Local Actions of Melatonin in Somatic Cells of the Testis
Abstract
:1. Introduction
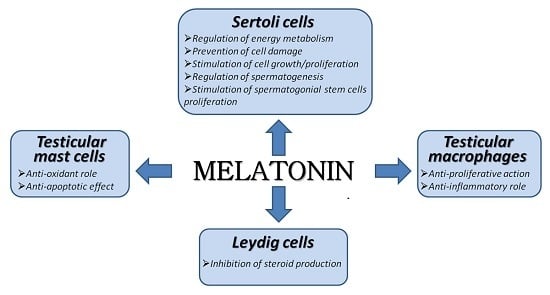
2. Melatonin in Leydig Cells
3. Melatonin in Sertoli Cells
4. Melatonin in Testicular Immune Cells
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Bartke, A. Male hamster reproductive endocrinology. In The Hamster, Reproduction and Behavior; Siegel, H.I., Ed.; Plenum: New York, NY, USA, 1985; pp. 73–98. [Google Scholar]
- Chowdhury, V.S.; Yamamoto, K.; Ubuka, T.; Bentley, G.E.; Hattori, A.; Tsutsui, K. Melatonin stimulates the release of gonadotropin-inhibitory hormone by the avian hypothalamus. Endocrinology 2010, 151, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Charreau, E.H.; Attramadal, A; Torjesen, P.A.; Purvis, K.; Calandra, R.; Hansson, V. Prolactin binding in rat testis: Specific receptors in interstitial cells. Mol. Cell. Endocrinol. 1977, 6, 303–307. [Google Scholar] [CrossRef]
- Lodge, J.; Salisbury, G. Seasonal variations and male reproductive efficiency. In The Testis; Johnson, A.D., Gomes, W.R., van Denmark, N.L., Eds.; Academic Press: New York, NY, USA, 1970; Volume 3, pp. 139–167. [Google Scholar]
- Minneman, K.P.; Wurtmann, R.J. Effects of pineal compounds on mammals. Life Sci. 1975, 17, 1189–1200. [Google Scholar] [CrossRef]
- Moore, R.Y. The innervation of the mammalian pineal gland. In The Pineal and Reproduction, Progress in Reproductive Biology; Reiter, R.J., Ed.; Karger: Basel, Switzerland, 1978; Volume 4, pp. 1–29. [Google Scholar]
- Reiter, R.J. The pineal and its hormones in the control of reproduction in mammals. Endocr. Rev. 1980, 1, 109–131. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J. Pineal melatonin: Cell biology of its synthesis and of its physiological interactions. Endocr. Rev. 1991, 12, 151–180. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J. Melatonin and human reproduction. Ann. Med. 1998, 30, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Jockers, R.; Delagrange, P.; Dubocovich, M.L.; Markus, R.P.; Renault, N.; Tosini, G.; Cecon, E.; Zlotos, D.P. Update on melatonin receptors: IUPHAR Review 20. Br. J. Pharmacol. 2016, 173, 2702–2725. [Google Scholar] [CrossRef] [PubMed]
- Drew, J.E.; Barrett, P.; Mercer, J.G.; Moar, K.M.; Canet, E.; Delagrange, P.; Morgan, P.J. Localization of the melatonin-related receptor in the rodent brain and peripheral tissues. J. Neuroendocrinol. 2001, 5, 453–458. [Google Scholar] [CrossRef]
- Frungieri, M.B.; Mayerhofer, A.; Zitta, K.; Pignataro, O.P.; Calandra, R.S.; Gonzalez-Calvar, S.I. Direct effect of melatonin on syrian hamster testes: Mel1a receptors, inhibition of androgen production, and interaction with the local corticotropin-releasing hormone (CRH) system. Endocrinology 2005, 146, 1541–1552. [Google Scholar] [CrossRef] [PubMed]
- Izzo, G.; Francesco, A.; Ferrara, D.; Campitiello, M.R.; Serino, I.; Minucci, S.; d’Istria, M. Expression of melatonin (MT1, MT2) and melatonin-related receptors in the adult rat testes and during development. Zygote 2010, 3, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Haldar, C. Melatonin membrane receptor (MT1R) expression and nitro-oxidative stress in testis of golden hamster, Mesocricetus auratus: An age-dependent study. Exp. Gerontol. 2015, 69, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Cardinali, D.P.; Lynch, H.J.; Wurtman, R.J. Binding of melatonin to human and rat plasma proteins. Endocrinology 1972, 91, 1213–1218. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J. Pineal control of reproduction. Prog. Clin. Biol. Res. 1981, 59B, 349–355. [Google Scholar] [PubMed]
