda, T.; Wu, S.; Shirasawa, H.; Yokosuka, O. Hepatitis C virus NS5A inhibitors and drug resistance mutations. World J. Gastroenterol. 2014, 20, 2902–2912. [Google Scholar] [CrossRef] [PubMed]
- Lawitz, E.; Poordad, F.F.; Pang, P.S.; Hyland, R.H.; Ding, X.; Mo, H.; Symonds, W.T.; McHutchison, J.G.; Membreno, F.E. Sofosbuvir and ledipasvir fixed-dose combination with and without ribavirin in treatment-naive and previously treated patients with genotype 1 hepatitis C virus infection (LONESTAR): An open-label, randomised, phase 2 trial. Lancet 2014, 383, 515–523. [Google Scholar] [CrossRef]
- Bourlière, M.; Bronowicki, J.P.; de Ledinghen, V.; Hézode, C.; Zoulim, F.; Mathurin, P.; Tran, A.; Larrey, D.G.; Ratziu, V.; Alric, L.; et al. Ledipasvir-sofosbuvir with or without ribavirin to treat patients with HCV genotype 1 infection and cirrhosis non-responsive to previous protease-inhibitor therapy: A randomised, double-blind, phase 2 trial (SIRIUS). Lancet Infect. Dis. 2015, 15, 397–404. [Google Scholar] [CrossRef]
- European Association of the Study of the Liver. 2011 European Association of the Study of the Liver hepatitis C virus clinical practice guidelines. Liver Int. 2012, 32, 2–8. [Google Scholar]
- European Association for Study of Liver. EASL Clinical Practice Guidelines: Management of hepatitis C virus infection. J. Hepatol. 2014, 60, 392–420. [Google Scholar]
- Fontaine, H.; Lazarus, A.; Pol, S.; Pecriaux, C.; Bagate, F.; Sultanik, P.; Boueyre, E.; Corouge, M.; Mallet, V.; Vallet-Pichard, A.; et al. Bradyarrhythmias Associated with Sofosbuvir Treatment. N. Engl. J. Med. 2015, 373, 1886–1888. [Google Scholar] [PubMed]
