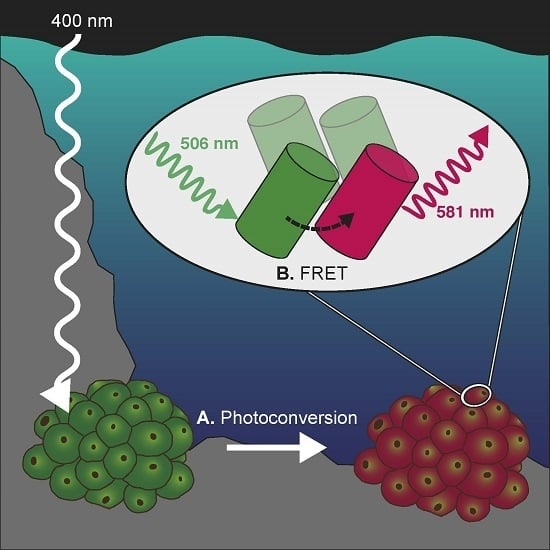
FRET-Mediated Long-Range Wavelength Transformation by Photoconvertible Fluorescent Proteins as an Efficient Mechanism to Generate Orange-Red Light in Symbiotic Deep Water Corals
Abstract
:1. Introduction
2. Results
2.1. Effects of Light Quality on Photoconvertible Red Fluorescent Proteins (pcRFPs)
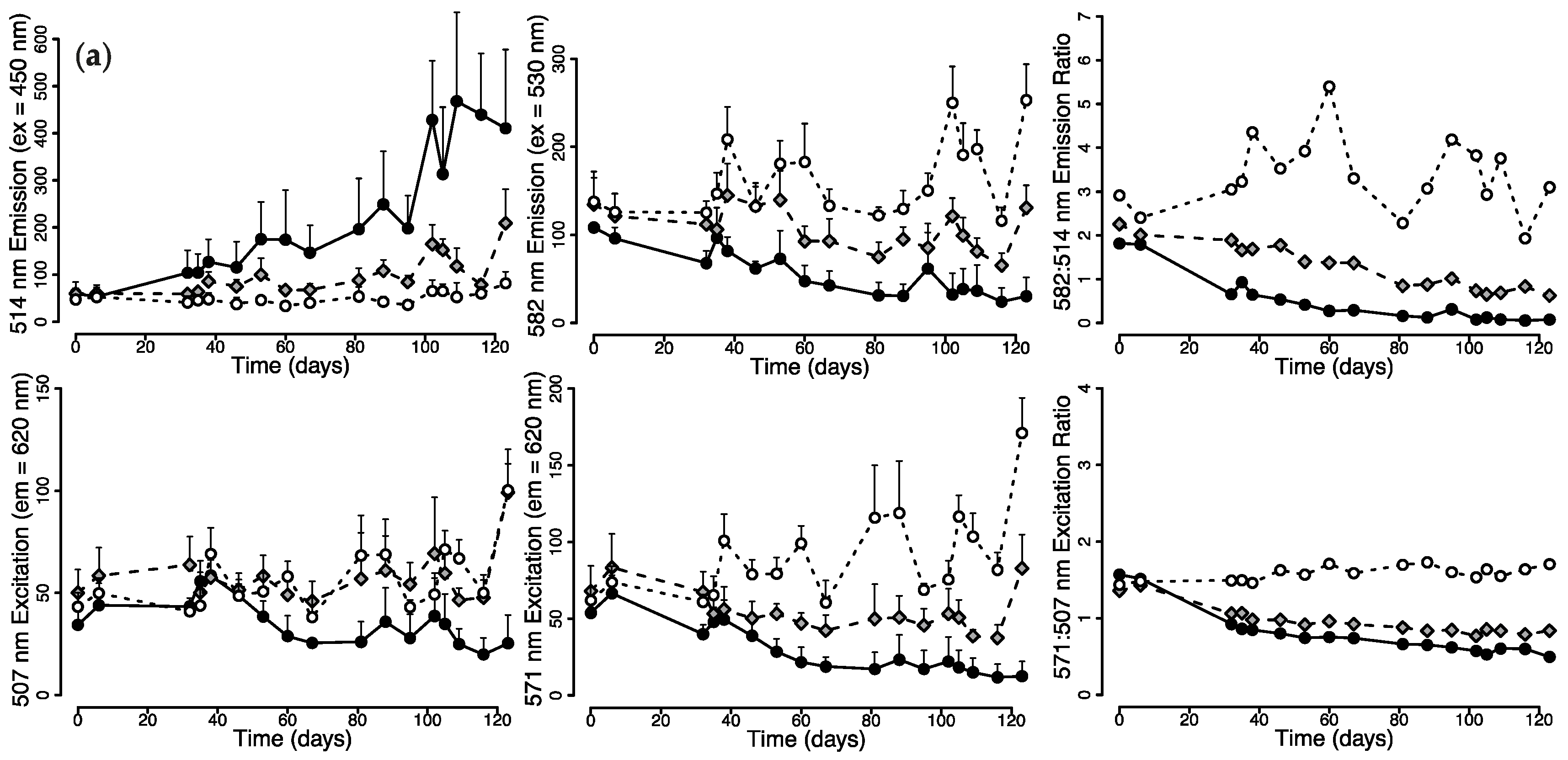
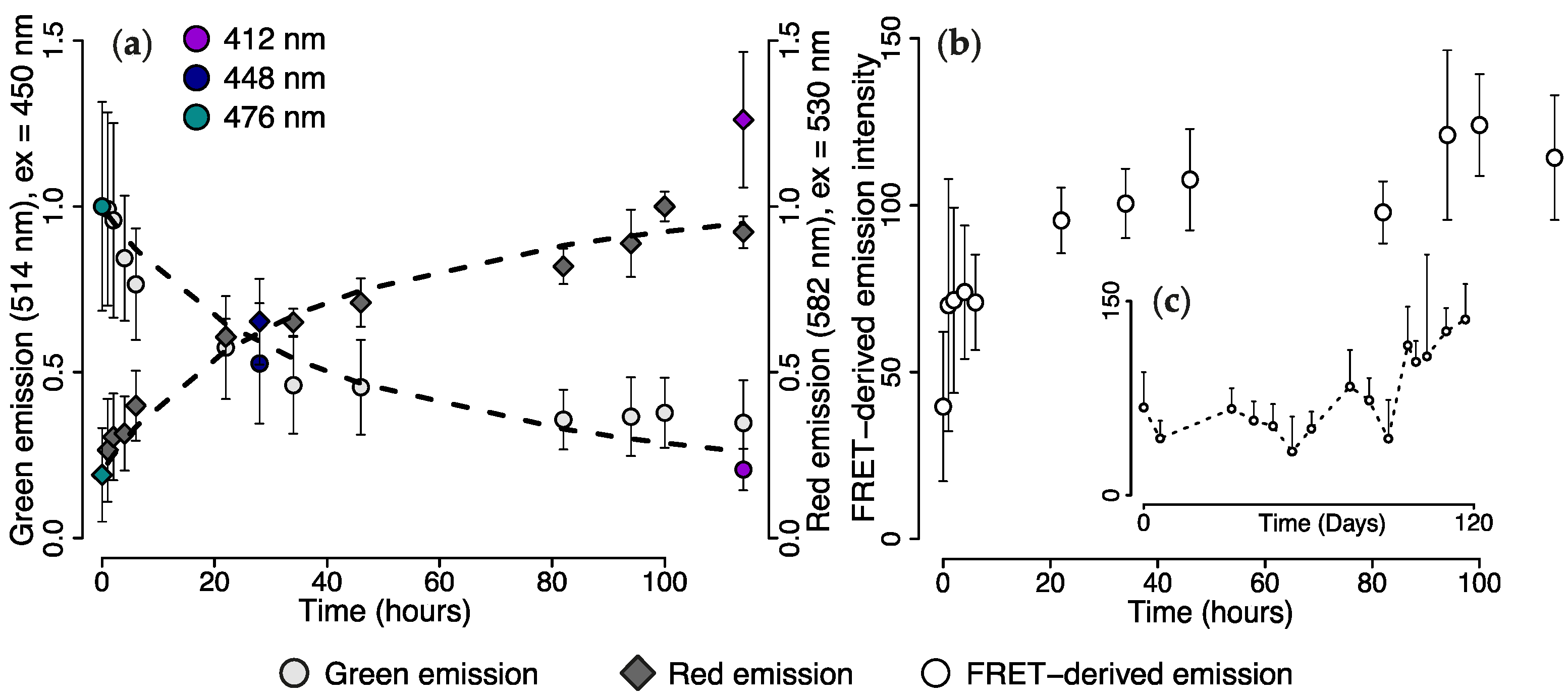
2.1.1. Changes in Live Tissue Fluorescence over Time
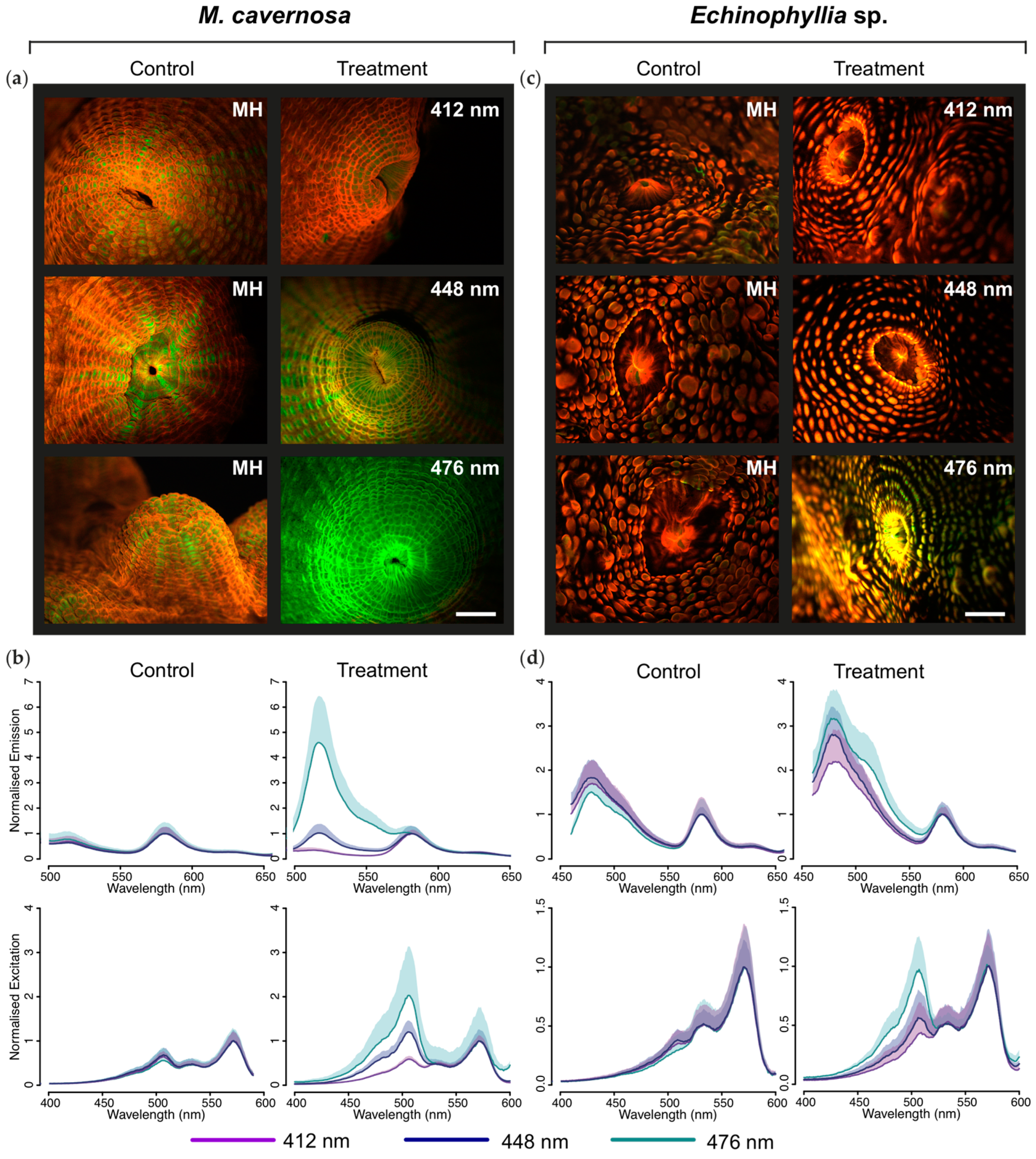
2.1.2. Microscopic and Spectroscopic Characterization of Tissue Fluorescence
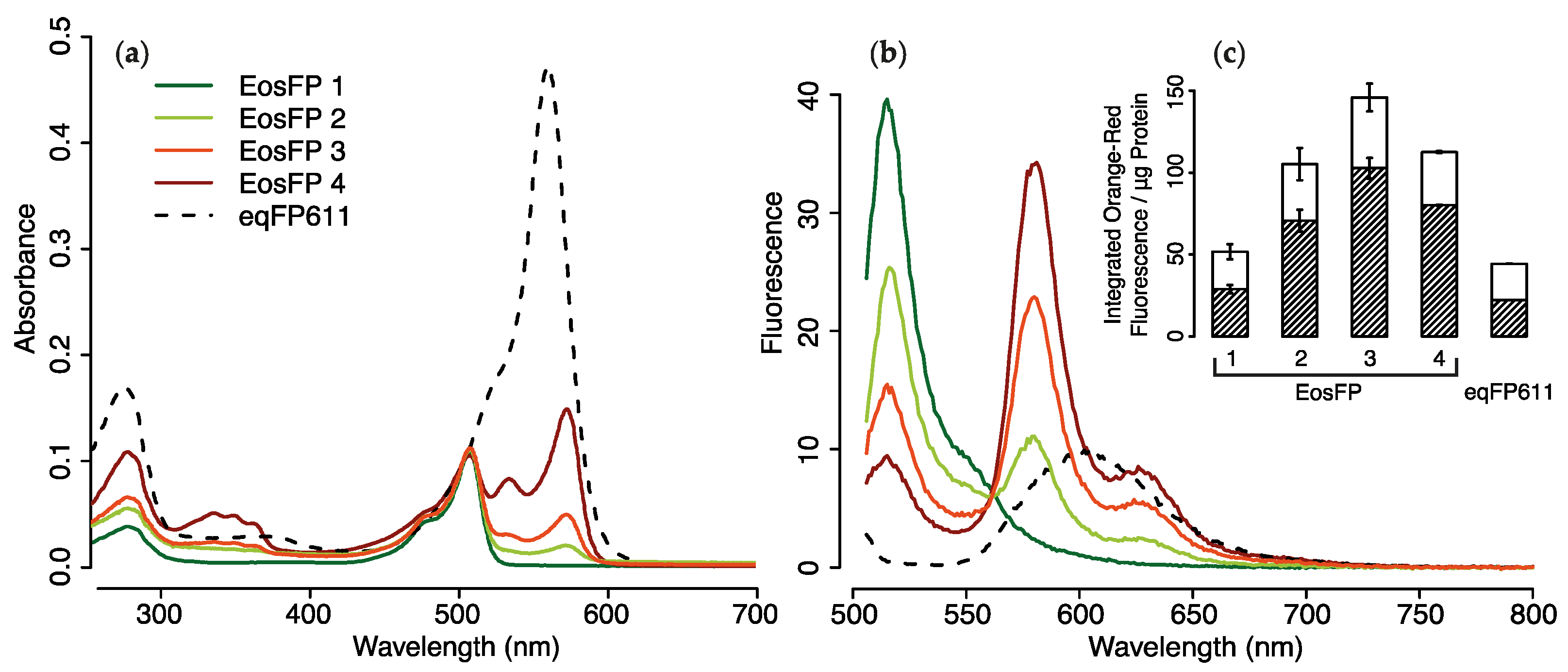
2.1.3. Purified pcRFPs
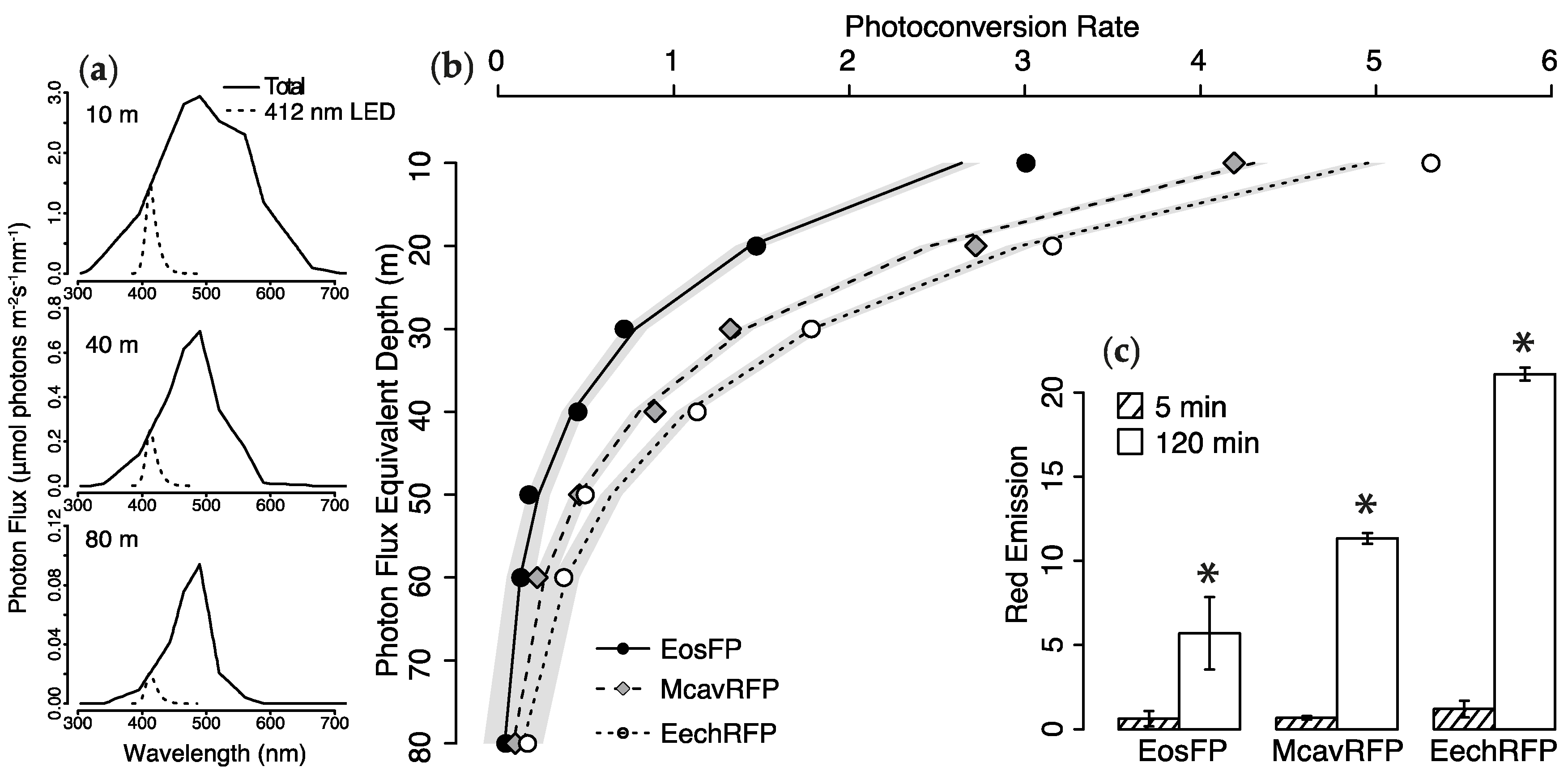
2.2. Photoconversion Along a Simulated Depth-Irradiance Gradient
2.3. Wavelength Transfer by Förster Resonance Energy Transfer (FRET)
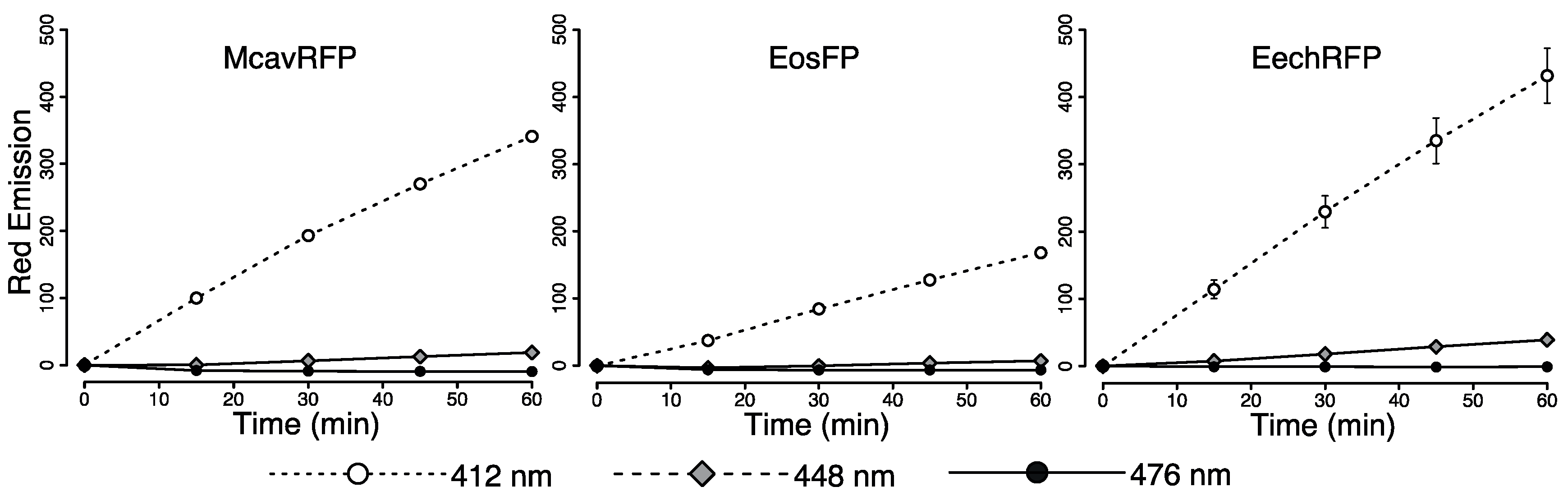
2.4. Efficiency of FRET-Mediated Wavelength Transfer
3. Discussion
3.1. Effects of Spectral Quality on Photoconversion of pcRFPs
3.2. Wavelength Transformation via FRET
4. Materials and Methods
4.1. Sample Culture and Aquarium Set up
4.2. Live Colony Fluorescence
4.3. Protein Expression and Purification
4.4. Photoconversion and Spectroscopy of Purified Proteins
4.5. Photoconversion Potential of Light Sources
4.6. Estimation of FRET in Live Colonies
4.7. Evaluation of Wavelength Conversion In Vitro
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dove, S.G.; Hoegh-Guldberg, O.; Ranganathan, S. Major colour patterns of reef-building corals are due to a family of GFP-like proteins. Coral Reefs 2001, 19, 197–204. [Google Scholar] [CrossRef]
- Oswald, F.; Schmitt, F.; Leutenegger, A.; Ivanchenko, S.; D’Angelo, C.; Salih, A.; Maslakova, S.; Bulina, M.; Schirmbeck, R.; Nienhaus, G.U.; et al. Contributions of host and symbiont pigments to the coloration of reef corals. FEBS J. 2007, 274, 1102–1122. [Google Scholar] [CrossRef] [PubMed]
