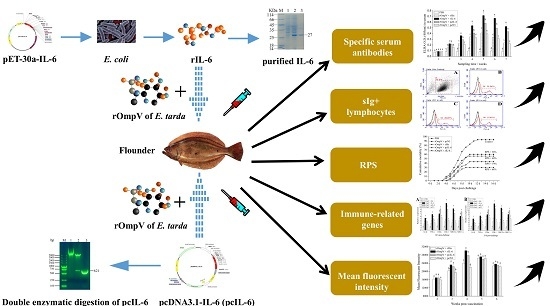
The Immune Adjuvant Effects of Flounder (Paralichthys olivaceus) Interleukin-6 on E. tarda Subunit Vaccine OmpV
Abstract
:1. Introduction
2. Results
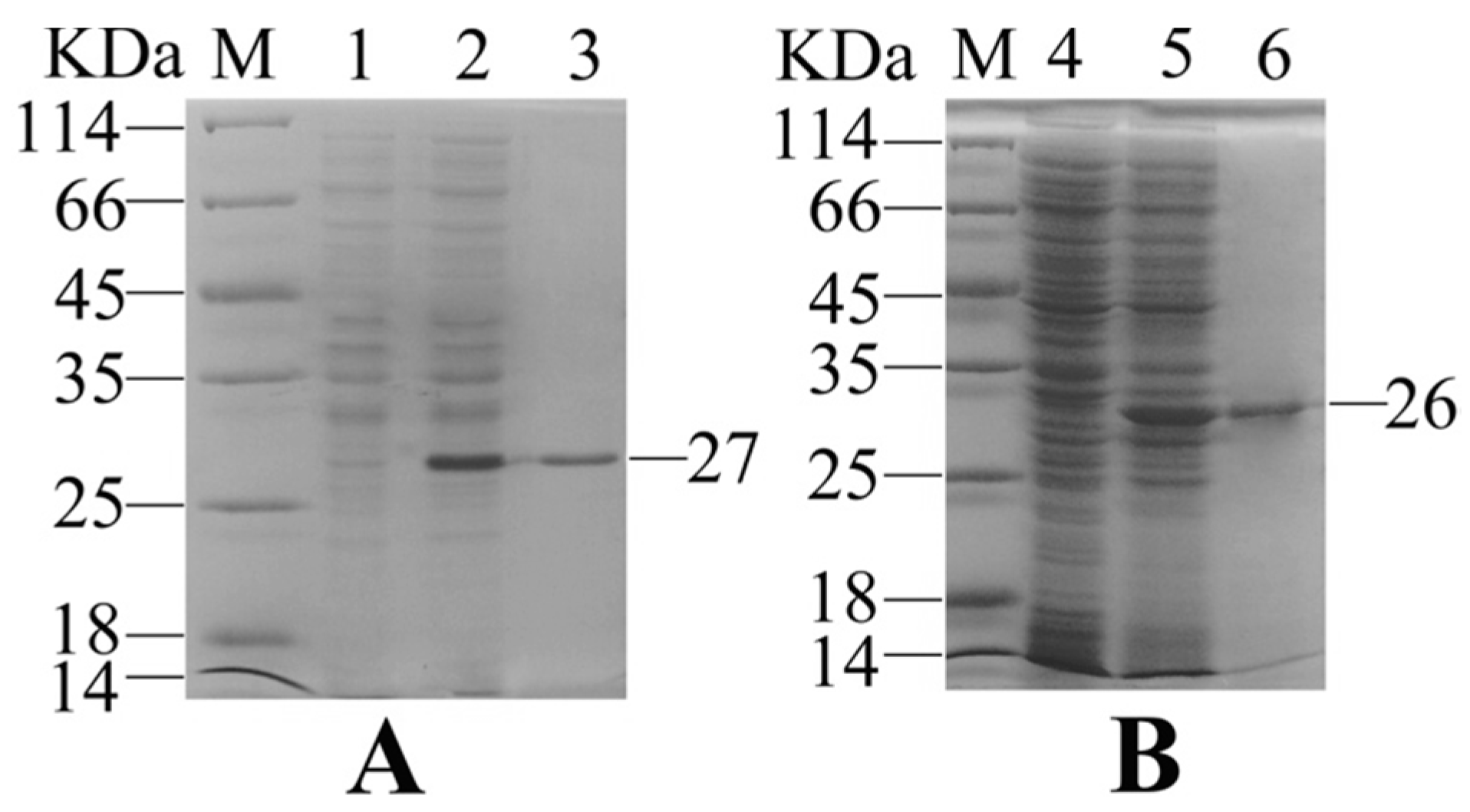
2.1. Expression and Purification of Recombinant Interleukin-6 and E. tarda Subunit Vaccine Recombinant Outer Membrant Protein V
2.2. Tissue Distribution and In Vivo Expression of pcDNA3.1-IL-6 (pcIL-6)
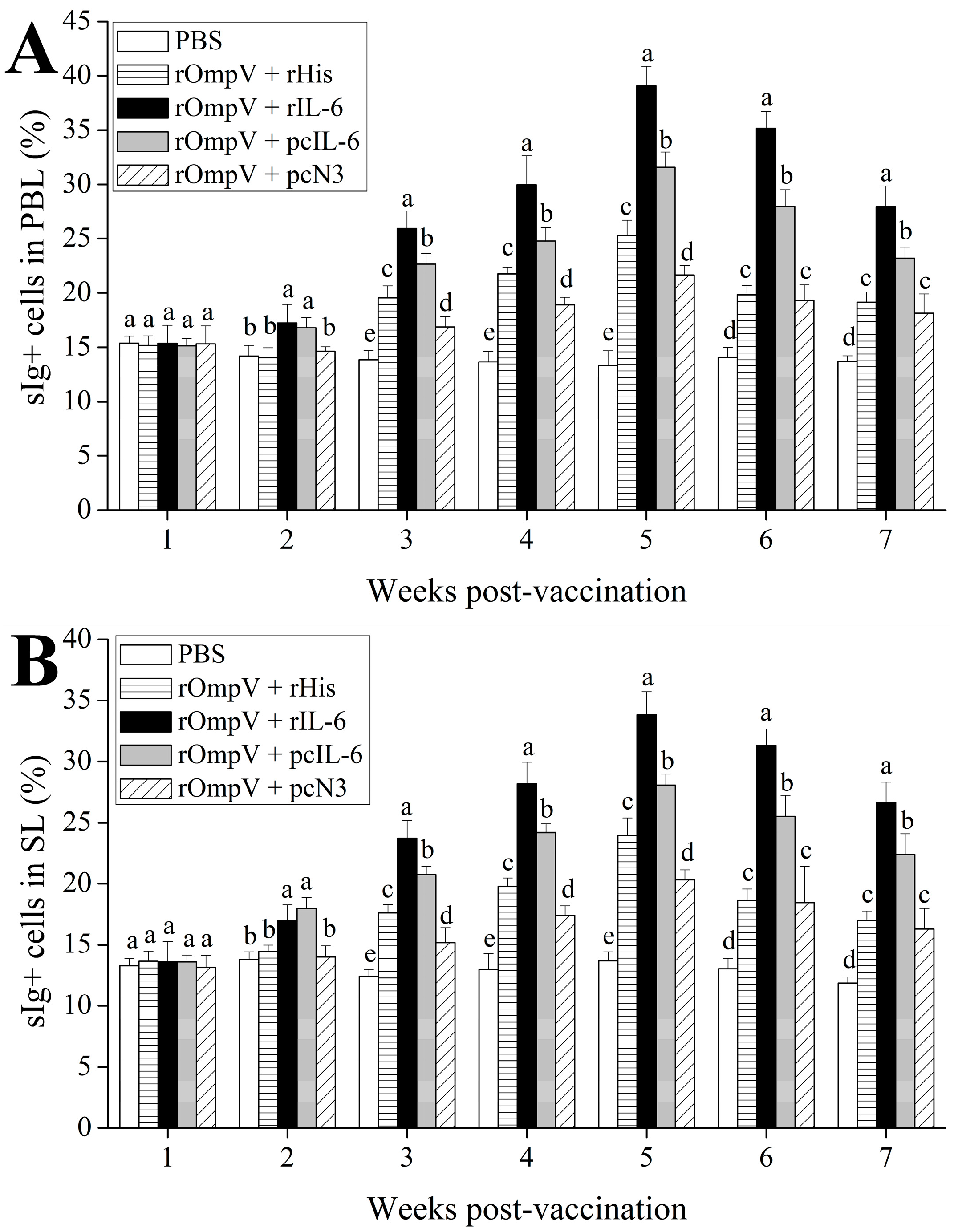
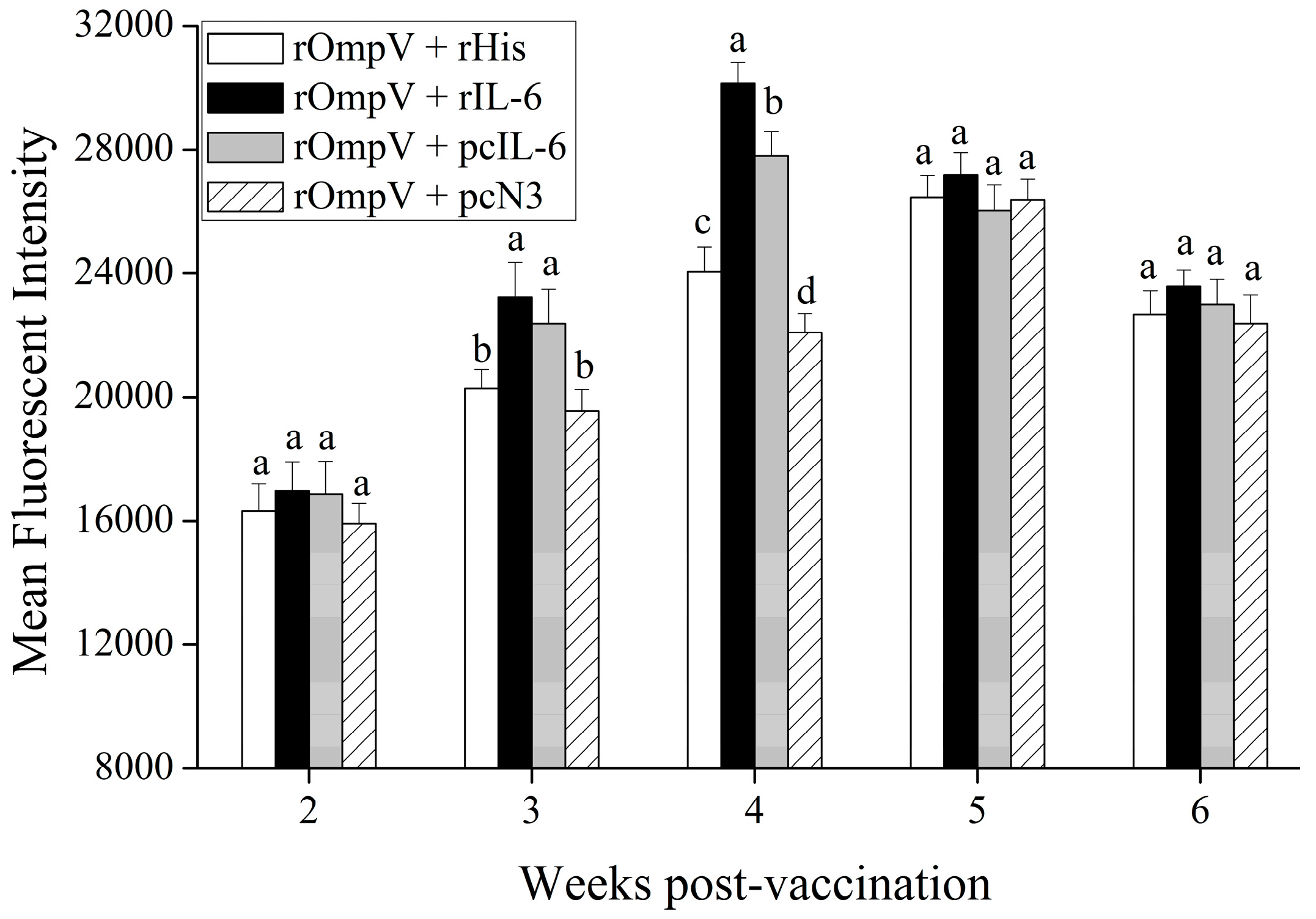
2.3. Response of Surface Membrane Immunoglobulin-Positive (sIg+) Lymphocytes after Vaccination
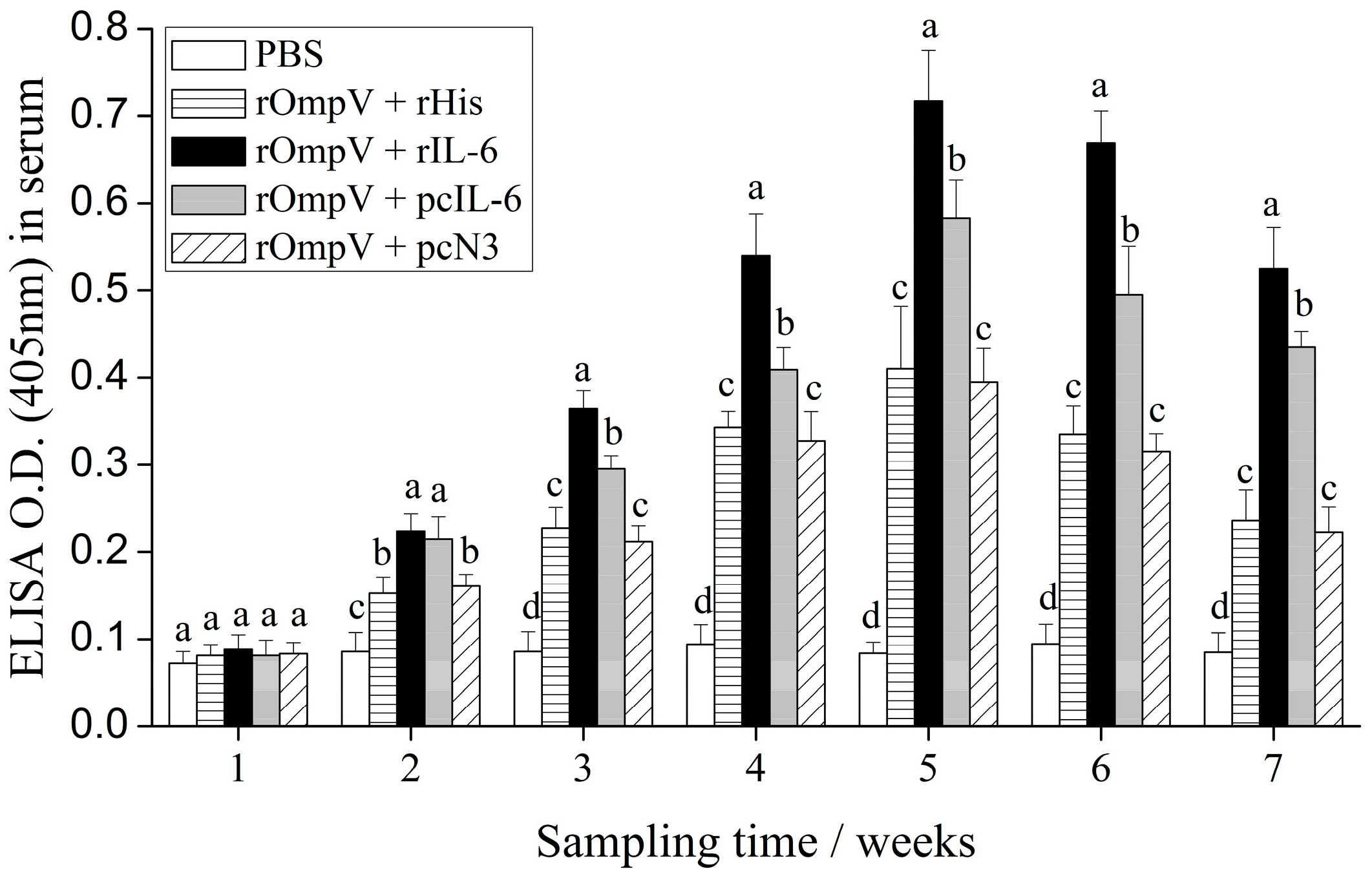
2.4. Response of Specific Serum Antibodies
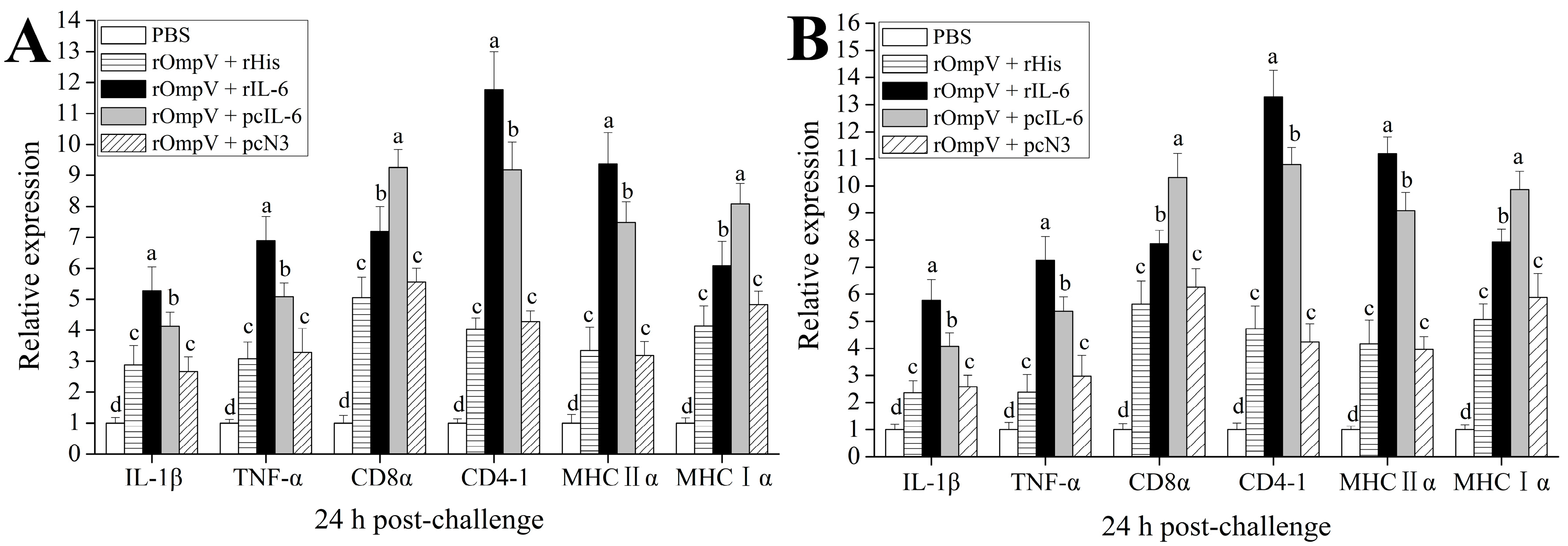
2.5. Expression of Immune-Related Genes
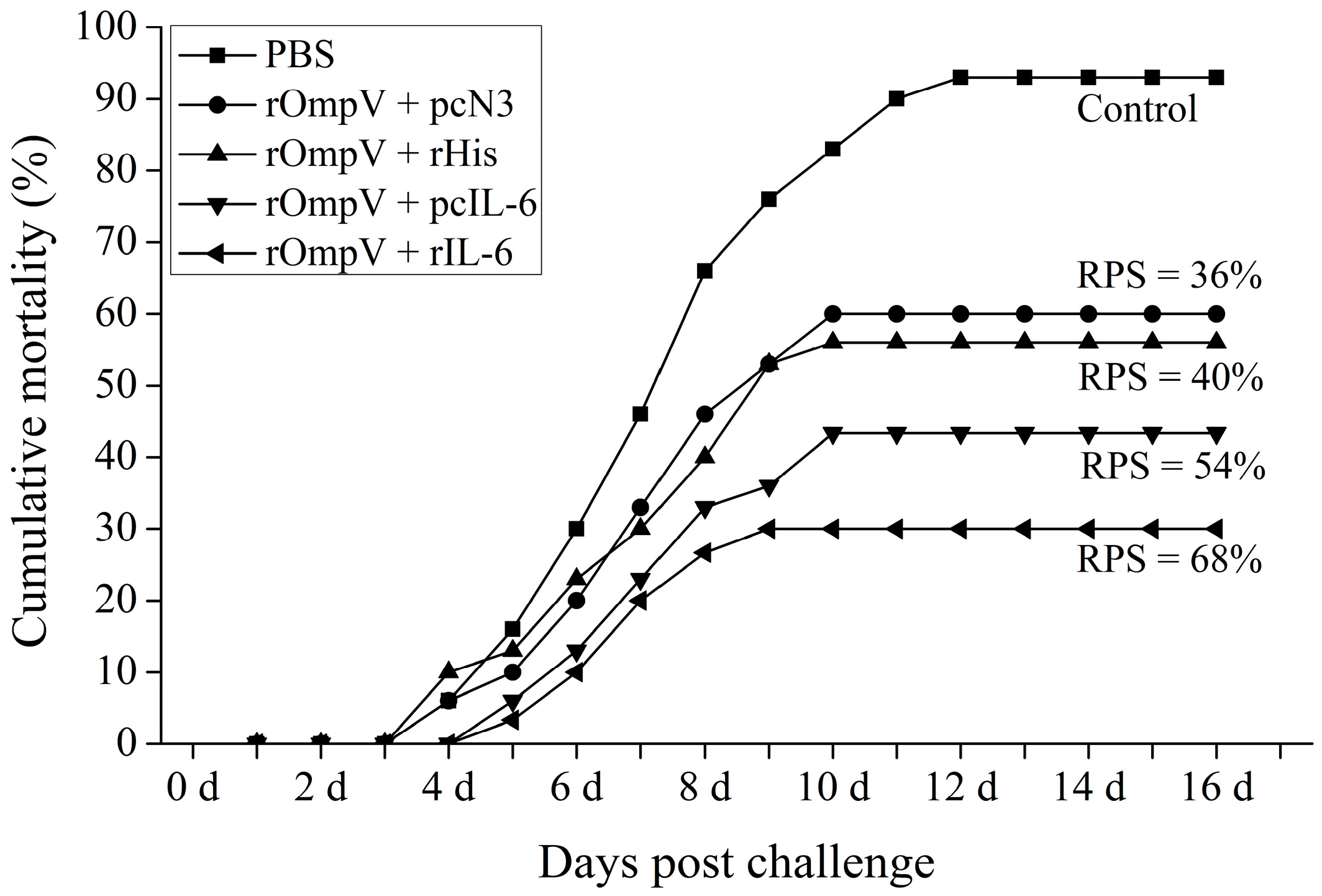
2.6. Protection against E. tarda Infection
3. Discussion
4. Materials and Methods
4.1. Expression, Purification, and Refolding of Recombinant Interleukin-6
4.2. Construction of the pcIL-6 Plasmid
4.3. Expression, Purification, and Refolding of Recombinant OmpV
4.4. Vaccination
4.5. Sampling
4.6. Distribution and Expression of pcIL-6 in Fish Tissues
4.7. Flow Cytometric Immunofluorescence Analysis
4.8. Detection of the Serum Antibodies against E. tarda by Enzyme-Linked Immunosorbent Assay
4.9. Analysis of the Expression of Immune-Related Genes by qRT-PCR
4.10. Challenge
4.11. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sun, K.; Zhang, W.W.; Hou, J.H.; Sun, L. Immunoprotective analysis of VhhP2, a Vibrio harveyi vaccine candidate. Vaccine 2009, 27, 2733–2740. [Google Scholar] [CrossRef] [PubMed]
- Sommerset, I.; Krossøy, B.; Biering, E.; Frost, P. Vaccines for fish in aquaculture. Expert Rev. Vaccines 2005, 4, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Park, S.B.; Aoki, T.; Jung, T.S. Pathogenesis of and strategies for preventing Edwardsiella tarda infection in fish. Vet. Res. 2012, 43, 67. [Google Scholar] [CrossRef] [PubMed]
- Brudeseth, B.E.; Wiulsrød, R.; Fredriksen, B.N.; Lindmo, K.; Løkling, K.E.; Bordevik, M.; Steine, N.; Klevan, A.; Gravningen, K. Status and future perspectives of vaccines for industrialised fin-fish farming. Fish Shellfish Immunol. 2013, 35, 1759–1768. [Google Scholar] [CrossRef] [PubMed]
