A Novel Therapeutic Approach in the Treatment of Pulmonary Arterial Hypertension: Allium ursinum Liophylisate Alleviates Symptoms Comparably to Sildenafil
Abstract
:1. Introduction
2. Results
2.1. Mass Spectrometry
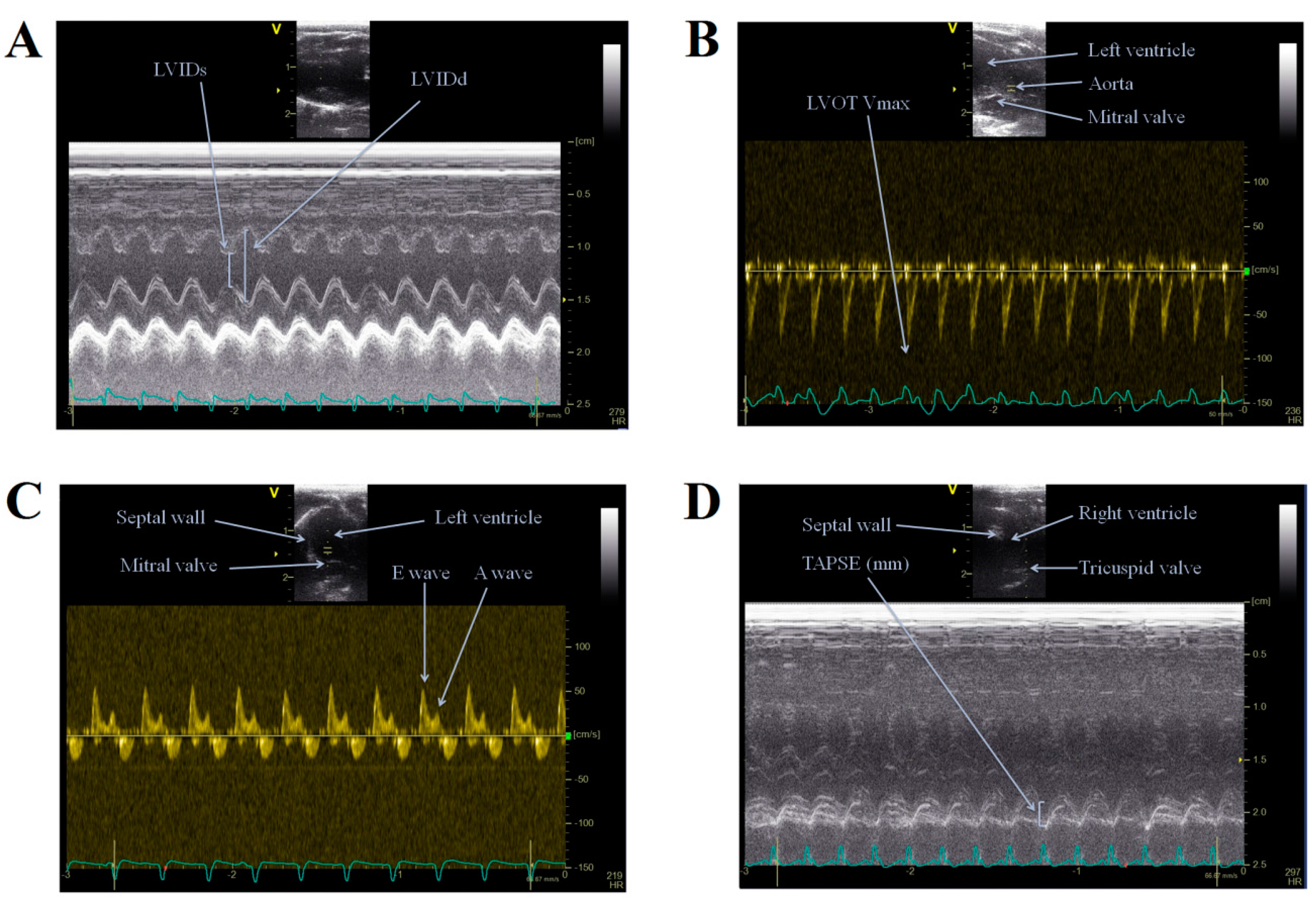
2.2. Echocardiography
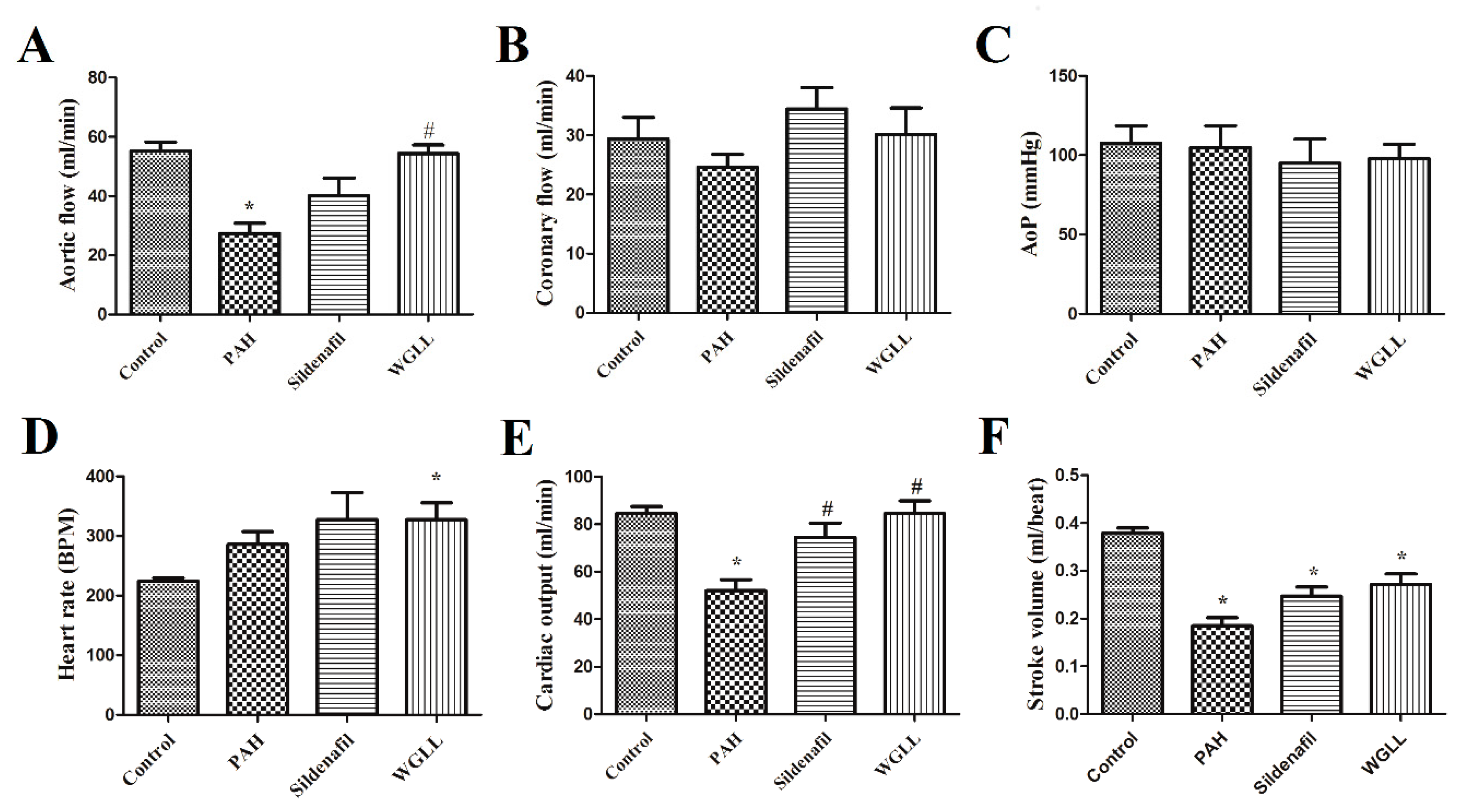
2.3. Effects of Monocrotaline (MCT) and Treatments on Isolated Left Ventricular Function
2.4. Body Mass
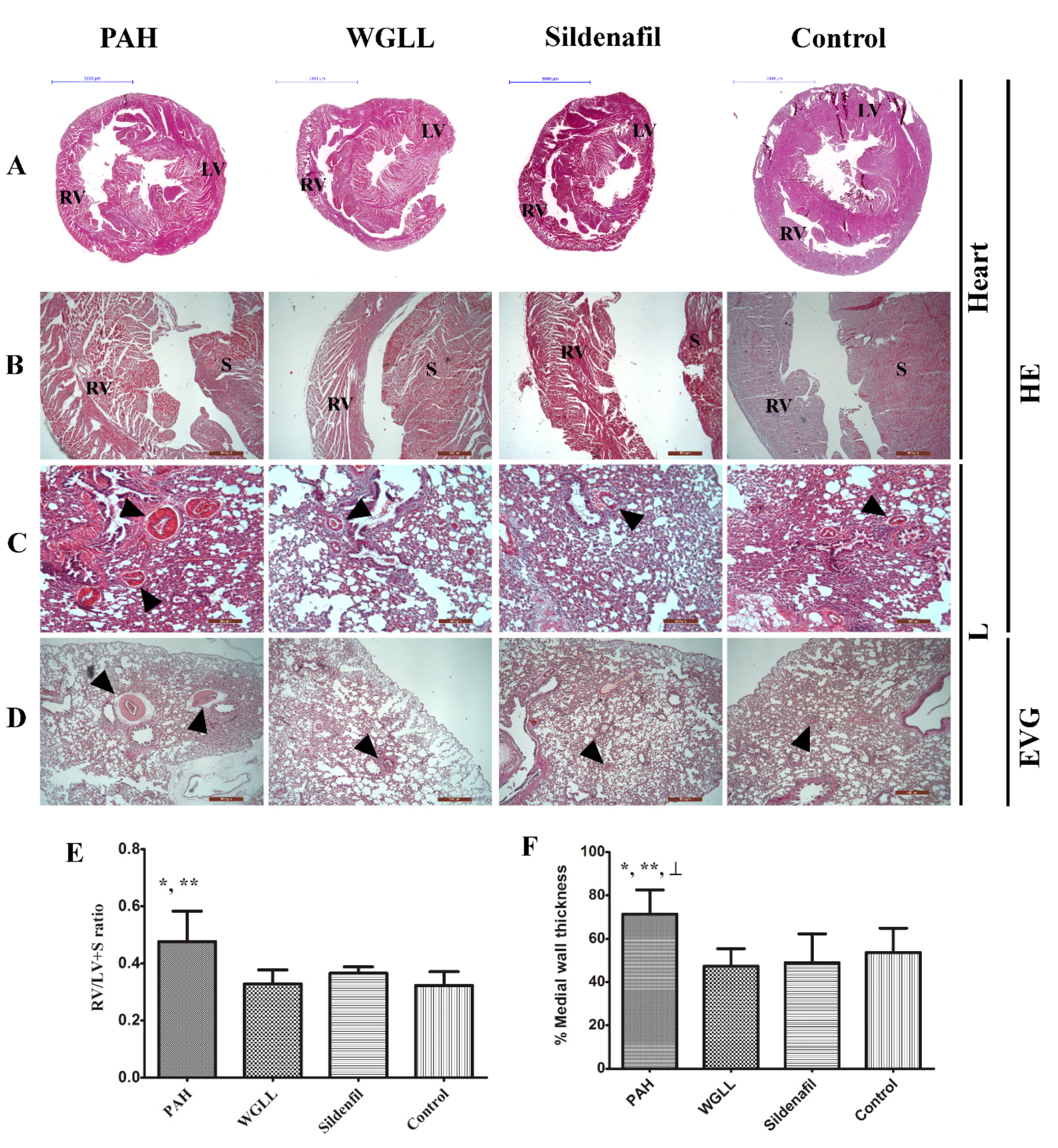
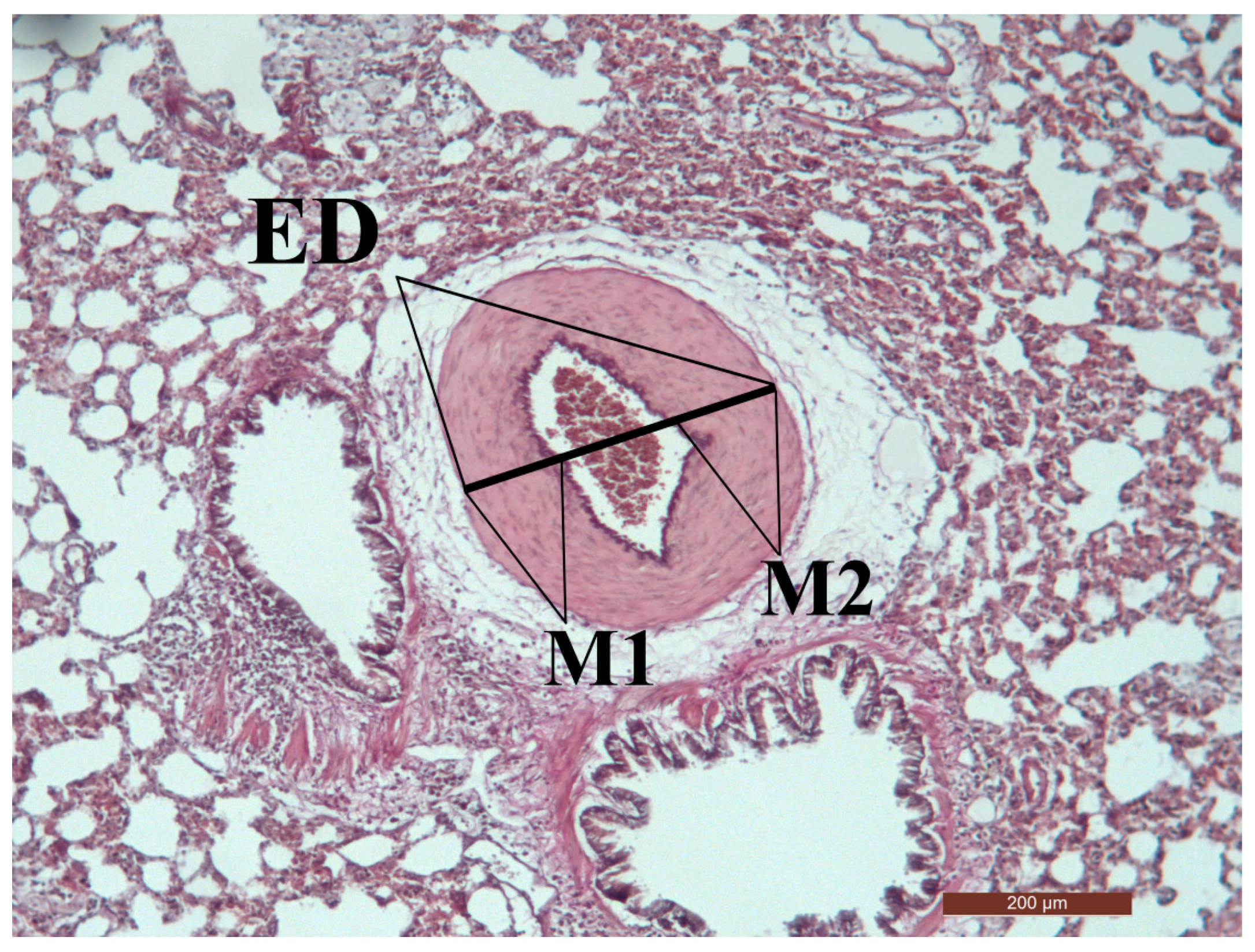
2.5. Microscopic Morphometry
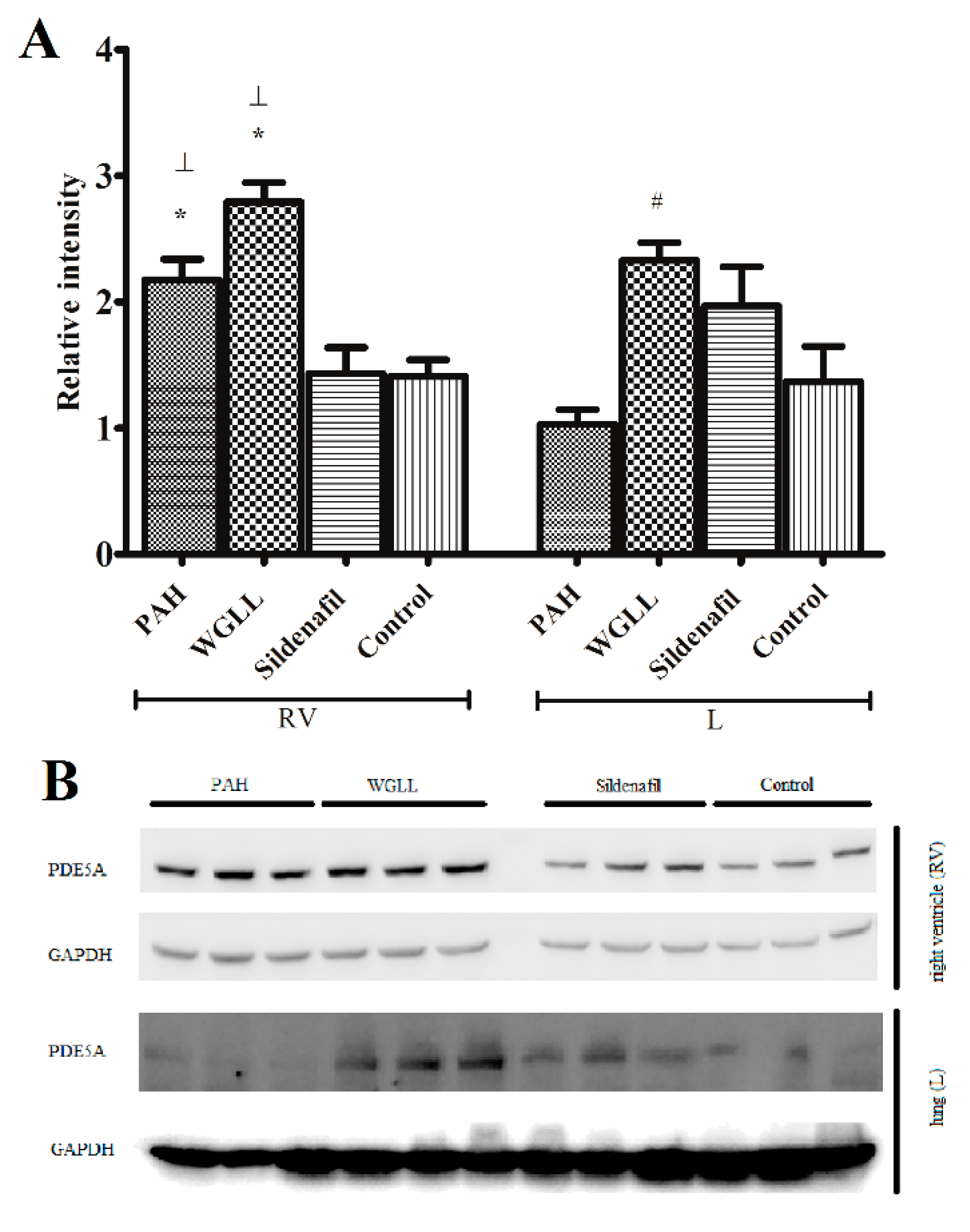
2.6. Western Blot
3. Discussion
4. Experimental section
4.1. Sample Liophyilisation and Mass Spectrometry
4.2. Study Design
4.3. Transthoracic Echocardiography (TTE)
4.4. Isolated Heart Parameters
4.5. Histological Evaluation of Myocardial and Lung Tissue Samples
4.6. Western Blot
4.7. Data Analysis and Statistical Procedures
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rubin, L.J. Primary pulmonary hypertension. N. Engl. J. Med. 1997, 336, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Moceri, P.; Baudouy, D.; Chiche, O.; Cerboni, P.; Bouvier, P.; Chaussade, C.; Ferrari, E. Imaging in pulmonary hypertension: Focus on the role of echocardiography. Arch. Cardiovasc. Dis. 2014, 107, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Vonk Noordegraaf, A.; Galie, N. The role of the right ventricle in pulmonary arterial hypertension. Eur. Respir. Rev. 2011, 20, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Pristera, N.; Musarra, R.; Schilz, R.; Hoit, B.D. The role of echocardiography in the evaluation of pulmonary arterial hypertension. Echocardiography 2016, 33, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Montani, D.; Savale, L.; Natali, D.; Jais, X.; Herve, P.; Garcia, G.; Humbert, M.; Simonneau, G.; Sitbon, O. Long-term response to calcium-channel blockers in non-idiopathic pulmonary arterial hypertension. Eur. Heart J. 2010, 31, 1898–1907. [Google Scholar] [CrossRef] [PubMed]
- Pepke-Zaba, J.; Morrell, N.W. The endothelin system and its role in pulmonary arterial hypertension (PAH). Thorax 2005, 60, 443–444. [Google Scholar] [CrossRef] [PubMed]
- Klinger, J.R. The nitric oxide/cGMP signaling pathway in pulmonary hypertension. Clin. Chest Med. 2007, 28, 143–167. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.A.; Ahmetaj-Shala, B.; Kirkby, N.S.; Wright, W.R.; Mackenzie, L.S.; Reed, D.M.; Mohamed, N. Role of prostacyclin in pulmonary hypertension. Glob. Cardiol. Sci. Pract. 2014, 2014, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Giaid, A.; Saleh, D. Reduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. N. Engl. J. Med. 1995, 333, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.R.; Kato, G.J.; Poljakovic, M.; Wang, X.; Blackwelder, W.C.; Sachdev, V.; Hazen, S.L.; Vichinsky, E.P.; Morris, S.M., Jr.; Gladwin, M.T. Dysregulated arginine metabolism, hemolysis-associated pulmonary hypertension, and mortality in sickle cell disease. JAMA 2005, 294, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Chester, A.H.; Yacoub, M.H. The role of endothelin-1 in pulmonary arterial hypertension. Glob. Cardiol. Sci. Pract. 2014, 2014, 62–78. [Google Scholar] [CrossRef] [PubMed]