- Alieva, N.O.; Konzen, K.A.; Field, S.F.; Meleshkevitch, E.A.; Hunt, M.E.; Beltran-Ramirez, V.; Miller, D.J.; Wiedenmann, J.; Salih, A.; Matz, M.V. Diversity and evolution of coral fluorescent proteins. PLoS ONE 2008, 3, e2680. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, C.; Smith, E.G.; Oswald, F.; Burt, J.; Tchernov, D.; Wiedenmann, J. Locally accelerated growth is part of the innate immune response and repair mechanisms in reef-building corals as detected by green fluorescent protein (GFP)-like pigments. Coral Reefs 2012, 31, 1045–1056. [Google Scholar] [CrossRef]
- Gittins, J.R.; D’Angelo, C.; Oswald, F.; Edwards, R.J.; Wiedenmann, J. Fluorescent protein-mediated colour polymorphism in reef corals: Multicopy genes extend the adaptation/acclimatization potential to variable light environments. Mol. Ecol. 2015, 24, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, O.; Johnson, F.H.; Saiga, Y. Extraction, purification and properties of aequorin, a bioluminescent protein from the luminous hydromedusan, Aequorea. J. Cell. Comp. Physiol. 1962, 59, 223–239. [Google Scholar] [CrossRef] [PubMed]
- Matz, M.V.; Fradkov, A.F.; Labas, Y.A.; Savitsky, A.P.; Zaraisky, A.G.; Markelov, M.L.; Lukyanov, S.A. Fluorescent proteins from nonbioluminescent Anthozoa species. Nat. Biotechnol. 1999, 17, 969–973. [Google Scholar] [CrossRef] [PubMed]
- Wiedenmann, J.; Elke, C.; Spindler, K.D.; Funke, W. Cracks in the β-can: Fluorescent proteins from Anemonia sulcata (Anthozoa, Actinaria). Proc. Natl. Acad. Sci. USA 2000, 97, 14091–14096. [Google Scholar] [CrossRef] [PubMed]
- Wiedenmann, J.; Schenk, A.; Röcker, C.; Girod, A.; Spindler, K.D.; Nienhaus, G.U. A far-red fluorescent protein with fast maturation and reduced oligomerization tendency from Entacmaea quadricolor (Anthozoa, Actinaria). Proc. Natl. Acad. Sci. USA 2002, 99, 11646–11651. [Google Scholar] [CrossRef] [PubMed]