- Reiter, R.J. The melatonin rhythm: Both a clock and a calendar. Experientia 1993, 49, 654–664. [Google Scholar] [CrossRef] [PubMed]
- Tijmes, M.; Pedraza, R.; Valladares, L. Melatonin in the rat testes: Evidence of local synthesis. Steroids 1996, 61, 65–68. [Google Scholar] [CrossRef]
- Stefulj, J.; Hortner, M.; Ghosh, M.; Schauenstein, K.; Rinner, I.; Wolfler, A.; Semmler, J.; Liebmann, P.M. Gene expression of the key enzymes of melatonin synthesis in extrapineal tissues of the rat. J. Pineal Res. 2001, 30, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Arto, M.; Hamilton, T.R.; Gallego, M.; Gaspar-Torrubia, E.; Aguilar, D.; Serrano-Blesa, E.; Abecia, J.A.; Pérez-Pé, R.; Muiño-Blanco, T.; Cebrián-Pérez, J.A.; et al. Evidence of melatonin synthesis in the ram reproductive tract. Andrology 2016, 4, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.S.; Leu, S.F.; Yang, H.Y.; Huang, B.M. Melatonin inhibits the expression of steroidogenic acute regulatory protein and steroidogenesis in MA-10 cells. J. Androl. 2001, 22, 245–254. [Google Scholar] [PubMed]
- Maitra, S.K.; Ray, A.K. Role of light in the mediation of acute effects of a single afternoon melatonin injection on steroidogenic activity of testis in the rat. J. Biosci. 2000, 25, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Pratis, K.; O’Donnell, L.; Ooi, G.T.; Stanton, P.G.; McLachlan, R.I.; Robertson, D.M. Differential regulation of rat testicular 5α-reductase type 1 and 2 isoforms by testosterone and FSH. J. Endocrinol. 2003, 176, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Heindel, J.J.; Jackson, F.L.; Berkowitz, A.S. Role of the pineal in the alteration of hamster Sertoli cell responsiveness to FSH during testicular regression. J. Androl. 1984, 5, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.P.; Windschuettl, S.; Matzkin, M.E.; Terradas, C.; Ponzio, R.; Puigdomenech, E.; Levalle, O.; Calandra, R.; Mayerhofer, A.; Frungieri, M.B. Melatonin in testes of infertile men: Evidence for anti-proliferative and anti-oxidant effects on local macrophage and mast cell populations. Andrology 2014, 2, 436–449. [Google Scholar] [CrossRef] [PubMed]
- Semercioz, A.; Onur, R.; Ogras, S.; Orhan, I. Effects of melatonin on testicular tissue nitric oxide level and antioxidant enzyme activities in experimentally induced left varicocele. Neurol. Endocrinol. Lett. 2003, 24, 86–90. [Google Scholar]
- Ateşşahin, A.; Sahna, E.; Türk, G.; Ceribaşi, A.O.; Yilmaz, S.; Yüce, A.; Bulmuş, O. Chemoprotective effect of melatonin against cisplatin-induced testicular toxicity in rats. J. Pineal. Res. 2006, 41, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Ilbey, Y.O.; Ozbek, E.; Simsek, A.; Otunctemur, A.; Cekmen, M.; Somay, A. Potential chemoprotective effect of melatonin in cyclo-phosphamide- and cisplatin-induced testicular damage in rats. Fertil. Steril. 2009, 92, 1124–1132. [Google Scholar] [CrossRef] [PubMed]
- Kanter, M. Protective effects of melatonin on testicular torsion/detorsion-induced ischemia-reperfusion injury in rats. Exp. Mol. Pathol. 2010, 89, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Lee, I.C.; Kim, S.H.; Moon, C.; Park, S.H.; Shin, D.H.; Kim, S.H.; Park, S.C.; Kim, H.C.; Kim, J.C. Melatonin attenuates doxorubicin-induced testicular toxicity in rats. Andrologia 2012, 44, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.L.; Wang, H.; Meng, C.; Zhao, X.F.; Zhang, C.; Zhang, Y.; Zhao, M.; Chen, Y.H.; Meng, X.H.; Xu, D.X. Melatonin alleviates cadmium-induced cellular stress and germ cell apoptosis in testes. J. Pineal Res. 2012, 52, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Lv, Z.; Xia, X.; Huang, D. Melatonin prevents testicular damage in hyperlipidaemic mice. Andrologia 2012, 44, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Rocha, C.S.; Martins, A.D.; Rato, L.; Silva, B.M.; Oliveira, P.F.; Alves, M.G. Melatonin alters the glycolytic profile of Sertoli cells: Implications for male fertility. Mol. Hum. Reprod. 