- Renet, S.; Chaumais, M.C.; Antonini, T.; Zhao, A.; Thomas, L.; Savoure, A.; Samuel, D.; Duclos-Vallée, J.C.; Algalarrondo, V. Extreme bradycardia after first doses of sofosbuvir and daclatasvir in patients receiving amiodarone: 2 cases including a rechallenge. Gastroenterology 2015, 149, 1378–1380. [Google Scholar] [CrossRef] [PubMed]
- Lagrutta, A.; Regan, C.P.; Zeng, H.; Imredy, J.P.; Koeplinger, K.; Morissette, P.; Liu, L.; Wollenberg, G.; Brynczka, C.; Lebrón, J.; et al. Cardiac drug-drug interaction between HCV-NS5B pronucleotide inhibitors and amiodarone is determined by their specific diastereochemistry. Sci. Rep. 2017, 7, 44820. [Google Scholar] [CrossRef] [PubMed]
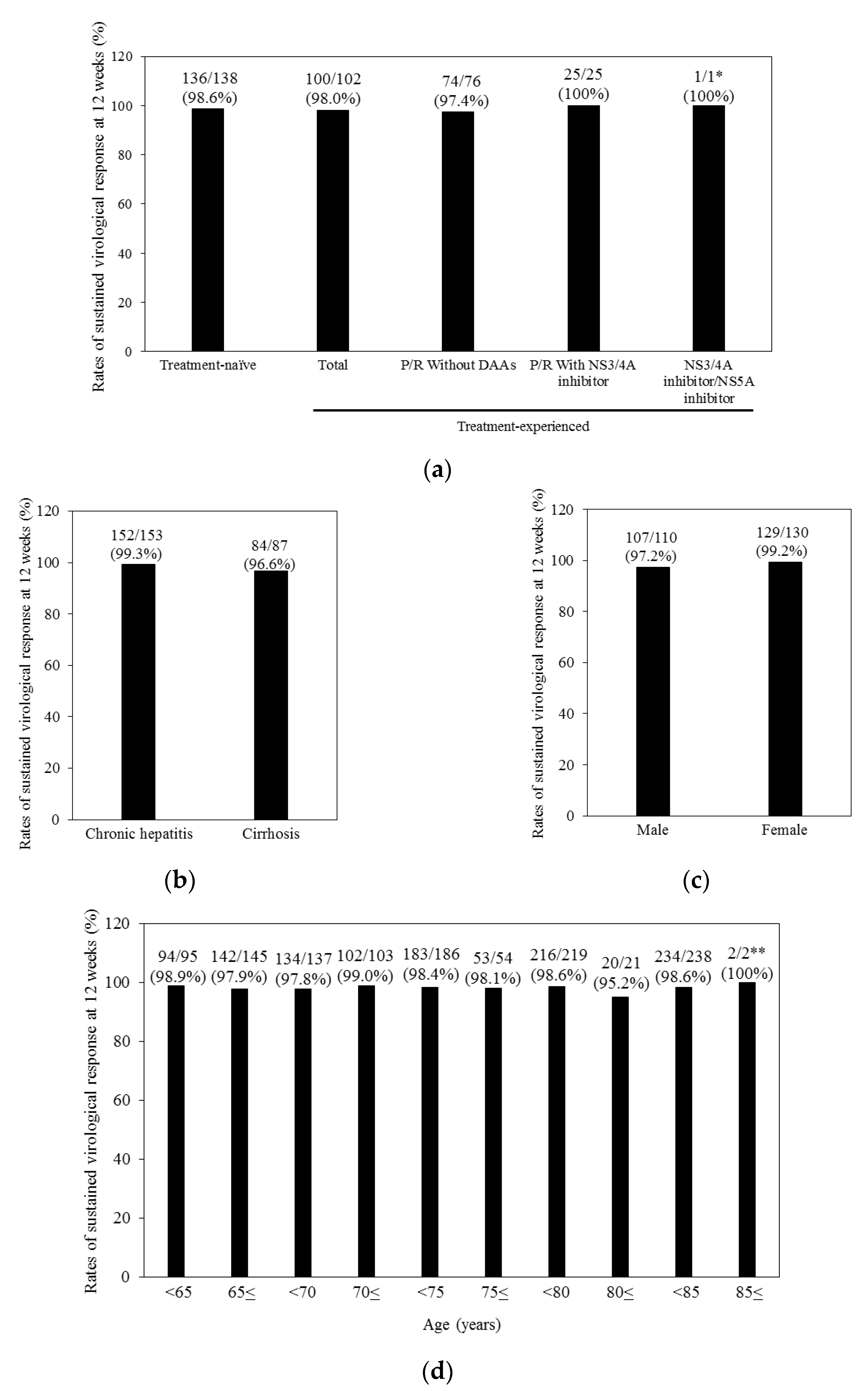
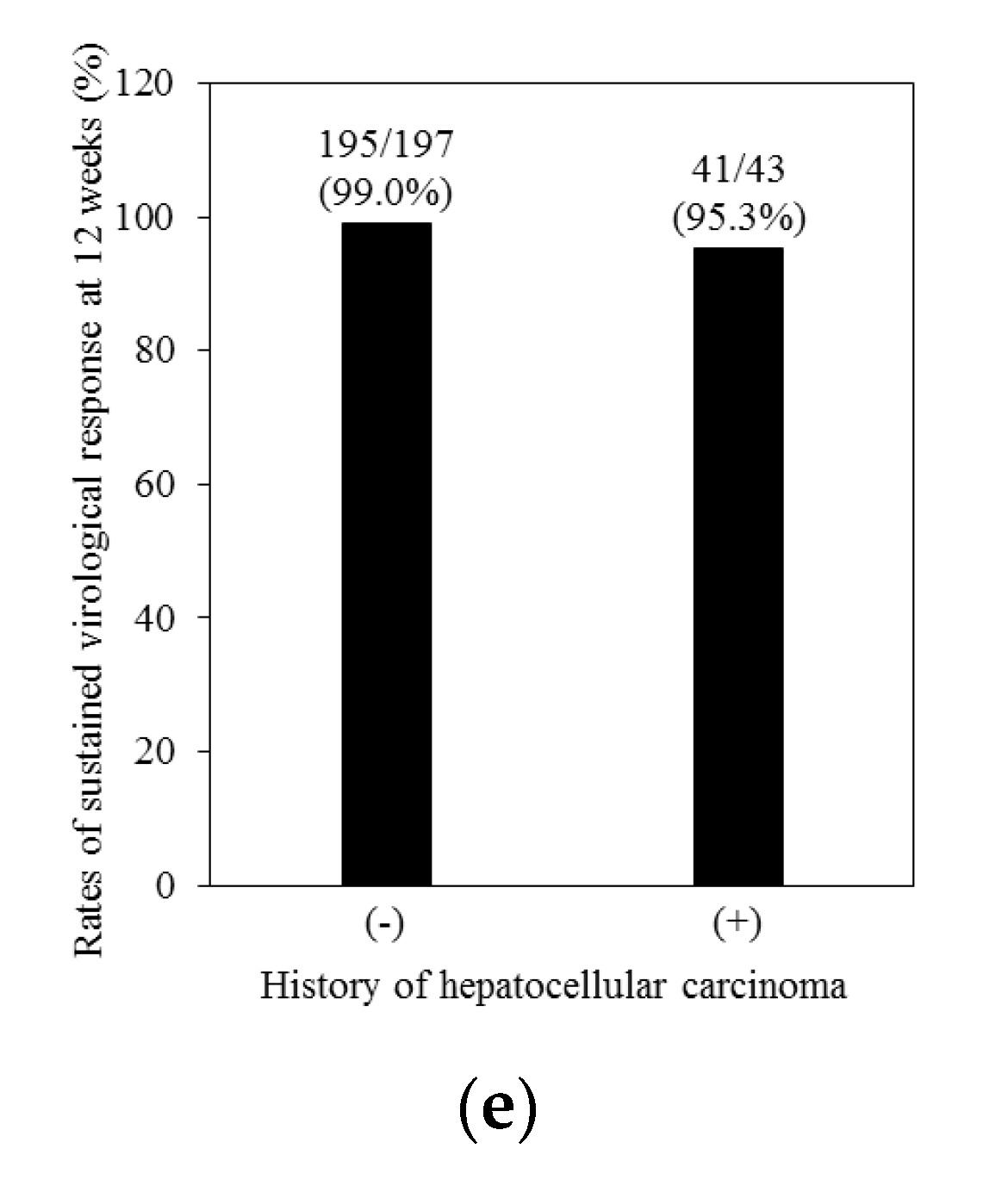
| Characteristics | All (n = 240) | Treatment-Naïve (n = 138) | Treatment-Experienced (n = 102) | p-Values 1 |
|---|---|---|---|---|
| Age (years) | 65.8 ± 11.6 | 67.5 ± 11.3 | 63.5 ± 11.6 | 0.00787 |
| Gender (male/female) | 110/130 | 59/79 | 51/51 | 0.328 |
| Interferon (naive/experienced) | 138/102 | 138/0 | 0/102 | N.A. |
| HCV GT (1a/1b/1) | 6/206/28 | 4/120/14 | 2/86/14 | 0.994 |
| HCV RNA (L/H) | 22/218 | 20/118 | 2/100 | 0.00194 |
| Body weight (kg) | 56.9 ± 10.2 | 55.9 ± 11.1 | 58.2 ± 8.8 | 0.0851 |
| Body length (cm) | 160 ± 9.5 | 160 ± 9.3 | 161 ± 9.7 | 0.420 |
| History of HCC +/− | 43/197 | 26/112 | 17/85 | 0.792 |
| Chronic hepatitis/cirrhosis | 153/87 | 88/50 | 65/37 | 0.897 |