- Wiedenmann, J.; Ivanchenko, S.; Oswald, F.; Nienhaus, G.U. Identification of GFP-like proteins in nonbioluminescent, azooxanthellate Anthozoa opens new perspectives for bioprospecting. Mar. Biotechnol. 2004, 6, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Moss, L.G.; Phillips, G.N. The molecular structure of green fluorescent protein. Nat. Biotechnol. 1996, 14, 1246–1251. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, O. Structure of the chromophore of Aequorea green fluorescent protein. FEBS Lett. 1979, 104, 220–222. [Google Scholar] [CrossRef]
- Shagin, D.A.; Barsova, E.V.; Yanushevich, Y.G.; Fradkov, A.F.; Lukyanov, K.A.; Labas, Y.A.; Semenova, T.N.; Ugalde, J.A.; Meyers, A.; Nunez, J.M.; et al. GFP-like proteins as ubiquitous metazoan superfamily: Evolution of functional features and structural complexity. Mol. Biol. Evol. 2004, 21, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Nienhaus, K.; Nienhaus, G.U.; Wiedenmann, J.; Nar, H. Structural basis for photo-induced protein cleavage and green-to-red conversion of fluorescent protein EosFP. Proc. Natl. Acad. Sci. USA 2005, 102, 9156–9159. [Google Scholar] [CrossRef] [PubMed]
- Nienhaus, G.U.; Wiedenmann, J. Structure, dynamics and optical properties of fluorescent proteins: Perspectives for marker development. ChemPhysChem 2009, 10, 1369–1379. [Google Scholar] [CrossRef] [PubMed]
- Cody, C.W.; Prasher, D.C.; Westler, W.M.; Prendergast, F.G.; Ward, W.W. Chemical structure of the hexapeptide chromophore of the Aequorea green-fluorescent protein. Biochemistry 1993, 32, 1212–1218. [Google Scholar] [CrossRef] [PubMed]
- Heim, R.; Prasher, D.C.; Tsien, R.Y. Wavelength mutations and posttranslational autoxidation of green fluorescent protein. Proc. Natl. Acad. Sci. USA 1994, 91, 12501–12504. [Google Scholar] [CrossRef] [PubMed]