2014, 20, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Espino, J.; Ortiz, Á.; Bejarano, I.; Lozano, G.M.; Monllor, F.; García, J.F.; Rodríguez, A.B.; Pariente, J.A. Melatonin protects human spermatozoa from apoptosis via melatonin receptor- and extracellular signal-regulated kinase-mediated pathways. Fertil. Steril. 2011, 95, 2290–2296. [Google Scholar] [CrossRef] [PubMed]
- Yie, S.M.; Daya, S.; Brown, G.M.; Deys, L.; YoungLai, E.V. Melatonin and aromatase stimulating activity of human seminal plasma. Andrologia 1991, 23, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Awad, H.; Halawa, F.; Mostafa, T.; Atta, H. Melatonin hormone profile in infertile males. Int. J. Androl. 2006, 29, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Frungieri, M.B.; Gonzalez-Calvar, S.I.; Rubio, M.; Ozu, M.; Lustig, L.; Calandra, R.S. Serotonin in golden hamster testes: Testicular levels, immunolocalization, and role during sexual development and photoperiodic regression-recrudescence transition. Neuroendocrinology 1999, 69, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Frungieri, M.B.; Zitta, K.; Pignataro, O.P.; Gonzalez-Calvar, S.I.; Calandra, R.S. Interactions between testicular serotoninergic, catecholaminergic, and corticotropin-releasing hormone systems modulating cAMP and testosterone production in the golden hamster. Neuroendocrinology 2002, 76, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Valenti, S.; Fazzuoli, L.; Giordano, G.; Giusti, M. Changes in binding of iodomelatonin to membranes of Leydig steroidogenesis after prolonged in vitro exposure to melatonin. Int. J. Androl. 2001, 24, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Valenti, S.; Guido, R.; Giusti, M.; Giordano, G. In vitro acute and prolonged effects of melatonin on purified rat Leydig cell steroidogenesis and adenosine 3′,5′-monophosphate production. Endocrinology 1995, 136, 5357–5362. [Google Scholar] [CrossRef] [PubMed]
- Frungieri, M.B.; Gonzalez-Calvar, S.I.; Calandra, R.S. Polyamine levels in testes and seminal vesicles from adult golden hamsters during gonadal regression recrudescence. J. Androl. 1996, 17, 683–691. [Google Scholar] [PubMed]
- Frungieri, M.B; Gonzalez-Calvar, S.I.; Bartke, A.; Calandra, R.S. Influence of age and photoperiod on steroidogenic function of the testis in the golden hamster. Int. J. Androl. 1999, 22, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Sinha Hikim, A.P.; Amador, A.G.; Bartke, A.; Russell, L.D. Structure/function relationships in active and inactive hamster Leydig cells: A correlative morphometric and endocrine study. Endocrinology 1989, 125, 1844–1856. [Google Scholar] [CrossRef] [PubMed]
- Sinha Hikim, A.P.; Chandrashekar, V.; Bartke, A.; Russell, L.D. Sentinels of Leydig cell structural and functional changes in golden hamsters in early testicular regression and recrudescence. Int. J. Androl. 1993, 16, 324–342. [Google Scholar] [CrossRef] [PubMed]
- Saez, J.M. Leydig cells: Endocrine, paracrine, and autocrine regulation. Endocr. Rev. 1994, 15, 574–626. [Google Scholar] [CrossRef] [PubMed]