- Barr, T.A.; Carlring, J.; Heath, A.W. Co-stimulatory agonists as immunological adjuvants. Vaccine 2006, 24, 3399–3407. [Google Scholar] [CrossRef] [PubMed]
- Storni, T.; Kündig, T.M.; Senti, G.; Johansen, P. Immunity in response to particulate antigen-delivery systems. Adv. Drug Deliv. Rev. 2005, 57, 333–355. [Google Scholar] [CrossRef] [PubMed]
- Schijns, V.E. Immunological concepts of vaccine adjuvant activity: Commentary. Curr. Opin. Immunol. 2000, 12, 456–463. [Google Scholar] [CrossRef]
- Zinkernagel, R.M.; Ehl, S.; Aichele, P.; Oehen, S.; Kündig, T.; Hengartner, H. Antigen localisation regulates immune responses in a dose-and time-dependent fashion: A geographical view of immune reactivity. Immunol. Rev. 1997, 156, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Matzinger, P. The danger model: A renewed sense of self. Science 2002, 296, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Matzinger, P. Tolerance, danger, and the extended family. Annu. Rev. Immunol. 1994, 12, 991–1045. [Google Scholar] [CrossRef] [PubMed]
- Janeway, C.A. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb. Symp. Quant. Biol. 1989, 54, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Janeway, C.A. The immune system evolved to discriminate infectious nonself from noninfectious self. Immunol. Today 1992, 13, 11–16. [Google Scholar] [CrossRef]
- Tafalla, C.; Bøgwald, J.; Dalmo, R.A. Adjuvants and immunostimulants in fish vaccines: Current knowledge and future perspectives. Fish Shellfish Immunol. 2013, 35, 1740–1750. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Brunn, C.; Ho, R.J.H. Cytokines as vaccine adjuvants. Current status and potential applications. Pharm. Biotechnol. 1995, 6, 625–643. [Google Scholar] [PubMed]
- Kishimoto, T. Interleukin-6. In The Cytokine Handbook, 4th ed.; Thomson, A.N., Lotze, M.T., Eds.; Two-Volume Set; Academic Press: San Diego, CA, USA, 2003; pp. 281–304. [Google Scholar]