- Nienhaus, G.U.; Nienhaus, K.; Hölzle, A.; Ivanchenko, S.; Renzi, F.; Oswald, F.; Wolff, M.; Schmitt, F.; Röcker, C.; Vallone, B.; et al. Photoconvertible fluorescent protein EosFP: Biophysical properties and cell biology applications. Photochem. Photobiol. 2006, 82, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Ando, R.; Hama, H.; Yamamoto-Hino, M.; Mizuno, H.; Miyawaki, A. An optical marker based on the UV-induced green-to-red photoconversion of a fluorescent protein. Proc. Natl. Acad. Sci. USA 2002, 99, 12651–12656. [Google Scholar] [CrossRef] [PubMed]
- Wiedenmann, J.; Ivanchenko, S.; Oswald, F.; Schmitt, F.; Röcker, C.; Salih, A.; Spindler, K.D.; Nienhaus, G.U. EosFP, a fluorescent marker protein with UV-inducible green-to-red fluorescence conversion. Proc. Natl. Acad. Sci. USA 2004, 101, 15905–15910. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, H.; Mal, T.K.; Tong, K.I.; Ando, R.; Furuta, T.; Ikura, M.; Miyawaki, A. Photo-induced peptide cleavage in the green-to-red conversion of a fluorescent protein. Mol. Cell 2003, 12, 1051–1058. [Google Scholar] [CrossRef]
- Labas, Y.A.; Gurskaya, N.G.; Yanushevich, Y.G.; Fradkov, A.F.; Lukyanov, K.A.; Lukyanov, S.A.; Matz, M.V. Diversity and evolution of the green fluorescent protein family. Proc. Natl. Acad. Sci. USA 2002, 99, 4256–4261. [Google Scholar] [CrossRef] [PubMed]
- Wacker, S.A.; Oswald, F.; Wiedenmann, J.; Knöchel, W. A green to red photoconvertible protein as an analyzing tool for early vertebrate development. Dev. Dyn. 2007, 236, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Wiedenmann, J.; Oswald, F.; Nienhaus, G.U. Fluorescent proteins for live cell imaging: Opportunities, limitations, and challenges. IUBMB Life 2009, 61, 1029–1042. [Google Scholar] [CrossRef] [PubMed]