| Liver stiffness (kPa) | 11.4 ± 12.7 | 10.8 ± 12.4 | 12.2 ± 13.1 | 0.399 |
| AST (IU/L) | 51.4 ± 32.0 | 48.1 ± 24.2 | 55.9 ± 39.9 | 0.0617 |
| ALT (IU/L) | 46.9 ± 40.2 | 41.6 ± 26.9 | 54.0 ± 52.5 | 0.0179 |
| Hemoglobin (g/dL) | 13.4 ± 1.6 | 13.4 ± 1.5 | 13.5 ± 1.6 | 0.620 |
| Platelets (×104/μL) | 15.3 ± 6.8 | 15.7 ± 7.5 | 14.9 ± 5.7 | 0.368 |
| eGFR (mL/min/1.73 m2) | 74.4 ± 17.9 | 73.2 ± 16.7 | 76.3 ± 19.6 | 0.188 |
| Characteristics | All (n = 240) | Treatment-Naïve (n = 138) | Treatment-Experienced (n = 102) | p-Values 1 |
|---|---|---|---|---|
| HCV undetectable no. (%) | ||||
| During treatment | ||||
| At 4 w | 177 (73.8) | 106 (76.8) | 71 (69.6) | 0.221 |
| At 8 w | 237 (98.8) | 136 (98.6) | 101 (99.0) | 0.791 |
| At 12 w | 239 (99.6) | 137 (99.3) | 102 (100) | 0.879 |
| After treatment | ||||
| Post 4 w | 238 (99.2) | 137 (99.3) | 101 (99.0) | 0.615 |
| Post 8 w | 236 (98.3) | 136 (98.6) | 100 (98.0) | 0.838 |
| Post 12 w | 236 (98.3) | 136 (98.6) | 100 (98.0) | 0.838 |
| Virological failure | ||||
| Discontinuation | 1 | 1/138 (0.7) | 0/102 (0) | 0.879 |
| Relapse | 2 | 0/138 (0) | 2/102 (2.0) | 0.350 |
| Lost due to AEs | 1 | 1/138 (0.7) | 0/102 (0) | 0.879 |
| No. | Age/Gender | Previous Treatment Response | GT | Cirrhosis/HCC | Efficacies | Adherence >80% | NS5A-L31 | NS5A-Y93 | NS5B-S282 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 66/Male | PegIFN/RBV null response | 1b | Yes/+ | Relapse (post 4 w) | Yes | M | M | W |
| 2 | 58/Male | IFN null response | 1b | Yes/− | Relapse (post 8 w) | Yes | M | W | W |