- Taga, T.; Kishimoto, T. Gp130 and the interleukin-6 family of cytokines. Annu. Rev. Immunol. 1997, 15, 797–819. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Takano, H.; Shimada, A.; Morita, T.; Yanagisawa, R.; Sakurai, M.; Sato, M.; Yoshino, S.; Yoshikawa, T. Cytoprotection by interleukin- 6 against liver injury induced by lipopolysaccharide. Int. J. Mol. Med. 2005, 15, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T. Interleukin 6 and its receptor: Ten years later. Int. Rev. Immunol. 1998, 16, 249–284. [Google Scholar] [CrossRef] [PubMed]
- Van Snick, J. Interleukin-6: An overview. Annu. Rev. Immunol. 1990, 8, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Su, B.; Wang, J.; Wang, X.; Jin, H.; Zhao, G.; Ding, Z.; Kang, Y.M.; Wang, B. The effects of IL-6 and TNF-α as molecular adjuvants on immune responses to FMDV and maturation of dendritic cells by DNA vaccination. Vaccine 2008, 26, 5111–5122. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Gao, R.; Meng, M.; Li, J.; Tan, M.; Shen, Y.; Wang, L.H.; Yin, X.; Wu, X.Y.; Xie, H.G.; et al. Regulating effects of porcine interleukin-6 gene and CpG motifs on immune responses to porcine trivalent vaccines in mice. Res. Vet. Sci. 2004, 77, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Pockley, A.G.; Montgomery, P.C. In vivo adjuvant effect of interleukins 5 and 6 on rat tear IgA antibody responses. Immunology 1991, 73, 19. [Google Scholar] [PubMed]
- Zhu, L.Y.; Nie, L.; Zhu, G.; Xiang, L.X.; Shao, J.Z. Advances in research of fish immune-relevant genes: A comparative overview of innate and adaptive immunity in teleosts. Dev. Comp. Immunol. 2013, 39, 39–62. [Google Scholar] [CrossRef] [PubMed]
- Savan, R.; Sakai, M. Genomics of fish cytokines. Comp. Biochem. Phys. D 2006, 1, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Taechavasonyoo, A.; Hirono, I.; Kondo, H. The immune-adjuvant effect of Japanese flounder Paralichthys olivaceus IL-1β. Dev. Comp. Immunol. 2013, 41, 564–568. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Wang, J.; Long, B.; Wang, K.; He, Y.; Yang, Q.; Chen, D.F.; Geng, Y.; Huang, X.L.; Ouyang, P.; et al. Molecular cloning, expression and the adjuvant effects of interleukin-8 of channel catfish (Ictalurus punctatus) against Streptococcus iniae. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Bird, S.; Zou, J.; Savan, R.; Kono, T.; Sakai, M.; Woo, J.; Secombes, C. Characterisation and expression analysis of an interleukin 6 homologue in the Japanese pufferfish, Fugu rubripes. Dev. Comp. Immunol. 2005, 29, 775–789. [Google Scholar] [CrossRef] [PubMed]