- Adam, V.; Mizuno, H.; Grichine, A.; Hotta, J.; Yamagata, Y.; Moeyaert, B.; Nienhaus, G.U.; Miyawaki, A.; Bourgeois, D.; Hofkens, J. Data storage based on photochromic and photoconvertible fluorescent proteins. J. Biotechnol. 2010, 149, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Wiedenmann, J.; Röcker, C.; Funke, W. The morphs of Anemonia aff. sulcata (Cnidaria, Anthozoa) in particular consideration of the ectodermal pigments. In Verhandlungen der Gesellschaft für Ökologie; Pfadenhauer, J., Ed.; Spektrum Akademischer Verlag: Heidelber, Germany, 1999; pp. 497–503. [Google Scholar]
- Salih, A.; Larkum, A.; Cox, G.; Kuhl, M.; Hoegh-Guldberg, O. Fluorescent pigments in corals are photoprotective. Nature 2000, 408, 850–853. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.G.; D’Angelo, C.; Salih, A.; Wiedenmann, J. Screening by coral green fluorescent protein (GFP)-like chromoproteins supports a role in photoprotection of zooxanthellae. Coral Reefs 2013, 32, 463–474. [Google Scholar] [CrossRef]
- Salih, A.; Cox, G.; Larkum, A.W.D. Cellular organisation and spectral diversity of GFP-like proteins in live coral cells studied by single and multi-photon imaging and microspectroscopy. In Multiphoton Microscopy in the Biomedical Sciences III, Proceedings of the SPIE 4963, San Jose, CA, USA, 25 January 2003; SPIE: Bellingham, WA, USA, 2003; pp. 194–200. [Google Scholar]
- D’Angelo, C.; Denzel, A.; Vogt, A.; Matz, M.V.; Oswald, F.; Salih, A.; Nienhaus, G.U.; Wiedenmann, J. Blue light regulation of host pigment in reef-building corals. Mar. Ecol. Prog. Ser. 2008, 364, 97–106. [Google Scholar] [CrossRef]
- Vogt, A.; D’Angelo, C.; Oswald, F.; Denzel, A.; Mazel, C.H.; Matz, M.V.; Ivanchenko, S.; Nienhaus, G.U.; Wiedenmann, J. A green fluorescent protein with photoswitchable emission from the deep sea. PLoS ONE 2008, 3, e3766. [Google Scholar] [CrossRef] [PubMed]
- Kahng, S.E.; Hochberg, E.J.; Apprill, A.; Wagner, D.; Luck, D.G.; Perez, D.; Bidigare, R.R. Efficient light harvesting in deep-water zooxanthellate corals. Mar. Ecol. Prog. Ser. 2012, 455, 65–77. [Google Scholar] [CrossRef]
