Metallothionein from Wild Populations of the African Catfish Clarias gariepinus: From Sequence, Protein Expression and Metal Binding Properties to Transcriptional Biomarker of Metal Pollution
Abstract
:1. Introduction
2. Results and Discussion
2.1. Study Area and Sampling Regime
2.2. Identification of C. gariepinus Metallothionein cDNA Sequence
2.3. Expression and Metal-Binding Properties of Recombinant CgMT
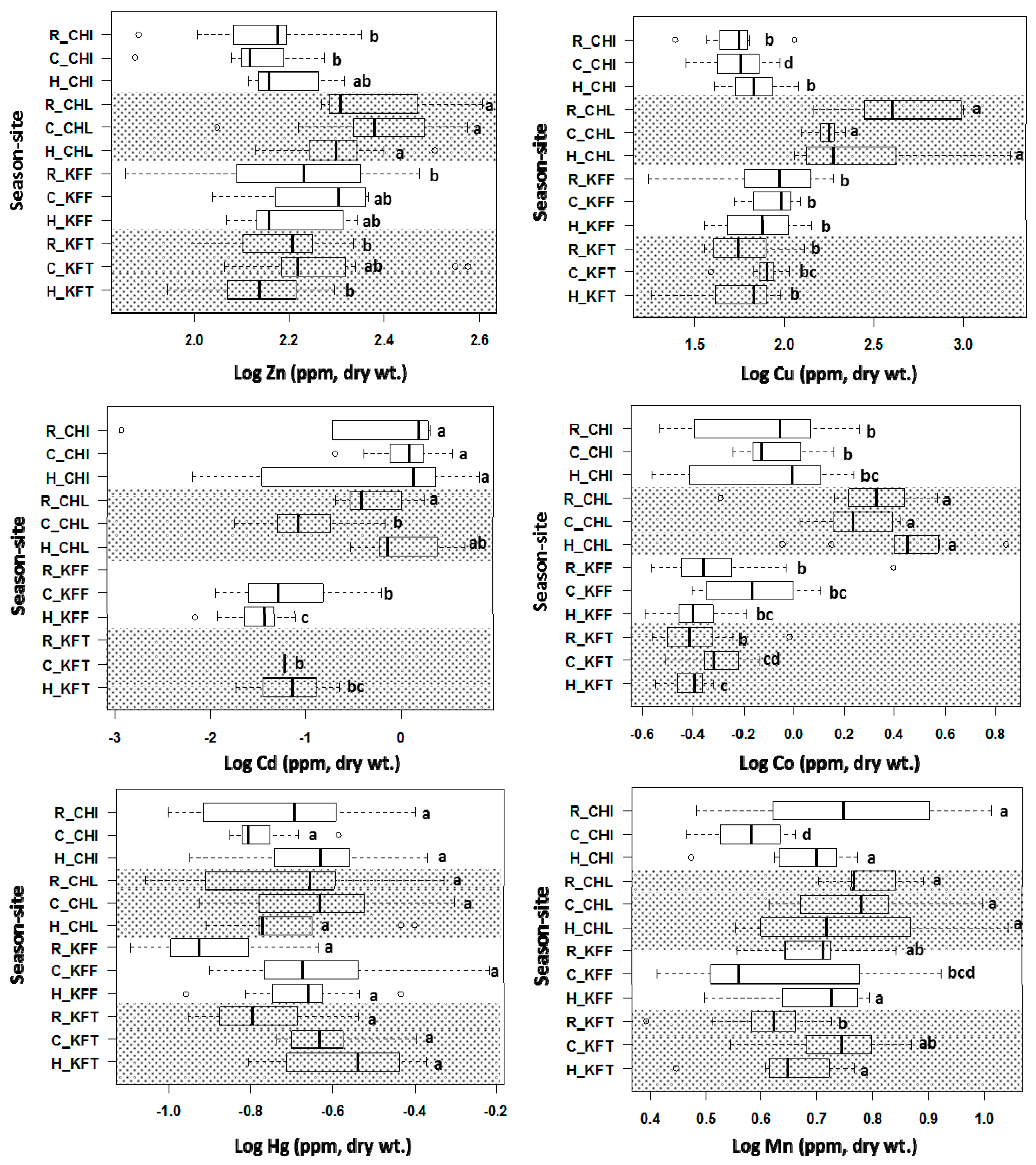
2.4. Concentrations of Heavy Metals in Fish Liver Samples
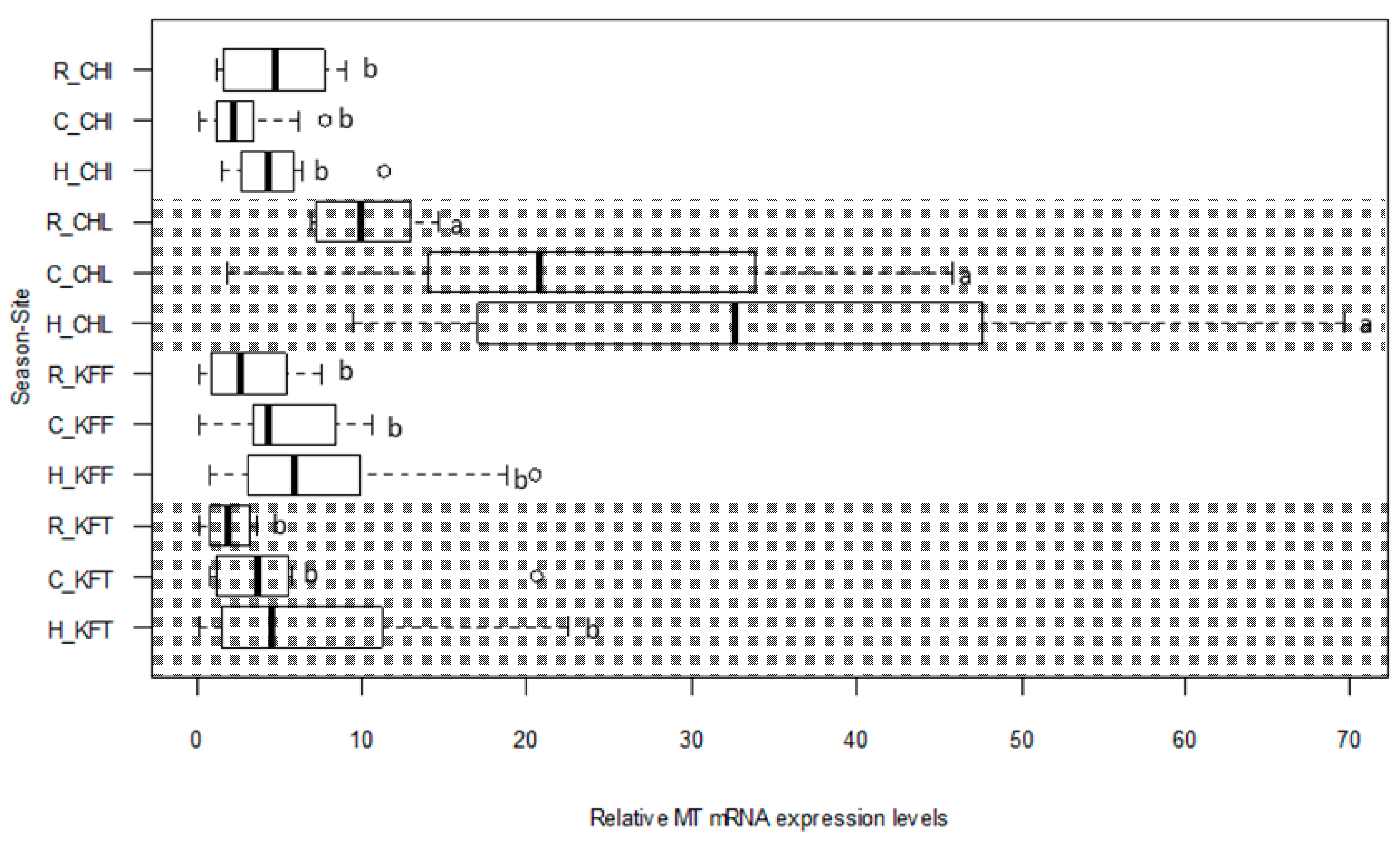
2.5. MT Gene Expression Levels in Liver of C. gariepinus
2.6. Suitability of C. gariepinus Liver MT mRNA as a Biomarker
3. Materials and Methods
3.1. Fish Collection, Preparation and Sampling
3.2. Total RNA Isolation and Reverse Transcription Reaction
3.3. Primer Design and Reverse Transcription Polymerase Chain Reaction (RT-PCR)
3.4. Expression and Purification of Recombinant CgMT
3.5. Analysis of Metal-Binding Stoichiometries of Recombinant CgMT
3.6. Quantitative Real-Time PCR
3.7. Heavy Metal Analysis in Fish Liver
3.8. Statistical Analyses
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AEX | Anion exchange chromatography |
| BLAST | Basic local alignment search tool |
| BLASTN | Nucleotide BLAST |
| BLASTP | Protein BLAST |
| cDNA | Complementary DNA |
| CDS | Coding sequence |
| CgMT | Clarias gariepinus metallothionein protein |
| cgMT | Clarias gariepinus metallothionein coding sequence |
| EDTA | 2,2′,2′′,2′′′-(Ethane-1,2-diyldinitrilo)tetraacetic acid |
| ESI-MS | Electrospray ionsation mass spectrometry |
| fMet | Formylated methionine |
| HSD | Honest significant difference |
| ICP-MS | Inductively-coupled plasma optical emission spectroscopy |
| ICP-MS | Inductively-coupled plasma mass spectrometry |
| LB | Lysogeny broth |
| mRNA | Messenger RNA |
| MT | Metallothionein |
| NCBI | National Center for Biotechnology Information |
| PCR | Polymerase chain reaction |
| PLI | Pollution load index |
| qPCR | Quantitative PCR |
| RI | Potential ecological risk index |
| RT-PCR | Reverse transcription PCR |
| SDS-PAGE | Sodium dodecyl sulphate polyacrylamide gel electrophoresis |
| SEC | Size-exclusion chromatography |
References
- Zhou, Q.; Zhang, J.; Fu, J.; Shi, J.; Jiang, G. Biomonitoring: An appealing tool for assessment of metal pollution in the aquatic ecosystem. Anal. Chim. Acta 2008, 606, 135–150. [Google Scholar] [CrossRef] [PubMed]
- McGeer, J.C.; Szebedinszky, C.; McDonald, D.G.; Wood, C.M. Effects of chronic sublethal exposure to waterborne Cu, Cd or Zn in rainbow trout 2: Tissue specific metal accumulation. Aquat. Toxicol. 2000, 50, 245–256. [Google Scholar] [CrossRef]
- Van der Oost, R.; Beyer, J.; Vermeulen, N.P.E. Fish bioaccumulation and biomarkers in environmental risk assessment: A review. Environ. Toxicol. Pharmacol. 2003, 13, 57–149. [Google Scholar] [CrossRef]