- Valenti, S.; Thellung, S.; Florio, T.; Giusti, M.; Schettini, G.; Giordano, G. A novel mechanism for the melatonin inhibition of testosterone secretion by rat Leydig cells: Reduction of GnRH-induced increase in cytosolic Ca2+. J. Mol. Endocrinol. 1999, 23, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Schulkin, J.; Denver, R.J. Evolutionarily conserved glucocorticoid regulation of corticotropin-releasing factor expression. Endocrinology 2008, 149, 2352–2360. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, A.; Tinajero, J.C.; Dufau, M.L. Corticotropin-releasing factor is produced by rat Leydig cells and has a major local antireproductive role in the testis. Endocrinology 1990, 127, 1541–1543. [Google Scholar] [CrossRef] [PubMed]
- Dufau, M.L.; Tinajero, J.C.; Fabbri, A. Corticotropin-releasing factor: An antireproductive hormone of the testis. FASEB J. 1993, 7, 299–307. [Google Scholar] [PubMed]
- Tsai-Morris, C.H.; Buczko, E.; Geng, Y.; Gamboa-Pinto, A.; Dufau, M.L. The genomic structure of the rat corticotropin releasing factor receptor. J. Biol. Chem. 1996, 271, 14519–14525. [Google Scholar] [PubMed]
- Heinrich, N.; Meyer, M.R.; Furkert, J.; Sasse, A.; Beyermann, M.; Bonigk, W.; Berger, H. Corticotropin-releasing factor (CRF) agonists stimulate testosterone production in mouse Leydig cells through CRF receptor-1. Endocrinology 1998, 139, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Ulisse, S.; Fabbri, A.; Dufau, M.L. Corticotropin releasing factor receptors and actions in the rat Leydig cell. J. Biol. Chem. 1989, 264, 2156–2163. [Google Scholar] [PubMed]
- Huang, B.M.; Stocco, D.M.; Hutson, J.C.; Norman, R.L. Corticotropin-releasing hormone stimulates steroidogenesis in mouse Leydig cells. Biol. Reprod. 1995, 53, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.P.; Matzkin, M.E.; Terradas, C.; Ponzio, R.; Puigdomenech, E.; Levalle, O.; Calandra, R.S.; Frungieri, M.B. New insights into melatonin/CRH signaling in hamster Leydig cells. Gen. Comp. Endocrinol. 2012, 178, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Cui, P.; Yu, M.; Luo, Z.; Dai, M.; Han, J.; Xiu, R.; Yang, Z. Intracellular signaling pathways involved in cell growth inhibition of human umbilical vein endothelial cells by melatonin. J. Pineal Res. 2008, 44, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Kimball, S.R.; Abbas, A.; Jefferson, L.S. Melatonin represses oxidative stress induced activation of the MAP kinase and mTOR signaling pathways in H4IIE hepatoma cells through inhibition of Ras. J. Pineal Res. 2008, 44, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; Genovese, T.; Caminiti, R.; Bramanti, P.; Meli, R.; Cuzzocrea, S. Melatonin reduces stress-activated/mitogen-activated protein kinases in spinal cord injury. J. Pineal Res. 2009, 46, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; McDowell, M.; Pava, M.J.; Smith, J.A.; Reiter, R.J.; Woodward, J.J.; Varma, A.K.; Ray, S.K.; Banik, N.L. The inhibition of apoptosis by melatonin in VSC4.1 motoneurons exposed to oxidative stress, glutamate excitotoxicity, or TNF-α toxicity involves membrane melatonin receptors. J. Pineal Res. 2010, 48, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.C.; Tang, K.Q.; Fu, C.Z.; Riaz, H.; Zhang, Q.; Zan, L.S. Melatonin regulates the development and function of bovine Sertoli cells via its receptors MT1 and MT2. Anim. Reprod. Sci. 2014, 147, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Lyon, M.F.; Glenister, P.H.; Lamoreux, M.L. Normal spermatozoa from androgen-resistant germ cells of chimaeric mice and the role of androgen in spermatogenesis. Nature 1975, 258, 620–622. [Google Scholar] [CrossRef] [PubMed]