- Eyal, G.; Wiedenmann, J.; Grinblat, M.; D’Angelo, C.; Kramarsky-Winter, E.; Treibitz, T.; Ben-Zvi, O.; Shaked, Y.; Smith, T.B.; Harii, S.; et al. Spectral diversity and regulation of coral fluorescence in a mesophotic reef habitat in the Red Sea. PLoS ONE 2015, 10, e0128697. [Google Scholar] [CrossRef] [PubMed]
- Roth, M.S.; Padilla-Gamiño, J.L.; Pochon, X.; Bidigare, R.R.; Gates, R.D.; Smith, C.M.; Spalding, H.L. Fluorescent proteins in dominant mesophotic reef-building corals. Mar. Ecol. Prog. Ser. 2015, 521, 63–79. [Google Scholar] [CrossRef]
- Leutenegger, A.; D’Angelo, C.; Matz, M.V.; Denzel, A.; Oswald, F.; Salih, A.; Nienhaus, G.U.; Wiedenmann, J. It’s cheap to be colorful. FEBS J. 2007, 274, 2496–2505. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, C.; Wiedenmann, J. An experimental mesocosm for long-term studies of reef corals. J. Mar. Biol. Assoc. 2012, 92, 769–775. [Google Scholar] [CrossRef]
- Field, S.F.; Matz, M.V. Retracing evolution of red fluorescence in GFP-like proteins from Faviina corals. Mol. Biol. Evol. 2010, 27, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Wolf, H.; Barisas, B.G.; Dietz, K.J.; Seidel, T. Kaede for detection of protein oligomerization. Mol. Plant 2013, 6, 1453–1462. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.P.; Baird, A.H.; Bellwood, D.R.; Card, M.; Connolly, S.R.; Folke, C.; Grosberg, R.; Hoegh-Guldberg, O.; Jackson, J.B.C.; Kleypas, J.; et al. Climate change, human impacts, and the resilience of coral reefs. Science 2003, 301, 929–933. [Google Scholar] [CrossRef] [PubMed]
- Glynn, P.W. Coral reef bleaching: Facts, hypotheses and implications. Glob. Chang. Biol. 1996, 2, 495–509. [Google Scholar] [CrossRef]
- Bongaerts, P.; Ridgway, T.; Sampayo, E.M.; Hoegh-Guldberg, O. Assessing the “deep reef refugia” hypothesis: Focus on Caribbean reefs. Coral Reefs 2010, 29, 309–327. [Google Scholar] [CrossRef]