© 2017 by the authors. Submitted for possible open access publication under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanda, T.; Yasui, S.; Nakamura, M.; Suzuki, E.; Arai, M.; Ooka, Y.; Ogasawara, S.; Chiba, T.; Saito, T.; Haga, Y.; et al. Real-World Experiences with the Combination Treatment of Ledipasvir plus Sofosbuvir for 12 Weeks in HCV Genotype 1-Infected Japanese Patients: Achievement of a Sustained Virological Response in Previous Users of Peginterferon plus Ribavirin with HCV NS3/4A Inhibitors. Int. J. Mol. Sci. 2017, 18, 906. https://doi.org/10.3390/ijms18050906
Kanda T, Yasui S, Nakamura M, Suzuki E, Arai M, Ooka Y, Ogasawara S, Chiba T, Saito T, Haga Y, et al. Real-World Experiences with the Combination Treatment of Ledipasvir plus Sofosbuvir for 12 Weeks in HCV Genotype 1-Infected Japanese Patients: Achievement of a Sustained Virological Response in Previous Users of Peginterferon plus Ribavirin with HCV NS3/4A Inhibitors. International Journal of Molecular Sciences. 2017; 18(5):906. https://doi.org/10.3390/ijms18050906
Chicago/Turabian StyleKanda, Tatsuo, Shin Yasui, Masato Nakamura, Eiichiro Suzuki, Makoto Arai, Yoshihiko Ooka, Sadahisa Ogasawara, Tetsuhiro Chiba, Tomoko Saito, Yuki Haga, and et al. 2017. "Real-World Experiences with the Combination Treatment of Ledipasvir plus Sofosbuvir for 12 Weeks in HCV Genotype 1-Infected Japanese Patients: Achievement of a Sustained Virological Response in Previous Users of Peginterferon plus Ribavirin with HCV NS3/4A Inhibitors" International Journal of Molecular Sciences 18, no. 5: 906. https://doi.org/10.3390/ijms18050906