- McLachlan, R.I.; Wreford, N.G.; O’Donnell, L.; de Kretser, D.M.; Robertson, D.M. The endocrine regulation of spermatogenesis: Independent roles for testosterone and FSH. J. Endocrinol. 1996, 148, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rashed, R.M.; Mohamed, I.K.; EL-Alfy, S.H. Effects of two different doses of melatonin on the spermatogenic cells of rat testes: A light and electron microscopic study. Egypt J. Histol. 2010, 33, 819–835. [Google Scholar]
- Mehraein, F.; Negahdar, F. Morphometric evaluation of seminiferous tubules in aged mice testes after melatonin administration. Cell J. 2011, 13, 1–4. [Google Scholar] [PubMed]
- Ulisse, S.; Jannini, E.A.; Pepe, M.; de Matteis, S.; D’Armiento, M. Thyroid hormone stimulates glucose transport and GLUT1 mRNA in rat Sertoli cells. Mol. Cell. Endocrinol. 1992, 87, 131–137. [Google Scholar] [CrossRef]
- Carosa, E.; Radico, C.; Giansante, N.; Rossi, S.; D’Adamo, F.; di Stasi, S.M.; Lenzi, A.; Jannini, E.A. Ontogenetic profile and thyroid hormone regulation of type-1 and type-8 glucose transporters in rat Sertoli cells. Int. J. Androl. 2005, 28, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Galardo, M.N.; Riera, M.F.; Pellizzari, E.H.; Cigorraga, S.B.; Meroni, S.B. The AMP-activated protein kinase activator, 5-aminoimidazole-4-carboxamide-1-b-d-ribonucleoside, regulates lactate production in rat Sertoli cells. J. Mol. Endocrinol. 2007, 39, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, R.P.; Brunengraber, H. Contributions of cytosolic and mitochondrial acetyl-CoA syntheses to the activation of lipogenic acetate in rat liver. Adv. Exp. Med. Biol. 1980, 132, 413–418. [Google Scholar] [PubMed]
- Aitken, R.J.; Roman, S.D. Antioxidant systems and oxidative stress in the testes. Oxid. Med. Cell Longev. 2008, 1, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Agbaje, I.M.; Rogers, D.A.; McVicar, C.M.; McClure, N.; Atkinson, A.B.; Mallidis, C.; Lewis, S.E. Insulin dependant diabetes mellitus: Implications for male reproductive function. Hum. Reprod. 2007, 22, 1871–1877. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.P.; Windschüttl, S.; Matzkin, M.E.; Rey-Ares, V.; Terradas, C.; Ponzio, R.; Puigdomenech, E.; Levalle, O.; Calandra, R.S.; Mayerhofer, A.; et al. Reactive oxygen species (ROS) production triggered by prostaglandin D2 (PGD2) regulates lactate dehydrogenase (LDH) expression/activity in TM4 Sertoli cells. Mol. Cell. Endocrinol. 2016, 434, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Vico, A.; Lardone, P.J.; Alvarez-Sánchez, N.; Rodríguez-Rodríguez, A.; Guerrero, J.M. Melatonin: Buffering the immune system. Int. J. Mol. Sci. 2013, 14, 8638–8683. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Melatonin and circadian oscillators in aging—A dynamic approach to the multiply connected players. In A Systems Biology Perspective; Interdiscipl. Top. Gerontol.; Yashin, A.I., Jazwinski, S.M., Eds.; Basel Karger: New Orleans, LA, USA, 2015; Volume 40, pp. 128–140. [Google Scholar] [CrossRef]
- Frungieri, M.B.; Calandra, R.S.; Rossi, S.P. Melatonin Induces the Expression of Cyclooxygenase 2 (Cox2) and Lipocalin-Type Prostaglandin D Synthase (L-PGDS) in Murine TM4 Sertoli Cells; Instituto de Biología y Medicina Experimental, CONICET: Buenos Aires, Argentina, 2017. [Google Scholar]
- Niu, B.; Li, B.; Wu, C.; Wu, J.; Yan, Y.; Shang, R.; Bai, C.; Li, G.; Hua, J. Melatonin promotes goat spermatogonia stem cells (SSCs) proliferation by stimulating glial cell line-derived neurotrophic factor (GDNF) production in Sertoli cells. Oncotarget 2016, 7, 77532–77542. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, J.M.; Reiter, R.J. A brief survey of pineal gland-immune system interrelationships. Endocr. Res. 1992, 18, 91–113. [Google Scholar] [CrossRef] [PubMed]