- Tuder, R.M.; Cool, C.D.; Geraci, M.W.; Wang, J.; Abman, S.H.; Wright, L.; Badesch, D.; Voelkel, N.F. Prostacyclin synthase expression is decreased in lungs from patients with severe pulmonary hypertension. Am. J. Respir. Crit. Care Med. 1999, 159, 1925–1932. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, A.C.; Dusek, A.C.; McMahon, T.J. Treatment-related biomarkers in pulmonary hypertension. Am. J. Respir. Cell Mol. Biol. 2015, 52, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Rubens, C.; Ewert, R.; Halank, M.; Wensel, R.; Orzechowski, H.D.; Schultheiss, H.P.; Hoeffken, G. Big endothelin-1 and endothelin-1 plasma levels are correlated with the severity of primary pulmonary hypertension. Chest 2001, 120, 1562–1569. [Google Scholar] [CrossRef] [PubMed]
- Vandeput, F.; Krall, J.; Ockaili, R.; Salloum, F.N.; Florio, V.; Corbin, J.D.; Francis, S.H.; Kukreja, R.C.; Movsesian, M.A. cGMP-hydrolytic activity and its inhibition by sildenafil in normal and failing human and mouse myocardium. J. Pharmacol. Exp. Ther. 2009, 330, 884–891. [Google Scholar] [CrossRef] [PubMed]
- Galie, N.; Ghofrani, H.A.; Torbicki, A.; Barst, R.J.; Rubin, L.J.; Badesch, D.; Fleming, T.; Parpia, T.; Burgess, G.; Branzi, A.; et al. Sildenafil citrate therapy for pulmonary arterial hypertension. N. Engl. J. Med. 2005, 353, 2148–2157. [Google Scholar] [CrossRef] [PubMed]
- Kukreja, R.C. Sildenafil and cardioprotection. Curr. Pharm. Des. 2013, 19, 6842–6847. [Google Scholar] [CrossRef] [PubMed]
- Vorhies, E.E.; Ivy, D.D. Drug treatment of pulmonary hypertension in children. Paediatr. Drugs 2014, 16, 43–65. [Google Scholar] [CrossRef] [PubMed]
- Galland-Decker, C.; Charmoy, A.; Jolliet, P.; Spertini, O.; Hugli, O.; Pantet, O. Progressive organ failure after ingestion of wild garlic juice. J. Emerg. Med. 2016, 50, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Oszmianski, J.; Kolniak-Ostek, J.; Wojdylo, A. Characterization and content of flavonol derivatives of Allium ursinum L. Plant. J. Agric. Food Chem. 2013, 61, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Sabha, D.; Hiyasat, B.; Grotzinger, K.; Hennig, L.; Schlegel, F.; Mohr, F.W.; Rauwald, H.W.; Dhein, S. Allium ursinum L.: Bioassay-guided isolation and identification of a galactolipid and a phytosterol exerting antiaggregatory effects. Pharmacology 2012, 89, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Hiyasat, B.; Sabha, D.; Grotzinger, K.; Kempfert, J.; Rauwald, J.W.; Mohr, F.W.; Dhein, S. Antiplatelet activity of Allium ursinum and