- Bonner, F.W.; Bridges, J.W. Toxicological aspects of trace elements. In Trace Elements in Health; Rose, J., Ed.; Butterworths: London, UK, 1983; pp. 1–20. [Google Scholar]
- Maret, W. The metals in the biological periodic system of the elements: Concepts and conjectures. Int. J. Mol. Sci. 2016, 17, 66. [Google Scholar] [CrossRef] [PubMed]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. EXS 2012, 101, 133–164. [Google Scholar] [PubMed]
- Hursthouse, A.S. The relevance of speciation in the remediation of soils and sediments contaminated by metallic elements—An overview and examples from central Scotland, UK. J. Environ. Monit. 2001, 3, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Basha, P.S.; Rani, A.U. Cadmium-induced antioxidant defense mechanism in freshwater teleost Oreochromis mossambicus (Tilapia). Ecotox. Environ. Saf. 2003, 56, 218–221. [Google Scholar] [CrossRef]
- Vilizzi, L.; Tarkan, A.S. Bioaccumulation of metals in common carp (Cyprinus carpio L.) from water bodies of Anatolia (Turkey): A review with implications for fisheries and human food consumption. Environ. Monit. Assess. 2016, 188, 243. [Google Scholar] [CrossRef] [PubMed]
- Duruibe, J.O.; Ogwuegbu, M.O.C.; Egwurugwu, J.N. Heavy metal pollution and human biotoxic effects. Int. J. Phys. Sci. 2007, 2, 112–118. [Google Scholar]
- Nogawa, K. Itai-itai disease and follow-up studies. In Cadmium in the Environment, Part II; Nriagu, J.O., Ed.; Wiley: New York, NY, USA, 1981; pp. 1–37. [Google Scholar]
- Pettersson, U.T.; Ingri, J. The geochemistry of Co and Cu in the Kafue River as it drains the Copperbelt mining area, Zambia. Chem. Geol. 2001, 177, 399–414. [Google Scholar] [CrossRef]
- Choongo, K.C.; Syakalima, M.S.; Mwase, M. Coefficient of condition in relation to copper levels in muscle of Serranochromis fish and sediment from the Kafue River, Zambia. Bull. Environ. Contam. Toxicol. 2005, 75, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Norrgren, L.; Pettersson, U.; Orn, S.; Bergqvist, P.A. Environmental monitoring of the Kafue River, located in the Copperbelt, Zambia. Arch. Environ. Contam. Toxicol. 2000, 38, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Sracek, O.; Kribek, B.; Mihaljevic, M.; Majer, V.; Veselovsky, F.; Vencelides, Z.; Nyambe, I. Mining-related contamination of surface water and sediments of the Kafue River drainage system in the Copperbelt district, Zambia: An example of a high neutralization capacity system. J. Geochem. Explor. 2012, 112, 174–188. [Google Scholar] [CrossRef]
- Mbewe, G.; Mutondo, M.; Maseka, K.; Sichilongo, K. Assessment of heavy-metal pollution in sediments and tilapia fish species in Kafue River of Zambia. Arch. Environ. Contam. Toxicol. 2016, 71, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Mwase, M.; Viktor, T.; Norrgren, L. Effects on tropical fish of soil sediments from Kafue River, Zambia. Bull. Environ. Contam. Toxicol. 1998, 61, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Van Gestel, C.A.M.; van Brummelen, T.C. Incorporation of the biomarker concept in ecotoxicology calls for a redefinition of terms. Ecotoxicology 1996, 5, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Kägi, J.H.R.; Vallee, B.L. Metallothionein: A cadmium- and zinc-containing protein from equine renal cortex. J. Biol. Chem. 1960, 235, 3460–3465. [Google Scholar] [PubMed]