- Calvo, J.R.; Rafii-el-Idrissi, M.; Pozo, D.; Guerrero, J.M. Immunomodulatory role of melatonin: Specific binding sites in human and rodent lymphoid cells. J. Pineal Res. 1995, 18, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, V.; Maestroni, G.J.M.; Cardinali, D.P.; Esquifino, A.I.; Perumal, S.R.; Miller, S.C. Melatonin, immune function and aging. Immun. Ageing 2005, 2, 17. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, M.D.; Mora-Santos, M.; Naji, L.; Carrascosa-Salmoral, M.P.; Naranjo, M.C.; Clavo, J.R. Evidence of melatonin synthesis and release by mast cells. Posible modulatory role on inflammation. Pharmacol. Res. 2010, 62, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Winnall, W.R.; Hedger, M.P. Phenotypic and functional heterogeneity of the testicular macrophage population: A new regulatory model. Reprod. Immunol. 2013, 97, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Yee, J.B.; Hutson, J.C. Testicular macrophages: Isolation, characterization and hormonal responsiveness. Biol. Reprod. 1983, 29, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.R.; Hedger, M.P.; Risbridger, G.P. The effect of testicular macrophages and interleukin-1 on testosterone production by purified adult rat Leydig cells cultured under in vitro maintenance conditions. Endocrinology 1993, 132, 186–192. [Google Scholar] [CrossRef] [PubMed]
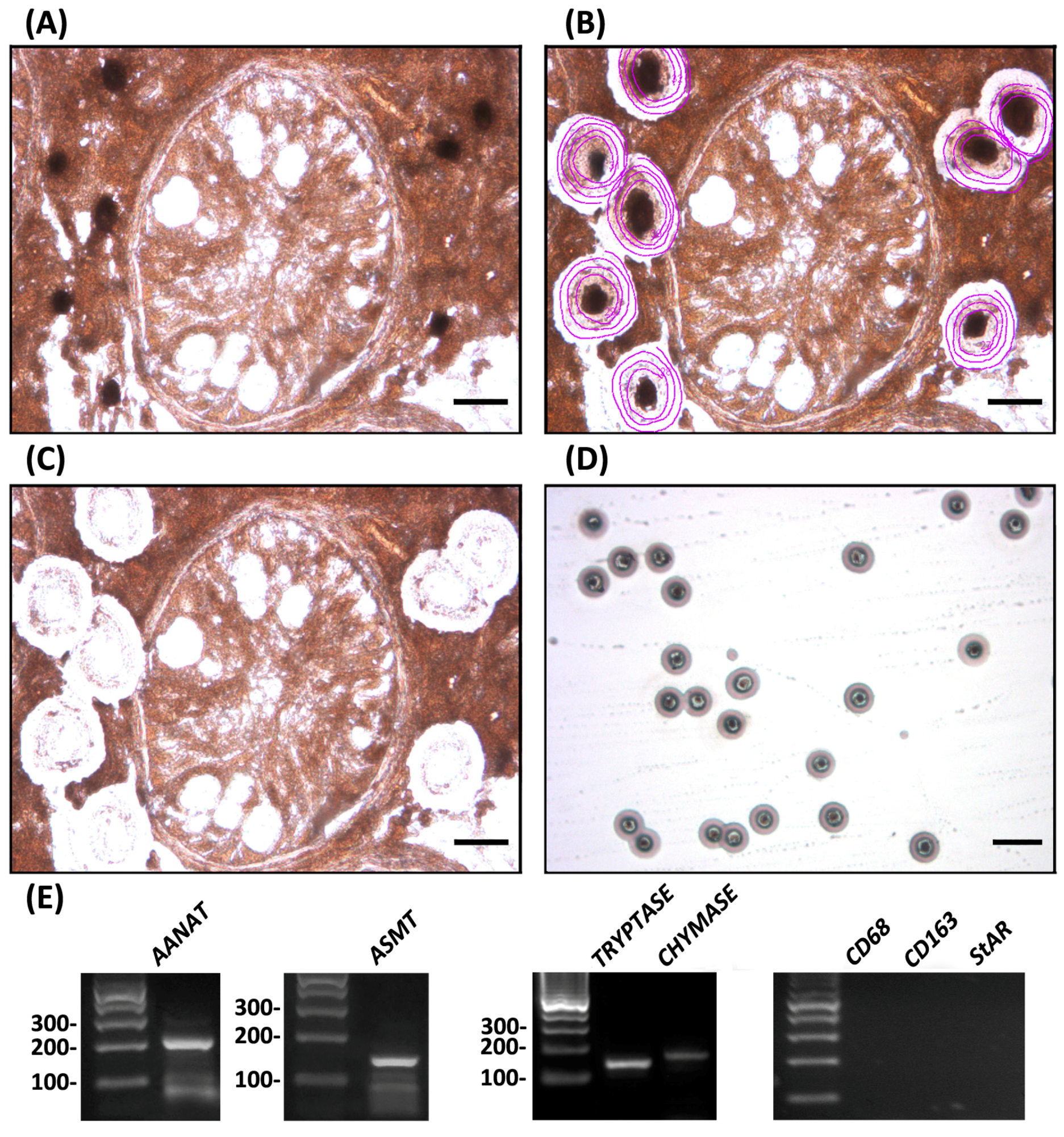
- Meineke, V.; Frungieri, M.B.; Jessberger, B.; Vogt, H.; Mayerhofer, A. Human testicular mast cells contain tryptase: Increased mast cell number and altered distribution in the testes of infertile men. Fertil. Steril. 2000, 74, 239–244. [Google Scholar] [CrossRef]
- Meroni, S.B.; Suburo, A.M.; Cigorraga, S.B. Interleukin-1β regulates nitric oxide production and γ-glutamyl transpeptidase activity in Sertoli cells. J. Androl. 2000, 21, 855–861. [Google Scholar] [PubMed]
- Frungieri, M.B.; Calandra, R.S.; Lustig, L.; Meineke, V.; Köhn, F.M.; Vogt, H.J.; Mayerhofer, A. Number, distribution pattern, and identification of macrophages in the testes of infertile men. Fertil. Steril. 2002, 78, 298–306. [Google Scholar] [CrossRef]
- Theas, M.S.; Rival, C.; Jarazo-Dietrich, S.; Jacobo, P.; Guazzone, V.A.; Lustig, L. Tumour necrosis factor alpha released by testicular macrophages induces apoptosis of germ cells in autoimmune orchitis. Hum. Reprod. 2008, 23, 1865–1872. [Google Scholar] [CrossRef] [PubMed]
- Jacobo, P.; Guazzone, V.A.; Theas, M.S.; Lustig, L. Testicular autoimmunity. Autoimmun. Rev. 2011, 10, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Winnall, W.R.; Muir, J.A.; Hedger, M.P. Differential responses of epithelial Sertoli cells of the rat testis to Toll-like receptor 2 and 4 ligands: Implications for studies of testicular inflammation using bacterial lipopolysaccharides. Innate Immun. 2011, 17, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mauriño, S.; Gonzalez-Haba, M.G.; Calvo, J.R.; Rafii-El-Idrissi, M.; Sanchez-Margalet, V.; Goberna, R.; Guerrero, J.M. Melatonin enhances IL-2, IL-6, and IFN-γ production by human circulating CD4+ cells: A possible nuclear receptor-mediated mechanism involving T helper type 1 lymphocytes and monocytes. J. Immunol. 1997, 159, 574–581. [Google Scholar] [PubMed]
- Morrey, K.M.; McLachlan, J.A.; Serkin, C.D.; Bakouche, O. Activation of human monocytes by the pineal hormone melatonin. J. Immunol. 1994, 153, 2671–2680. [Google Scholar] [PubMed]