Allium sativum. Pharmacology 2009, 83, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Sendl, A.; Elbl, G.; Steinke, B.; Redl, K.; Breu, W.; Wagner, H. Comparative pharmacological investigations of Allium ursinum and Allium sativum. Planta Med. 1992, 58, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Stajner, D.; Popovic, B.M.; Canadanovic-Brunet, J.; Stajner, M. Antioxidant and scavenger activities of Allium ursinum. Fitoterapia 2008, 79, 303–305. [Google Scholar] [CrossRef] [PubMed]
- Reuter, H.D. Allium sativum and allium ursinum: Part 2 pharmacology and medicinal application. Phytomedicine 1995, 2, 73–91. [Google Scholar] [CrossRef]
- Mohamadi, A.; Jarrell, S.T.; Shi, S.J.; Andrawis, N.S.; Myers, A.; Clouatre, D.; Preuss, H.G. Effects of wild versus cultivated garlic on blood pressure and other parameters in hypertensive rats. Heart Dis. 2000, 2, 3–9. [Google Scholar] [PubMed]
- Preuss, H.G.; Clouatre, D.; Mohamadi, A.; Jarrell, S.T. Wild garlic has a greater effect than regular garlic on blood pressure and blood chemistries of rats. Int. Urol. Nephrol. 2001, 32, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Rietz, B.; Isensee, H.; Strobach, H.; Makdessi, S.; Jacob, R. Cardioprotective actions of wild garlic (Allium ursinum) in ischemia and reperfusion. Mol. Cell. Biochem. 1993, 119, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Bombicz, M.; Priksz, D.; Varga, B.; Gesztelyi, R.; Kertesz, A.; Lengyel, P.; Balogh, P.; Csupor, D.; Hohmann, J.; Bhattoa, H.P.; et al. Anti-atherogenic properties of allium ursinum liophylisate: Impact on lipoprotein homeostasis and cardiac biomarkers in hypercholesterolemic rabbits. Int. J. Mol. Sci. 2016, 17, 1284. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, M.; Mimaki, Y.; Kameyama, A.; Sashida, Y.; Nikaido, T. Steroidal saponins from Allium chinense and their inhibitory activities on cyclic AMP phosphodiesterase and Na+/K+ ATPase. Phytochemistry 1995, 40, 1071–1076. [Google Scholar] [CrossRef]
- Lines, T.C.; Ono, M. FRS 1000, an extract of red onion peel, strongly inhibits phosphodiesterase 5A (PDE 5A). Phytomedicine 2006, 13, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Carotenuto, A.; de Feo, V.; Fattorusso, E.; Lanzotti, V.; Magno, S.; Cicala, C. The flavonoids of Allium ursinum. Phytochemistry 1996, 41, 531–536. [Google Scholar] [CrossRef]
- Leporatti, M.L.; Ivancheva, S. Preliminary comparative analysis of medicinal plants used in the traditional medicine of bulgaria and italy. J. Ethnopharmacol. 2003, 87, 123–142. [Google Scholar] [CrossRef]
- Sytar, O.; Bruckova, K.; Hunkova, E.; Zivcak, M.; Konate, K.; Brestic, M. The application of multiplex fluorimetric sensor for the analysis of flavonoids content in the medicinal herbs family asteraceae, lamiaceae, rosaceae. Biol. Res. 2015, 48, 5. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, A.; Mikhova, B.; Najdenski, H.; Tsvetkova, I.; Kostova, I. Chemical composition and antimicrobial activity of wild garlic Allium ursinum of Bulgarian origin. Nat. Prod. Commun. 2009, 4, 1059–1062. [Google Scholar] [PubMed]