- Nam, B.H.; Byon, J.Y.; Kim, Y.O.; Park, E.M.; Cho, Y.C.; Cheong, J. Molecular cloning and characterisation of the flounder (Paralichthys olivaceus) interleukin-6 gene. Fish Shellfish Immunol. 2007, 23, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Iliev, D.B.; Castellana, B.; MacKenzie, S.; Planas, J.V.; Goetz, F.W. Cloning and expression analysis of an IL-6 homolog in rainbow trout (Oncorhynchus mykiss). Mol. Immunol. 2007, 44, 1803–1807. [Google Scholar] [CrossRef] [PubMed]
- Castellana, B.; Iliev, D.B.; Sepulcre, M.P.; MacKenzie, S.; Goetz, F.W.; Mulero, V.; Planas, J.V. Molecular characterization of interleukin-6 in the gilthead seabream (Sparus aurata). Mol. Immunol. 2008, 45, 3363–3370. [Google Scholar] [CrossRef] [PubMed]
- Øvergård, A.C.; Nepstad, I.; Nerland, A.H.; Patel, S. Characterisation and expression analysis of the Atlantic halibut (Hippoglossus hippoglossus L.) cytokines: IL-1β, IL-6, IL-11, IL-12β and IFNγ. Mol. Boil. Rep. 2012, 39, 2201–2213. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Li, C.; Yu, Z.X.; Zou, P.F.; Meng, Q.X.; Yao, C.L. Molecular and immune response characterizations of IL-6 in large yellow croaker (Larimichthys crocea). Fish Shellfish Immunol. 2016, 50, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Tang, X.; Sheng, X.; Xing, J.; Zhan, W. Comparative study of the vaccine potential of six outer membrane proteins of Edwardsiella tarda and the immune responses of flounder (Paralichthys olivaceus) after vaccination. Vet. Immunol. Immunopathol. 2017, 185, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Muraguchi, A.; Kishimoto, T.; Miki, Y.; Kuritani, T.; Kaieda, T.; Yoshizaki, K.; Yamamura, Y. T cell-replacing factor-(TRF) induced IgG secretion in a human B blastoid cell line and demonstration of acceptors for TRF. J. Immunol. 1981, 127, 412–416. [Google Scholar] [PubMed]
- Faris, M.; Kokot, N.; Stahl, N.; Nel, A.E. Involvement of Stat3 in interleukin-6-induced IgM production in a human B-cell line. Immunology 1997, 90, 350. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.M.; Maehr, T.; Diaz-Rosales, P.; Secombes, C.J.; Wang, T. Bioactivity studies of rainbow trout (Oncorhynchus mykiss) interleukin-6: Effects on macrophage growth and antimicrobial peptide gene expression. Mol. Immunol. 2011, 48, 1903–1916. [Google Scholar] [CrossRef] [PubMed]