- Olsson, P.-E. Toxicology of Aquatic Pollution: Physiological, Molecular and Cellular Approaches; Society for Experimental Biology Seminar Series; Taylor, E.W., Ed.; Cambridge University Press: Cambridge, UK, 1996; pp. 187–204. [Google Scholar]
- Roesijadi, G. Metallothionein induction as a measure of response to metal exposure in aquatic animals. Environ. Health Perspect. 1994, 102, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Linde, A.R.; Sanchez-Galan, S.; Valles-Mota, P.; Garcia-Vazquez, E. Metallothionein as bioindicator of freshwater metal pollution: European eel and brown trout. Ecotoxicol. Environ. Saf. 2001, 49, 60–63. [Google Scholar] [CrossRef] [PubMed]
- George, S.; Gubbins, M.; MacIntosh, A.; Reynolds, W.; Sabine, V.; Scott, A.; Thain, J. A comparison of pollutant biomarker responses with transcriptional responses in European flounders (Platicthys flesus) subjected to estuarine pollution. Mar. Environ. Res. 2004, 58, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Tom, M.; Chen, N.; Segev, M.; Herut, B.; Rinkevich, B. Quantifying fish metallothionein transcript by real time PCR for its utilization as an environmental biomarker. Mar. Pollut. Bull. 2004, 48, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Viarengo, A.; Burlando, B.; Dondero, F.; Marro, A.; Fabbri, R. Metallothionein as a tool in biomonitoring programmes. Biomarkers 1999, 4, 455–466. [Google Scholar]
- Sarkar, A.; Ray, D.; Shrivastava, A.N.; Sarker, S. Molecular biomarkers: Their significance and application in marine pollution monitoring. Ecotoxicology 2006, 15, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Hauser-Davis, R.A.; de Campos, R.C.; Ziolli, R.L. Fish metalloproteins as biomarkers of environmental contamination. In Reviews of Environmental Contamination and Toxicology; Whitacre, D.M., Ed.; Springer: New York, NY, USA, 2012; Volume 218, pp. 101–123. [Google Scholar]
- Hemmadi, V. Metallothionein—A potential biomarker to assess the metal contamination in marine fishes. Int. J. Bioassays 2016, 5, 4961–4973. [Google Scholar] [CrossRef]
- Blindauer, C.A.; Leszczyszyn, O.I. Metallothioneins: Unparalleled diversity in structures and functions for metal ion homeostasis and more. Nat. Prod. Rep. 2010, 27, 720–741. [Google Scholar] [CrossRef] [PubMed]
- Kägi, J.H. Overview of metallothionein. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1991; Volume 205, pp. 613–626. [Google Scholar]