- Barjavel, M.J.; Mamdouh, Z.; Raghbate, N.; Bakouche, O. Differential expression of the melatonin receptor in human monocytes. J. Immunol. 1998, 160, 1191–1197. [Google Scholar] [PubMed]
- Lissoni, P.; Rovelli, F.; Brivio, F.; Brivio, O.; Fumagalli, L. Circadian secretions of IL-2, IL-12, IL-6 and IL-10 in relation to the light/dark rhythm of the pineal hormone melatonin in healthy humans. Nat. Immun. 1998, 16, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, J.; Singh, J.; Lea, R.W.; Skwarlo-Sonta, K. Effect of melatonin on phagocytic activity and intracellular free calcium concentration in testicular macrophages from normal and streptozotocin-induced diabetic rats. Mol. Cell. Biochem. 2005, 275, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Izzo, G.; d'Istria, M.; Serino, I.; Minucci, S. Inhibition of the increased 17β-estradiol-induced mast cell number by melatonin in the testis of the frog Rana esculenta, in vivo and in vitro. J. Exp. Biol. 2004, 207, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Irani, A.M.; Schwartz, L.B. Human mast cell heterogeneity. Allergy Proc. 1994, 15, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Weidinger, S.; Mayerhofer, A.; Frungieri, M.B.; Meineke, V.; Ring, J.; Kohn, F.M. Mast cell–sperm interaction: Evidence for tryptase and proteinase-activated receptors in the regulation of sperm motility. Hum. Reprod. 2003, 18, 2519–2524. [Google Scholar] [CrossRef] [PubMed]
- Frungieri, M.B.; Weidinger, S.; Meineke, V.; Kohn, F.M.; Mayerhofer, A. Proliferative action of mast cell tryptase is mediated by PAR2, COX2, prostaglandins and PPARγ: Possible relevance to human fibrotic disorders. Proc. Natl. Acad. Sci. USA 2002, 99, 15072–15077. [Google Scholar] [CrossRef] [PubMed]
- Frungieri, M.B.; Albrecht, M.; Raemsch, R.; Mayerhofer, A. The action of the mast cell product tryptase on cyclooxygenase-2 (COX2) and subsequent fibroblast proliferation involves activation of the extracellular signal-regulated kinase isoforms 1 and 2 (erk1/2). Cell Signal. 2005, 17, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Schell, C.; Albrecht, M.; Spillner, S.; Mayer, C.; Kunz, L.; Kohn, F.M.; Schwarzer, U.; Mayerhofer, A. 5-Deoxy-δ 12–14-prostaglandin-J2 induces hypertrophy and loss of contractility in human testicular peritubular cells: Implications for human male fertility. Endocrinology 2010, 151, 1257–1268. [Google Scholar] [CrossRef] [PubMed]
- Adam, M.; Schwarzer, J.U.; Kohn, F.M.; Strauss, L.; Poutanen, M.; Mayerhofer, A. Mast cell tryptase stimulates production of decorin by human testicular peritubular cells: Possible role of decorin in male infertility by interfering with growth factor signaling. Hum. Reprod. 2011, 26, 2613–2625. [Google Scholar] [CrossRef] [PubMed]
- Spinnler, K.; Frohlich, T.; Arnold, G.J.; Kunz, L.; Mayerhofer, A. Human tryptase cleaves pro-nerve growth factor (Pro-NGF): Hints of local, mast cell-dependent regulation of NGF/pro-NGF action. J. Biol. Chem. 2011, 286, 31707–31713. [Google Scholar] [CrossRef] [PubMed]
- Pérez, C.V.; Theas, M.S.; Jacobo, P.V.; Jarazo-Dietrich, S.; Guazzone, V.A.; Lustig, L. Dual role of immune cells in the testis: Protective or pathogenic for germ cells? Spermatogenesis 2013, 3, e23870. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, A.; Misro, M.M.; Aggarwal, A.; Sharma, R.K.; Nandan, D. Pathways involved in testicular germ cell apoptosis induced by H2O2 in vitro. FEBS J. 2009, 276, 870–881. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Osuna, C.; Gitto, E. Actions of melatonin in the reduction of oxidative stress. J. Biomed. Sci. 2000, 7, 444–458. [Google Scholar] [CrossRef] [PubMed]