- Field, S.; Bulina, M.; Kelmanson, I.; Bielawski, J.; Matz, M. Adaptive evolution of multicolored fluorescent proteins in reef-building corals. J. Mol. Evol. 2006, 62, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Schlichter, D.; Fricke, H.W.; Weber, W. Light harvesting by wavelength transformation in a symbiotic coral of the Red Sea twilight zone. Mar. Biol. 1986, 91, 403–407. [Google Scholar] [CrossRef]
- Clarke, G.L.; James, H.L. Laboratory analysis of the selective absorption of light by sea water. J. Opt. Soc. Am. 1939, 29, 43–55. [Google Scholar] [CrossRef]
- Jerlov, N.G. Marine Optics; Elsevier: Amsterdam, The Netherlands, 1976. [Google Scholar]
- Salih, A.; Wiedenmann, J.; Matz, M.; Larkum, A.W.; Cox, G. Photoinduced activation of GFP-like proteins in tissues of reef corals. In Genetically Engineered Probes for Biomedical Applications, Proceedings of the SPIE 6098, San Jose, CA, USA, 24 January 2006; SPIE: Bellingham, WA, USA, 2006; pp. 60980B-1–60980B-12. [Google Scholar]
- Kirk, J.T.O. Light and Photosynthesis in Aquatic Ecosystems; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Gordon, G.W.; Berry, G.; Liang, X.H.; Levine, B.; Herman, B. Quantitative fluorescence resonance energy transfer measurements using fluorescence microscopy. Biophys. J. 1998, 74, 2702–2713. [Google Scholar] [CrossRef]
- Hoppe, A.; Christensen, K.; Swanson, J.A. Fluorescence Resonance Energy Transfer-based stoichiometry in living cells. Biophys. J. 2002, 83, 3652–3664. [Google Scholar] [CrossRef]
- Müller, S.M.; Galliardt, H.; Schneider, J.; Barisas, B.G.; Seidel, T. Quantification of Förster resonance energy transfer by monitoring sensitized emission in living plant cells. Front. Plant Sci. 2013, 4, 413. [Google Scholar] [CrossRef] [PubMed]
- Baird, G.S.; Zacharias, D.A.; Tsien, R.Y. Biochemistry, mutagenesis, and oligomerization of DsRed, a red fluorescent protein from coral. Proc. Natl. Acad. Sci. USA 2000, 97, 11984–11989. [Google Scholar] [CrossRef] [PubMed]