- Savai, R.; Al-Tamari, H.M.; Sedding, D.; Kojonazarov, B.; Muecke, C.; Teske, R.; Capecchi, M.R.; Weissmann, N.; Grimminger, F.; Seeger, W.; et al. Pro-proliferative and inflammatory signaling converge on FoxO1 transcription factor in pulmonary hypertension. Nat. Med. 2014, 20, 1289–1300. [Google Scholar] [CrossRef] [PubMed]
- Thibault, H.B.; Kurtz, B.; Raher, M.J.; Shaik, R.S.; Waxman, A.; Derumeaux, G.; Halpern, E.F.; Bloch, K.D.; Scherrer-Crosbie, M. Noninvasive assessment of murine pulmonary arterial pressure: Validation and application to models of pulmonary hypertension. Circ. Cardiovasc. Imaging 2010, 3, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.M.; Mocanu, M.M.; Yellon, D.M. Retrograde heart perfusion: The langendorff technique of isolated heart perfusion. J. Mol. Cell. Cardiol. 2011, 50, 940–950. [Google Scholar] [CrossRef] [PubMed]
- Vidavalur, R.; Swarnakar, S.; Thirunavukkarasu, M.; Samuel, S.M.; Maulik, N. Ex vivo and in vivo approaches to study mechanisms of cardioprotection targeting ischemia/reperfusion (i/r) injury: Useful techniques for cardiovascular drug discovery. Curr. Drug Discov. Technol. 2008, 5, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Vecsernyes, M.; Szokol, M.; Bombicz, M.; Priksz, D.; Gesztelyi, R.; Fulop, G.A.; Varga, B.; Juhasz, B.; Haines, D.; Tosaki, A. α-MSH induces vasodilatation and exerts cardioprotection via the heme-oxygenase pathway in rat hearts. J. Cardiovasc. Pharmacol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Juhasz, B.; Varga, B.; Czompa, A.; Bak, I.; Lekli, I.; Gesztelyi, R.; Zsuga, J.; Kemeny-Beke, A.; Antal, M.; Szendrei, L.; et al. Postischemic cardiac recovery in heme oxygenase-1 transgenic ischemic/reperfused mouse myocardium. J. Cell. Mol. Med. 2011, 15, 1973–1982. [Google Scholar] [CrossRef] [PubMed]
- Nogueira-Ferreira, R.; Vitorino, R.; Ferreira, R.; Henriques-Coelho, T. Exploring the monocrotaline animal model for the study of pulmonary arterial hypertension: A network approach. Pulm. Pharmacol. Ther. 2015, 35, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.J.; Marsboom, G.; Archer, S.L. Rodent models of group 1 pulmonary hypertension. Handb. Exp. Pharmacol. 2013, 218, 105–149. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Arroyo, J.G.; Farkas, L.; Alhussaini, A.A.; Farkas, D.; Kraskauskas, D.; Voelkel, N.F.; Bogaard, H.J. The monocrotaline model of pulmonary hypertension in perspective. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 302, L363–L369. [Google Scholar] [CrossRef] [PubMed]