- Vašák, M. Advances in metallothionein structure and functions. J. Trace Elem. Med. Biol. 2005, 19, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Capdevila, M.; Bofill, R.; Palacios, O.; Atrian, S. State-of-the-art of metallothioneins at the beginning of the 21st century. Coord. Chem. Rev. 2012, 256, 46–62. [Google Scholar] [CrossRef]
- Sutherland, D.E.; Stillman, M.J. The “magic numbers” of metallothionein. Metallomics 2011, 3, 444–463. [Google Scholar] [CrossRef] [PubMed]
- Capdevila, M.; Atrian, S. Metallothionein protein evolution: A miniassay. J. Biol. Inorg. Chem. 2011, 16, 977–989. [Google Scholar] [CrossRef] [PubMed]
- Blindauer, C.A. Metallothioneins. In Binding, Transport and Storage of Metal Ions in Biological Cells; Maret, W., Wedd, W., Eds.; Royal Society of Chemistry: London, UK, 2014; Volume 2, pp. 606–665. [Google Scholar]
- Vergani, L. Metallothioneins in aquatic organisms: Fish, crustaceans, molluscs, and echinoderms. In Metal Ions in Life Sciences; Sigel, A., Sigel, H., Sigel, R.K.O., Eds.; Royal Society of Chemistry: Cambridge, UK, 2009; Volume 5, pp. 199–237. [Google Scholar]
- Maret, W. Cellular zinc and redox states converge in the metallothionein/thionein pair. J. Nutr. 2003, 133, 1460S–1462S. [Google Scholar] [PubMed]
- Buico, A.; Cassino, C.; Dondero, F.; Vergani, L.; Osella, D. Radical scavenging abilities of fish MT-A and mussel MT-10 metallothionein isoforms: An ESR study. J. Inorg. Biochem. 2008, 102, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Amiard, J.C.; Amiard-Triquet, C.; Barka, S.; Pellerin, J.; Rainbow, P.S. Metallothioneins in aquatic invertebrates: Their role in metal detoxification and their use as biomarkers. Aquat. Toxicol. 2006, 76, 160–202. [Google Scholar] [CrossRef] [PubMed]
- Bracken, W.M.; Klaassen, C.D. Induction of metallothionein in rat primary hepatocyte cultures: Evidence for direct and indirect induction. J. Toxicol. Environ. Health 1987, 22, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Cherian, M.G.; Nordberg, M. Cellular adaptation in metal toxicology and metallothionein. Toxicology 1983, 28, 1–15. [Google Scholar] [CrossRef]
- Thiele, D.J. Metal-regulated transcription in eukaryotes. Nucleic Acids Res. 1992, 20, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Olsson, P.E.; Kille, P. Functional comparison of the metal-regulated transcriptional control regions of metallothionein genes from cadmium-sensitive and tolerant fish species. Biochim. Biophys. Acta 1997, 1350, 325–334. [Google Scholar] [CrossRef]
- Olsson, P.E.; Gerpe, M.; Kling, P. Functional and regulatory aspects of teleost metallothionein. In Metallothionein IV; Klaassen, C.D., Ed.; Birkhäuser Verlag AG: Basel, Switzerland, 1999; pp. 179–185. [Google Scholar]
- Davis, S.R.; Cousins, R.J. Metallothionein expression in animals: A physiological perspective on function. J. Nutr. 2000, 130, 1085–1088. [Google Scholar] [PubMed]