- Kaneda, M.; Odaka, T.; Suetake, H.; Tahara, D.; Miyadai, T. Teleost IL-6 promotes antibody production through STAT3 signaling via IL-6R and gp130. Dev. Comp. Immunol. 2012, 38, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Abós, B.; Wang, T.; Castro, R.; Granja, A.G.; Leal, E.; Havixbeck, J.; Luque, A.; Barreda, D.R.; Secombes, C.J.; Tafalla, C. Distinct differentiation programs triggered by IL-6 and LPS in teleost IgM+ B cells in the absence of germinal centers. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Irvine, K.R.; Rao, J.B.; Rosenberg, S.A.; Restifo, N.P. Cytokine enhancement of DNA immunization leads to effective treatment of established pulmonary metastases. J. Immunol. 1996, 156, 238–245. [Google Scholar] [PubMed]
- Larsen, D.L.; Dybdahl-Sissoko, N.; McGregor, M.W.; Drape, R.; Neumann, V.; Swain, W.F.; Olsen, C.W. Coadministration of DNA encoding interleukin-6 and hemagglutinin confers protection from influenza virus challenge in mice. J. Virol. 1998, 72, 1704–1708. [Google Scholar] [PubMed]
- Tarte, K.; Zhan, F.; de Vos, J.; Klein, B.; Shaughnessy, J. Gene expression profiling of plasma cells and plasmablasts: Toward a better understanding of the late stages of B-cell differentiation. Blood 2003, 102, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Schneider, P.; Takatsuka, H.; Wilson, A.; Mackay, F.; Tardivel, A.; Lens, S.; Cachero, T.G.; Finke, D.; Beermann, F.; Tschopp, J. Maturation of marginal zone and follicular B cells requires B cell activating factor of the tumor necrosis factor family and is independent of B cell maturation antigen. J. Exp. Med. 2001, 194, 1691–1698. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, B.P.; Cascalho, M.; Noelle, R.J. Short-lived and long-lived bone marrow plasma cells are derived from a novel precursor population. J. Exp. Med. 2002, 195, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Bromage, E.S.; Kaattari, I.M.; Zwollo, P.; Kaattari, S.L. Plasmablast and plasma cell production and distribution in trout immune tissues. J. Immunol. 2004, 173, 7317–7323. [Google Scholar] [CrossRef] [PubMed]