- Gan, C.; Lankhaar, J.W.; Marcus, J.T.; Westerhof, N.; Marques, K.M.; Bronzwaer, J.G.; Boonstra, A.; Postmus, P.E.; Vonk-Noordegraaf, A. Impaired left ventricular filling due to right-to-left ventricular interaction in patients with pulmonary arterial hypertension. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H1528–H1533. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.K.; Lee, H.; Kim, K.C.; Hong, Y.M. The effect of sildenafil on right ventricular remodeling in a rat model of monocrotaline-induced right ventricular failure. Korean J. Pediatr. 2016, 59, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Yoshiyuki, R.; Tanaka, R.; Fukushima, R.; Machida, N. Preventive effect of sildenafil on right ventricular function in rats with monocrotaline-induced pulmonary arterial hypertension. Exp. Anim. 2016, 65, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Jasinska-Stroschein, M.; Owczarek, J.; Luczak, A.; Orszulak-Michalak, D. The beneficial impact of fasudil and sildenafil on monocrotaline-induced pulmonary hypertension in rats: A hemodynamic and biochemical study. Pharmacology 2013, 91, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Farah, C.; Kleindienst, A.; Bolea, G.; Meyer, G.; Gayrard, S.; Geny, B.; Obert, P.; Cazorla, O.; Tanguy, S.; Reboul, C. Exercise-induced cardioprotection: A role for enos uncoupling and no metabolites. Basic Res. Cardiol. 2013, 108, 389. [Google Scholar] [CrossRef] [PubMed]
- Itter, G.; Jung, W.; Schoelkens, B.A.; Linz, W. The isolated working heart model in infarcted rat hearts. Lab. Anim. 2005, 39, 178–193. [Google Scholar] [CrossRef] [PubMed]
- Buyukakilli, B.; Gurgul, S.; Citirik, D.; Hallioglu, O.; Ozeren, M.; Tasdelen, B. Determination of the effects of pulmonary arterial hypertension and therapy on the cardiovascular system of rats by impedance cardiography. Croat. Med. J. 2014, 55, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.J.; Thenappan, T.; Rich, S.; Tian, L.; Archer, S.L.; Gomberg-Maitland, M. Association of serum creatinine with abnormal hemodynamics and mortality in pulmonary arterial hypertension. Circulation 2008, 117, 2475–2483. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.Y.; Tsai, K.B.; Hsu, J.H.; Shin, S.J.; Wu, J.R.; Yeh, J.L. Liraglutide prevents and reverses monocrotaline-induced pulmonary arterial hypertension by suppressing ET-1 and enhancing enOS/SGC/PKG pathways. Sci. Rep. 2016, 6, 31788. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.H.; Chung, S.Y.; Chua, S.; Chai, H.T.; Sheu, J.J.; Chen, Y.L.; Chen, C.H.; Chang, H.W.; Tong, M.S.; Sung, P.H.; et al. Effect of early administration of lower dose versus high dose of fresh mitochondria on reducing monocrotaline-induced pulmonary artery hypertension in rat. Am. J. Transl. Res. 2016, 8, 5151–5168. [Google Scholar] [PubMed]
- Lewis, G.D.; Shah, R.; Shahzad, K.; Camuso, J.M.; Pappagianopoulos, P.P.; Hung, J.; Tawakol, A.; Gerszten, R.E.; Systrom, D.M.; Bloch, K.D.; et al. Sildenafil improves exercise capacity and quality of life in patients with systolic heart failure and secondary pulmonary hypertension. Circulation 2007, 116, 1555–1562. [Google Scholar] [CrossRef] [PubMed]