- He, P.F.; Xu, M.X.; Ren, H.W. Cloning and functional characterization of 5′-upstream region of metallothionein-I gene from crucian carp (Carassius cuvieri). Int. J. Biochem. Cell Biol. 2007, 39, 832–841. [Google Scholar] [CrossRef] [PubMed]
- Bury, N.R.; Chung, M.J.; Sturm, A.; Walker, P.A.; Hogstrand, C. Cortisol stimulates the zinc signaling pathway and expression of metallothioneins and ZnT1 in rainbow trout gill epithelial cells. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R623–R629. [Google Scholar] [CrossRef] [PubMed]
- Mayer, G.D.; Leach, A.; Kling, P.; Olsson, P.E.; Hogstrand, C. Activation of the rainbow trout metallothionein-A promoter by silver and zinc. Comp. Biochem. Physiol. B 2003, 134, 181–188. [Google Scholar] [CrossRef]
- Bourdineaud, J.P.; Baudrimont, M.; Gonzalez, P.; Moreau, J.L. Challenging the model for induction of metallothionein gene expression. Biochimie 2006, 88, 1787–1792. [Google Scholar] [CrossRef] [PubMed]
- Zafarullah, M.; Olsson, P.E.; Gedamu, L. Endogenous and heavy-metal-ion-induced metallothionein gene-expression in salmonid tissues and cell lines. Gene 1989, 83, 85–93. [Google Scholar] [CrossRef]
- Van Campenhout, K.; Infante, H.G.; Hoff, P.T.; Moens, L.; Goemans, G.; Belpaire, C.; Adams, F.; Blust, R.; Bervoets, L. Cytosolic distribution of Cd, Cu and Zn, and metallothionein levels in relation to physiological changes in Gibel carp (Carassius auratus gibelio) from metal-impacted habitats. Ecotoxicol. Environ. Saf. 2010, 73, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Hayes, R.A.; Regondi, S.; Winter, M.J.; Butler, P.J.; Agradi, E.; Taylor, E.W.; Chipman, J.K. Cloning of a chub metallothionein cDNA and development of competitive RT-PCR of chub metallothionein mRNA as a potential biomarker of heavy metal exposure. Mar. Environ. Res. 2004, 58, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Petrlova, J.; Krizkova, S.; Zitka, O.; Hubalek, J.; Prusa, R.; Adam, V.; Wang, J.; Beklova, M.; Sures, B.; Kizek, R. Utilizing a chronopotentiometric sensor technique for metallothionein determination in fish tissues and their host parasites. Sens. Actuator B 2007, 127, 112–119. [Google Scholar] [CrossRef]
- Aich, A.; Chattopadhyay, B.; Mukhopadhyay, S.K. Immunolocalization of metallothionein in hepatocytes of guppy fish (Poecilia reticulata) exposed to tannery effluent: A biomarker study. Chemosphere 2017, 169, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Langston, W.J.; Chesman, B.S.; Burt, G.R.; Pope, N.D.; McEvoy, J. Metallothionein in liver of eels Anguilla anguilla from the Thames estuary: An indicator of environmental quality? Mar. Environ. Res. 2002, 53, 263–293. [Google Scholar] [CrossRef]