- Uyttenhove, C.; Coulie, P.G.; Van Snick, J. T cell growth and differentiation induced by interleukin-HP1/IL-6, the murine hybridoma/plasmacytoma growth factor. J. Exp. Med. 1988, 167, 1417–1427. [Google Scholar] [CrossRef] [PubMed]
- Takai, Y.; Wong, G.G.; Clark, S.C.; Burakoff, S.J.; Herrmann, S.H. B cell stimulatory factor-2 is involved in the differentiation of cytotoxic T lymphocytes. J. Immunol. 1988, 140, 508–512. [Google Scholar] [PubMed]
- Okada, M.; Kitahara, M.; Kishimoto, S.; Matsuda, T.; Hirano, T.; Kishimoto, T. BSF-2/IL-6 functions as a killer helper factor in the in vitro induction of cytotoxic T cells. Int. J. Immunol. 1988, 10, 131. [Google Scholar]
- Heppell, J.; Davis, H.L. Application of DNA vaccine technology to aquaculture. Adv. Drug Deliv. Rev. 2000, 43, 29–43. [Google Scholar] [CrossRef]
- Barouch, D.H.; Letvin, N.L.; Seder, R.A. The role of cytokine DNAs as vaccine adjuvants for optimizing cellular immune responses. Immunol. Rev. 2004, 202, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Pressley, M.E.; Phelan, P.E.; Witten, P.E.; Mellon, M.T.; Kim, C.H. Pathogenesis and inflammatory response to Edwardsiella tarda infection in the zebrafish. Dev. Comp. Immunol. 2005, 29, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Dan, X.M.; Zhang, T.W.; Li, Y.W.; Li, A.X. Immune responses and immune-related gene expression profile in orange-spotted grouper after immunization with Cryptocaryon irritans vaccine. Fish Shellfish Immunol. 2013, 34, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Tsumoto, K.; Shinoki, K.; Kondo, H.; Uchikawa, M.; Juji, T.; Kumagai, I. Highly efficient recovery of functional single-chain Fv fragments from inclusion bodies overexpressed in Escherichia coli by controlled introduction of oxidizing reagent—Application to a human single-chain Fv fragment. J. Immunol. Methods 1998, 219, 119–129. [Google Scholar] [CrossRef]
- Umetsu, M.; Tsumoto, K.; Hara, M.; Ashish, K.; Goda, S.; Adschiri, T.; Kumagai, I. How additives influence the refolding of immunoglobulin-folded proteins in a stepwise dialysis system spectroscopic evidence for highly efficient refolding of a single-chain Fv fragment. J. Biol. Chem. 2003, 278, 8979–8987. [Google Scholar] [CrossRef] [PubMed]