- Elias-Al-Mamun, M.; Satoh, K.; Tanaka, S.-I.; Shimizu, T.; Nergui, S.; Miyata, S.; Fukumoto, Y.; Shimokawa, H. Combination therapy with fasudil and sildenafil ameliorates monocrotaline-induced pulmonary hypertension and survival in rats. Circ. J. 2014, 78, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Kuang, T.; Wang, J.; Pang, B.; Huang, X.; Burg, E.D.; Yuan, J.X.J.; Wang, C. Combination of sildenafil and simvastatin ameliorates monocrotaline-induced pulmonary hypertension in rats. Pulm. Pharmacol. Ther. 2010, 23, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Nagendran, J.; Archer, S.L.; Soliman, D.; Gurtu, V.; Moudgil, R.; Haromy, A.; St. Aubin, C.; Webster, L.; Rebeyka, I.M.; Ross, D.B.; et al. Phosphodiesterase type 5 is highly expressed in the hypertrophied human right ventricle, and acute inhibition of phosphodiesterase type 5 improves contractility. Circulation 2007, 116, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Kass, D.A.; Champion, H.C.; Beavo, J.A. Phosphodiesterase type 5: Expanding roles in cardiovascular regulation. Circ. Res. 2007, 101, 1084–1095. [Google Scholar] [CrossRef] [PubMed]
- Hanson, K.A.; Ziegler, J.W.; Rybalkin, S.D.; Miller, J.W.; Abman, S.H.; Clarke, W.R. Chronic pulmonary hypertension increases fetal lung cgmp phosphodiesterase activity. Am. J. Physiol. 1998, 275, L931–L941. [Google Scholar] [PubMed]
- Lochhead, A.; Nekrasova, E.; Arshavsky, V.Y.; Pyne, N.J. The regulation of the cGMP-binding cGMP phosphodiesterase by proteins that are immunologically related to gamma subunit of the photoreceptor cGMP phosphodiesterase. J. Biol. Chem. 1997, 272, 18397–18403. [Google Scholar] [CrossRef] [PubMed]
- Corbin, J.D.; Beasley, A.; Blount, M.A.; Francis, S.H. High lung PDE5: A strong basis for treating pulmonary hypertension with PDE5 inhibitors. Biochem. Biophys. Res. Commun. 2005, 334, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, B.; Pangallo, M.; Ruffenach, G.; Cunningham, C.M.; Perron, J.C.; Kolluru, S.; Eghbali, M.; Gupta, V. Advances in treatment of pulmonary arterial hypertension: Patent review. Expert Opin. Ther. Pat. 2017. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; VanPatten, S.; Lakshminrusimha, S.; Patel, H.; Coleman, T.R.; Al-Abed, Y. Effects of novel muscarinic M3 receptor ligand C1213 in pulmonary arterial hypertension models. Physiol. Rep. 2016, 4. [Google Scholar] [CrossRef] [PubMed]
- Leopold, J.A.; Maron, B.A. Molecular mechanisms of pulmonary vascular remodeling in pulmonary arterial hypertension. Int. J. Mol. Sci. 2016, 17, 761. [Google Scholar] [CrossRef] [PubMed]
- Ankri, S.; Mirelman, D. Antimicrobial properties of allicin from garlic. Microbes Infect. 1999, 1, 125–129. [Google Scholar] [CrossRef]