- Rodriguez-Cea, A.; Arias, A.R.L.; de la Campa, M.R.; Moreira, J.C.; Sanz-Medel, A. Metal speciation of metallothionein in white sea catfish, Netuma barba, and pearl cichlid, Geophagus brasiliensis, by orthogonal liquid chromatography coupled to ICP-MS detection. Talanta 2006, 69, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Van Campenhout, K.; Infante, H.G.; Goemans, G.; Belpaire, C.; Adams, F.; Blust, R.; Bervoets, L. A field survey of metal binding to metallothionein and other cytosolic ligands in liver of eels using an on-line isotope dilution method in combination with size exclusion (SE) high pressure liquid chromatography (HPLC) coupled to inductively coupled plasma time-of-flight mass spectrometry (ICP-TOFMS). Sci. Total Environ. 2008, 394, 379–389. [Google Scholar] [PubMed]
- Vasconcelos, M.H.; Tam, S.C.; Hesketh, J.E.; Reid, M.; Beattie, J.H. Metal- and tissue-dependent relationship between metallothionein mRNA and protein. Toxicol. Appl. Pharmacol. 2002, 182, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Scudiero, R.; Carginale, V.; Riggio, M.; Capasso, C.; Capasso, A.; Kille, P.; diPrisco, G.; Parisi, E. Difference in hepatic metallothionein content in antarctic red-blooded and haemoglobinless fish: Undetectable metallothionein levels in haemoglobinless fish is accompanied by accumulation of untranslated metallothionein mRNA. Biochem. J. 1997, 322, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Ghedira, J.; Jebali, J.; Bouraoui, Z.; Banni, M.; Guerbej, H.; Boussetta, H. Metallothionein and metal levels in liver, gills and kidney of Sparus aurata exposed to sublethal doses of cadmium and copper. Fish Physiol. Biochem. 2010, 36, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.N.; Volz, D.C.; Freedman, J.H.; Di Giulio, R.T. Differential display of hepatic mRNA from killifish (Fundulus heteroclitus) inhabiting a superfund estuary. Aquat. Toxicol. 2005, 73, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, S.M.M.; Ikenaka, Y.; Muzandu, K.; Choongo, K.; Oroszlany, B.; Teraoka, H.; Mizuno, N.; Ishizuka, M. Heavy metal accumulation in lake sediments, fish (Oreochromis niloticus and Serranochromis thumbergi), and crayfish (Cherax quadricarinatus) in Lake Itezhi-tezhi and Lake Kariba, Zambia. Arch. Environ. Contam. Toxicol. 2010, 59, 291–300. [Google Scholar] [CrossRef] [PubMed]
- M’Kandawire, E.; Syakalima, M.; Muzandu, K.; Pandey, G.; Simuunza, M.; Nakayama, S.M.M.; Kawai, Y.K.; Ikenaka, Y.; Ishizuka, M. The nucleotide sequence of metallothioneins (MT) in liver of the Kafue lechwe (Kobus leche kafuensis) and their potential as biomarkers of heavy metal pollution of the Kafue River. Gene 2012, 506, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Coetzee, L.; du Preez, H.H.; van Vuren, J.H.J. Metal concentrations in Clarias gariepinus and Labeo umbratus from the Olifants and Klein Olifants river, Mpumalanga, South Africa: Zinc, copper, manganese, lead, chromium, nickel, aluminium and iron. Water SA 2002, 28, 433–448. [Google Scholar] [CrossRef]
- Skelton, P.H. A Complete Guide to the Freshwater Fishes of Southern Africa; Struik Publishers: Cape Town, South Africa, 2001. [Google Scholar]