- Asano, R.; Kudo, T.; Makabe, K.; Tsumoto, K.; Kumagai, I. Antitumor activity of interleukin-21 prepared by novel refolding procedure from inclusion bodies expressed in Escherichia coli. FEBS Lett. 2002, 528, 70–76. [Google Scholar] [CrossRef]
- Aida, Y.; Pabst, M.J. Removal of endotoxin from protein solutions by phase separation using Triton X-114. J. Immunol. Methods 1990, 132, 191–195. [Google Scholar] [CrossRef]
- Kozak, M. An analysis of 5′-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987, 15, 8125–8148. [Google Scholar] [CrossRef] [PubMed]
- Kozak, M. An analysis of vertebrate mRNA sequences: Intimations of translational control. J. Cell. Biol. 1991, 115, 887–903. [Google Scholar] [CrossRef] [PubMed]
- Kozak, M. Downstream secondary structure facilitates recognition of initiator codons by eukaryotic ribosomes. Proc. Natl. Acad. Sci. USA 1990, 87, 8301–8305. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.; Sun, L.; Hood, D.W.; Moxon, E.R. Involvement of genes of genome maintenance in the regulation of phase variation frequencies in Neisseria meningitidis. Microbiology 2004, 150, 3001–3012. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Sheng, X.; Xing, J.; Zhan, W. Effect of temperature on immune response of Japanese flounder (Paralichthys olivaceus) to inactivated lymphocystis disease virus (LCDV). Fish Shellfish Immunol. 2011, 30, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhan, W.; Xing, J.; Sheng, X. Production, characterisation and applicability of monoclonal antibodies to immunoglobulin of Japanese flounder (Paralichthys olivaceus). Fish Shellfish Immunol. 2007, 23, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Zhan, W.; Sheng, X.; Chi, H. Immune response of Japanese flounder Paralichthys olivaceus to outer membrane protein of Edwardsiella tarda. Fish Shellfish Immunol. 2010, 28, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Amend, D.F. Potency testing of fish vaccines. International symposium on fish biologics: Serodiagnostics and vaccines. Dev. Biol. Stand. 1981, 49, 447–454. [Google Scholar]




| No. | Primer Name | Primer Sequence (5′→3′) a | Source |