- Mayo, J.C.; Sainz, R.M.; Antolin, I.; Herrera, F.; Martin, V.; Rodriguez, C. Melatonin regulation of antioxidant enzyme gene expression. Cell Mol. Life Sci. 2002, 59, 1706–1713. [Google Scholar] [CrossRef] [PubMed]
- Pandi-Perumal, S.R.; BaHammam, A.S.; Brown, G.M.; Spence, D.W.; Bharti, V.K.; Kaur, C.; Hardeland, R.; Cardinali, D.P. Melatonin antioxidative defense: Therapeutical implications for aging and neurodegenerative processes. Neurotox. Res. 2013, 23, 267–300. [Google Scholar] [CrossRef] [PubMed]

| Cell Types | Species/Cell Lines | Melotonin Effects | Receptor Types | Melatonin Concentrations | References | |
|---|---|---|---|---|---|---|
| Leydig cell | mouse | Inhibition of steroid production | Inhibition of StAR protein expression | Mt1 and/or Mt2 | 10 nM to 1 µM | [21] |
| MA-10 | Inhibition of progesterone production | Mt1 and/or Mt2 | 10 nM to 1 µM | [21] | ||
| Rat | Inhibition of steroid production | Inhibition of androgen production | - | 4 pM to 4 µM | [40] | |
| Inhibition of GnRH-dependent intracellular Ca2+release | - | 0.2 pM | [46] | |||
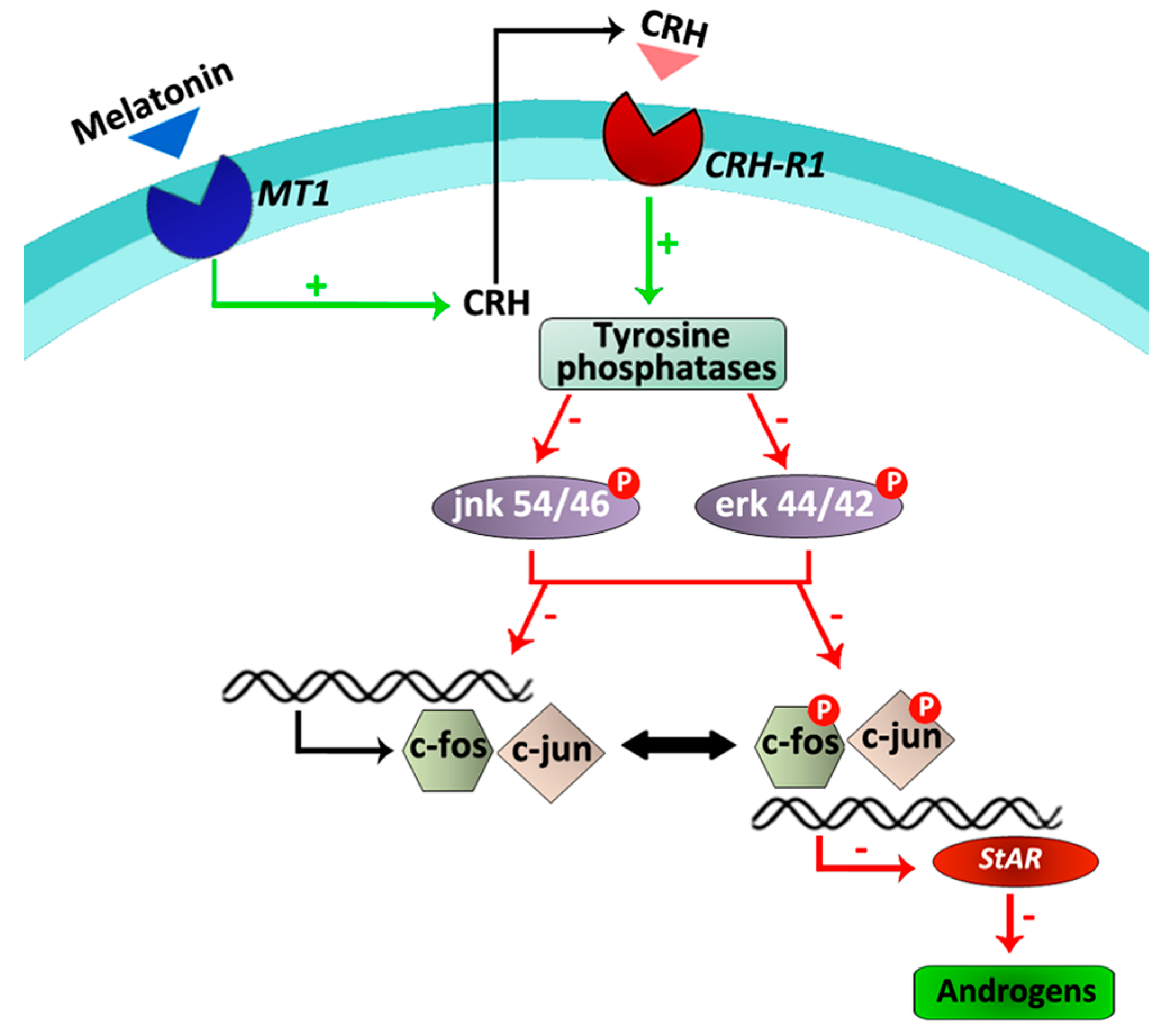
| Hamster | Inhibition of steroid production | Stimulation of CRH production | Mt1 | 1 and 10 µM | [12,54] | |
| Stimulation of tyrosine phosphatases activity | Mt1 | 1 and 10 µM | [54] | |||
| Inhibition of erk and jnk phosphorylation | Mt1 | 1 and 10 µM | [54] | |||
| Downregulation of c-fos and c-jun expression | Mt1 | 1 and 10 µM | [54] | |||
| Inhibition of StAR, Hsd3b and Hsd17b3 expression | Mt1 | 1 and 10 µM | [12,54] | |||
| Inhibition of cAMP generation | Mt1 | 1 µM | [12] | |||
| Inhibition of androgen production | Mt1 | 100 pM to 1 µM | [12,54] | |||
| Sertoli cell | Rat | Regulation of energy metabolism | Inhibition of basal lactate production | Mt1 and Mt2 | 1 mM | [33] |
| Upregulation of the insulin-stimulated lactate generation | Mt1 and Mt2 | 1 mM | [33] | |||
| Stimulation of GLUT1 protein expression and glucose consumption | 1 mM | [33] | ||||
| Inhibition of LDH protein expression and activity | Mt1 and Mt2 | 1 mM | [33] | |||
| Stimulation of acetate production | 1 mM | [33] | ||||
| Prevention of testicular damage | Regulation of intracellular redox state | Mt1 and Mt2 | 1 mM | [33] | ||
| Hamster | Prevention of testicular damage | Stimulation of the responsiveness to FSH during testicular development | - | 25 µg daily injection (1 to 15 wk) | [24] | |
| Bovine | Stimulation of cell growth/proliferation | Downregulation of mRNA P21 expression | Mt1 and Mt2 | 0.3 to 1 nM | [59] | |
| Regulation of spermatogenesis | Upregulation of Cyclin D1, Cyclin E, Pdgfa, Dhh, Ocludin and Claudin expression | Mt1 and Mt2 | 0.3 to 1 nM | [59] | ||
| Goat | Stimulation of SSCs proliferation | Stimulation of GDNF production | Mt1 and Mt2 | 1 nM and 1 µM | [73] | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frungieri, M.B.; Calandra, R.S.; Rossi, S.P. Local Actions of Melatonin in Somatic Cells of the Testis. Int. J. Mol. Sci. 2017, 18, 1170. https://doi.org/10.3390/ijms18061170
Frungieri MB, Calandra RS, Rossi SP. Local Actions of Melatonin in Somatic Cells of the Testis. International Journal of Molecular Sciences. 2017; 18(6):1170. https://doi.org/10.3390/ijms18061170
Chicago/Turabian StyleFrungieri, Mónica Beatriz, Ricardo Saúl Calandra, and Soledad Paola Rossi. 2017. "Local Actions of Melatonin in Somatic Cells of the Testis" International Journal of Molecular Sciences 18, no. 6: 1170. https://doi.org/10.3390/ijms18061170