- Olojo, E.A.A.; Olurin, K.B.; Mbaka, G.; Oluwemimo, A.D. Histopathology of the gill and liver tissues of the African catfish Clarias gariepinus exposed to lead. Afr. J. Biotechnol. 2005, 4, 117–122. [Google Scholar]
- Jooste, A.; Marr, S.M.; Addo-Bediako, A.; Luus-Powell, W.J. Sharptooth catfish shows its metal: A case study of metal contamination at two impoundments in the Olifants River, Limpopo river system, South Africa. Ecotoxicol. Environ. Saf. 2015, 112, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Du Preez, G.; Wepener, V. Influence of mining pollution on metal bioaccumulation and biomarker responses in cave dwelling fish, Clarias gariepinus. Bull. Environ. Contam. Toxicol. 2016, 97, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Campana, O.; Sarasquete, C.; Blasco, J. Effect of lead on ala-d activity, metallothionein levels, and lipid peroxidation in blood, kidney, and liver of the toadfish Halobatrachus didactylus. Ecotoxicol. Environ. Saf. 2003, 55, 116–125. [Google Scholar] [CrossRef]
- Chowdhury, M.J.; Pane, E.F.; Wood, C.M. Physiological effects of dietary cadmium acclimation and waterborne cadmium challenge in rainbow trout: Respiratory, ionoregulatory, and stress parameters. Comp. Biochem. Physiol. C 2004, 139, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Schlenk, D.; Zhang, Y.S.; Nix, J. Expression of hepatic metallothionein messenger-RNA in feral and caged fish species correlates with muscle mercury levels. Ecotoxicol. Environ. Saf. 1995, 31, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Olsvik, P.A.; Gundersen, P.; Andersen, R.A.; Zachariassen, K.E. Metal accumulation and metallothionein in two populations of brown trout, Salmo trutta, exposed to different natural water environments during a run-off episode. Aquat. Toxicol. 2000, 50, 301–316. [Google Scholar] [CrossRef]
- Pathiratne, A.; Chandrasekera, L.; Pathiratne, K.A.S. Use of biomarkers in Nile tilapia (Oreochromis niloticus) to assess the impacts of pollution in Bolgoda lake, an urban water body in Sri Lanka. Environ. Monit. Assess. 2009, 156, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Ahmad, I.; Maria, V.L.; Serafim, A.; Bebianno, M.J.; Pacheco, M.; Santos, M.A. Hepatic metallothionein concentrations in the golden grey mullet (Liza aurata)—Relationship with environmental metal concentrations in a metal-contaminated coastal system in Portugal. Mar. Environ. Res. 2010, 69, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Kille, P.; Stephens, P.E.; Kay, J. Elucidation of cDNA sequences for metallothioneins from rainbow-trout, stone loach and pike liver using the polymerase chain-reaction. Biochim. Biophys. Acta 1991, 1089, 407–410. [Google Scholar] [CrossRef]
- Kambole, M.S. Managing the water quality of the Kafue River. Phys. Chem. Earth 2003, 28, 1105–1109. [Google Scholar] [CrossRef]
- Sheppe, W.A. Effects of human activities on Zambia Kafue Flats ecosystems. Environ. Conserv. 1985, 12, 49–57. [Google Scholar] [CrossRef]