- Kuguru, B.; Chadwick, N.E.; Achituv, Y.; Zandbank, K.; Tchernov, D. Mechanisms of habitat segregation between corallimorpharians: Photosynthetic parameters and Symbiodinium types. Mar. Ecol. Prog. Ser. 2008, 369, 115–129. [Google Scholar] [CrossRef]
- Kuhl, M.; Cohen, Y.; Dalsgaard, T.; Jorgensen, B.B.; Revsbech, N.P. Microenvironment and photosynthesis of zooxanthellae in scleractinian corals studied with microsensors for O2, pH and light. Mar. Ecol. Prog. Ser. 1995, 117, 159–172. [Google Scholar] [CrossRef]
- Al-Horani, F.A.; Al-Moghrabi, S.M.; de Beer, D. Microsensor study of photosynthesis and calcification in the scleractinian coral, Galaxea fascicularis: Active internal carbon cycle. J. Exp. Mar. Biol. Ecol. 2003, 288, 1–15. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bollati, E.; Plimmer, D.; D’Angelo, C.; Wiedenmann, J. FRET-Mediated Long-Range Wavelength Transformation by Photoconvertible Fluorescent Proteins as an Efficient Mechanism to Generate Orange-Red Light in Symbiotic Deep Water Corals. Int. J. Mol. Sci. 2017, 18, 1174. https://doi.org/10.3390/ijms18071174
Bollati E, Plimmer D, D’Angelo C, Wiedenmann J. FRET-Mediated Long-Range Wavelength Transformation by Photoconvertible Fluorescent Proteins as an Efficient Mechanism to Generate Orange-Red Light in Symbiotic Deep Water Corals. International Journal of Molecular Sciences. 2017; 18(7):1174. https://doi.org/10.3390/ijms18071174
Chicago/Turabian StyleBollati, Elena, Daniel Plimmer, Cecilia D’Angelo, and Jörg Wiedenmann. 2017. "FRET-Mediated Long-Range Wavelength Transformation by Photoconvertible Fluorescent Proteins as an Efficient Mechanism to Generate Orange-Red Light in Symbiotic Deep Water Corals" International Journal of Molecular Sciences 18, no. 7: 1174. https://doi.org/10.3390/ijms18071174