|---|---|---|---|
| 1 | IL-6-F | CGGGATCCGCTCCAGTCGAATACGAGCCCAC (BamHI) | DQ267937.1 |
| 2 | IL-6-R | CCCAAGCTTTGTCATTTGGTAAGAGGGATGGA (HindII) | |
| 3 | 18sRNA-F | GGTCTGTGATGCCCTTAGATGTC | EF126037 |
| 4 | 18sRNA-R | AGTGGGGTTCAGCGGGTTAC | |
| 5 | pcN3-IL-6-F | CCCAAGCTTACCATGGCTCCAGTCGAATACGA (HindIIII) | DQ267937.1 |
| 6 | pcN3-IL-6-R | CGGGATCCTAATGTCATTTGGTAAGAGGGAT (BamHI) | |
| 7 | pcDNAG-F | TAATACGACTCACTATAGGG | Invitrogen |
| 8 | pcDNAG-R | TAGAAGGCACAGTCGAGG | |
| 9 | OmpV-F | CGGGATCCGAGGGCCAGACGGTTTCTCTG (BamHI) | ETAE 1239 |
| 10 | OmpV-R | CCAAGCTTGAAGCGGTAGCCGACACCCAGG (HindII) | |
| 11 | IL-1β-F | CTGTCGTTCTGGGCATCAAA | AB720983 |
| 12 | IL-1β-R | AACAGAAATCGCACCATCTCACT | |
| 13 | TNFα-F | GTCCTGGCGTTTTCTTGGTA | AB040448 |
| 14 | TNFα-R | CTTGGCTCTGCTGCTGATTT | |
| 15 | MHCIα-F | AGACCACAGGCTGTTATCACCA | AB126921 |
| 16 | MHCIα-R | TCTTCCCATGCTCCACGAA | |
| 17 | MHCIIα-F | ACAGGGACGGAACTTATCAACG | AY997530 |
| 18 | MHCIIα-R | TCATCGGACTGGAGGGAGG | |
| 19 | CD4-1-F | CCAGTGGTCCCCACCTAAAA | AB643634 |
| 20 | CD4-1-R | CACTTCTGGGACGGTGAGATG | |
| 21 | CD8α-F | CCTCTCCCCATACATTGATTCC | AB082957 |
| 22 | CD8α-R | CCGAGCTTTGCTGAAGGACTT |
| Group | Treatment |
|---|---|
| 1 | 100 μL PBS |
| 2 | 200 μg rOmpV + 20 μg rHis |
| 3 | 200 μg rOmpV + 20 μg rIL-6 |
| 4 | 200 μg rOmpV + 20 μg pcN3 |
| 5 | 200 μg rOmpV + 20 μg pcIL-6 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, M.; Tang, X.; Sheng, X.; Xing, J.; Zhan, W. The Immune Adjuvant Effects of Flounder (Paralichthys olivaceus) Interleukin-6 on E. tarda Subunit Vaccine OmpV. Int. J. Mol. Sci. 2017, 18, 1445. https://doi.org/10.3390/ijms18071445
Guo M, Tang X, Sheng X, Xing J, Zhan W. The Immune Adjuvant Effects of Flounder (Paralichthys olivaceus) Interleukin-6 on E. tarda Subunit Vaccine OmpV. International Journal of Molecular Sciences. 2017; 18(7):1445. https://doi.org/10.3390/ijms18071445
Chicago/Turabian StyleGuo, Ming, Xiaoqian Tang, Xiuzhen Sheng, Jing Xing, and Wenbin Zhan. 2017. "The Immune Adjuvant Effects of Flounder (Paralichthys olivaceus) Interleukin-6 on E. tarda Subunit Vaccine OmpV" International Journal of Molecular Sciences 18, no. 7: 1445. https://doi.org/10.3390/ijms18071445