- Sendl, A.; Schliack, M.; Loser, R.; Stanislaus, F.; Wagner, H. Inhibition of cholesterol synthesis in vitro by extracts and isolated compounds prepared from garlic and wild garlic. Atherosclerosis 1992, 94, 79–85. [Google Scholar] [CrossRef]
- Farkas, A.; Molnar, R.; Morschhauser, T.; Hahn, I. Variation in nectar volume and sugar concentration of Allium ursinum L. ssp. ucrainicum in three habitats. Sci. World J. 2012, 2012, 138579. [Google Scholar] [CrossRef] [PubMed]
- Karasu-Minareci, E.; Ozbudak, I.H.; Ozbilim, G.; Sadan, G. Acute effects of vardenafil on pulmonary artery responsiveness in pulmonary hypertension. Sci. World J. 2012, 2012, 718279. [Google Scholar] [CrossRef] [PubMed]
- Varga, B.; Gesztelyi, R.; Bombicz, M.; Haines, D.; Szabo, A.M.; Kemeny-Beke, A.; Antal, M.; Vecsernyes, M.; Juhasz, B.; Tosaki, A. Protective effect of α-melanocyte-stimulating hormone (α-MSH) on the recovery of ischemia/reperfusion (I/R)-induced retinal damage in a rat model. J. Mol. Neurosci. 2013, 50, 558–570. [Google Scholar] [CrossRef] [PubMed]
- Opie, L.H. Coronary flow rate and perfusion pressure as determinants of mechanical function and oxidative metabolism of isolated perfused rat heart. J. Physiol. 1965, 180, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Xie, Q.; Pan, G.; Wang, J.; Wang, M. Mesenchymal stem cells participate in angiogenesis and improve heart function in rat model of myocardial ischemia with reperfusion. Eur. J. Cardiothorac. Surg. 2006, 30, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Porvasnik, S.L.; Germain, S.; Embury, J.; Gannon, K.S.; Jacques, V.; Murray, J.; Byrne, B.J.; Shacham, S.; Al-Mousily, F. PRX-08066, a novel 5-hydroxytryptamine receptor 2B antagonist, reduces monocrotaline-induced pulmonary arterial hypertension and right ventricular hypertrophy in rats. J. Pharmacol. Exp. Ther. 2010, 334, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, A.; Kalasz, J.; Pasztor, E.T.; Toth, A.; Papp, Z.; Dhalla, N.S.; Barta, J. Myosin heavy chain and cardiac troponin T damage is associated with impaired myofibrillar ATPase activity contributing to sarcomeric dysfunction in Ca2+-paradox rat hearts. Mol. Cell. Biochem. 2017. [Google Scholar] [CrossRef] [PubMed]

| Structure | PubChem CID | Common Name | Input m/z | Exact m/z | Δ | Formula | Ion |
|---|---|---|---|---|---|---|---|
| 74640 | 24211973 | Kaempferol-3-O-rutinoside | 617.4 | 617.1472 | 0.2528 | C27H30O15Na | [M + Na]+ |
| 74640 | 24211973 | Kaempferol-3-O-rutinoside | 633.3 | 633.1212 | 0.1788 | C27H30O15K | [M + K]+ |
| 46189 | 5280459 | Quercitrin | 471.2 | 471.0898 | 0.1102 | C21H20O11Na | [M + Na]+ |
| 46189 | 5280459 | Quercitrin | 487.2 | 487.0637 | 0.1363 | C21H20O11K | [M + K]+ |
| 47726 | 5748554 | Juglanin | 441.4 | 441.0792 | 0.3208 | C20H18O10Na | [M + Na]+ |
| 47726 | 5748554 | Juglanin | 457.3 | 457.0531 | 0.2469 | C20H18O10K | [M + K]+ |
| 68247 | 160270 | Dracorubin | 527.2 | 527.1255 | 0.0745 | C32H24O5K | [M + K]+ |
| 68247 | 160270 | Dracorubin | 511.2 | 511.1516 | 0.0484 | C32H24O5Na | [M + Na]+ |
| 48987 | 11289628 | Blumeatin | 341.2 | 341.0422 | 0.1578 | C16H14O6K | [M + K]+ |
| 48987 | 11289628 | Blumeatin | 325.2 | 325.0683 | 0.1317 | C16H14O6Na | [M + Na]+ |
| Parameter | Control | PAH | Sildenafil | WGLL |
|---|---|---|---|---|
| LV Ejection Fraction (%) | 73.39 ± 3.638 | 82.61 ± 2.911 | 76.93 ± 2.294 | 76.13 ± 2.327 |
| LV Fractional Shortening (%) | 38.64 ± 3.268 | 47.75 ± 3.432 | 40.84 ± 2.177 | 40.49 ± 2.059 |
| LV mass (g) | 1.388 ± 0.085 | 1.343 ± 0.037 | 1.487 ± 0.036 | 1.469 ± 0.057 |
| Stroke volume (mL) | 0.459 ± 0.070 | 0.456 ± 0.056 | 0.445 ± 0.081 | 0.452 ± 0.032 |
| HR (bpm) | 269.5 ± 16.21 | 274.2 ± 9.37 | 264.3 ± 10.39 | 243.1 ± 9.76 |
| LVOT maxPG (mmHg) | 2.225 ± 0.247 | 2.415 ± 0.260 | 2.401 ± 0.381 | 2.128 ± 0.179 |
| LVOT meanPG (mmHg) | 1.032 ± 0.112 | 1.067 ± 0.096 | 1.169 ± 0.185 | 0.876 ± 0.074 |
| LVOT Vmax (m/s) | 0.753 ± 0.041 | 0.746 ± 0.445 | 0.748 ± 0.082 | 0.720 ± 0.032 |
| LVOT Vmean (m/s) | 0.432 ± 0.025 | 0.451 ± 0.018 | 0.463 ± 0.045 | 0.398 ± 0.017 |
| Lat S’ (cm/s) | 32.29 ± 1.782 | 40.51 ± 2.710 | 50.39 ± 2.278 * | 39.36 ± 3.565 |
| MV E vel (cm/s) | 65.81 ± 3.860 | 64.71 ± 3.046 | 58.09 ± 2.906 | 63.56 ± 2.484 |
| MV A vel (cm/s) | 40.15 ± 4.253 | 37.54 ± 5.484 | 30.82 ± 1.054 | 39.19 ± 2.385 |
| MV E/A ratio | 1.841 ± 0.167 | 1.856 ± 0.165 | 1.903 ± 0.052 | 1.745 ± 0.129 |
| MAPSE (mm) | 2.085 ± 0.089 | 1.961 ± 0.098 | 1.905 ± 0.057 | 1.944 ± 0.150 |
| TAPSE (mm) | 2.308 ± 0.074 | 1.697 ± 0.098 * | 2.390 ± 0.069 # | 2.021 ± 0.071 # |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bombicz, M.; Priksz, D.; Varga, B.; Kurucz, A.; Kertész, A.; Takacs, A.; Posa, A.; Kiss, R.; Szilvassy, Z.; Juhasz, B. A Novel Therapeutic Approach in the Treatment of Pulmonary Arterial Hypertension: Allium ursinum Liophylisate Alleviates Symptoms Comparably to Sildenafil. Int. J. Mol. Sci. 2017, 18, 1436. https://doi.org/10.3390/ijms18071436
Bombicz M, Priksz D, Varga B, Kurucz A, Kertész A, Takacs A, Posa A, Kiss R, Szilvassy Z, Juhasz B. A Novel Therapeutic Approach in the Treatment of Pulmonary Arterial Hypertension: Allium ursinum Liophylisate Alleviates Symptoms Comparably to Sildenafil. International Journal of Molecular Sciences. 2017; 18(7):1436. https://doi.org/10.3390/ijms18071436
Chicago/Turabian StyleBombicz, Mariann, Daniel Priksz, Balazs Varga, Andrea Kurucz, Attila Kertész, Akos Takacs, Aniko Posa, Rita Kiss, Zoltan Szilvassy, and Bela Juhasz. 2017. "A Novel Therapeutic Approach in the Treatment of Pulmonary Arterial Hypertension: Allium ursinum Liophylisate Alleviates Symptoms Comparably to Sildenafil" International Journal of Molecular Sciences 18, no. 7: 1436. https://doi.org/10.3390/ijms18071436