- Choongo, E.H. Economic Aspects of the SADC Rural Industries Programme in Zambia; Zambian Alliance of Women: Lusaka, Zambia, 1994. [Google Scholar]
- Soucek, D.J.; Cherry, D.S.; Currie, R.J.; Latimer, H.A.; Trent, G.C. Laboratory to field validation in an integrative assessment of an acid mine drainage-impacted watershed. Environ. Toxicol. Chem. 2000, 19, 1036–1043. [Google Scholar] [CrossRef]
- Von der Heyden, C.J.; New, M.G. Sediment chemistry: A history of mine contaminant remediation and an assessment of processes and pollution potential. J. Geochem. Explor. 2004, 82, 35–57. [Google Scholar] [CrossRef]
- M’kandawire, E.; Choongo, K.; Yabe, J.; Mwase, M.; Saasa, N.; Nakayama, S.M.M.; Bortey-Sam, N.; Blindauer, C.A. Sediment metal contamination in the Kafue River of Zambia and ecological risk assessment. Bull. Environ. Contam. Toxicol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Olafson, R.W.; Thompson, J.A. Isolation of heavy-metal binding-proteins from marine vertebrates. Mar. Biol. 1974, 28, 83–86. [Google Scholar] [CrossRef]
- Quiniou, S.M.; Waldbieser, G.C.; Duke, M.V. A first generation BAC-based physical map of the channel catfish genome. BMC Genom. 2007, 8, 40. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, C.; Kim, J.; Kim, W.K.; Shin, H.S.; Lim, E.S.; Lee, J.W.; Kim, S.; Kim, K.T.; Lee, S.K.; et al. Cloning metallothionein gene in Zacco platypus and its potential as an exposure biomarker against cadmium. Environ. Monit. Assess. 2015, 187, 7. [Google Scholar] [CrossRef] [PubMed]
- Blindauer, C.A. Metallothioneins with unusual residues: Histidines as modulators of zinc affinity and reactivity. J. Inorg. Biochem. 2008, 102, 507–521. [Google Scholar] [CrossRef] [PubMed]
- Vašák, M.; McClelland, C.E.; Hill, H.A.O.; Kägi, J.H.R. Role of lysine side-chains n metallothionein. Experientia 1985, 41, 30–34. [Google Scholar] [CrossRef]
- Scudiero, R.; Temussi, P.A.; Parisi, E. Fish and mammalian metallothioneins: A comparative study. Gene 2005, 345, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Capasso, C.; Carginale, V.; Crescenzi, O.; Di Maro, D.; Parisi, E.; Spadaccini, R.; Temussi, P.A. Solution structure of MT_nc, a novel metallothionein from the Antarctic fish Notothenia coriiceps. Structure 2003, 11, 435–443. [Google Scholar] [CrossRef]
- Capasso, C.; Carginale, V.; Scudiero, R.; Crescenzi, O.; Spadaccini, R.; Temussi, P.A.; Parisi, E. Phylogenetic divergence of fish and mammalian metallothionein: Relationships with structural diversification and organismal temperature. J. Mol. Evol. 2003, 57, S250–S257. [Google Scholar] [CrossRef] [PubMed]
- Kille, P.; Stephens, P.; Cryer, A.; Kay, J. The expression of a synthetic rainbow-trout metallothionein gene in Escherichia coli. Biochim. Biophys. Acta 1990, 1048, 178–186. [Google Scholar] [CrossRef]
- Bofill, R.; Capdevila, M.; Atrian, S. Independent metal-binding features of recombinant metallothioneins convergently draw a step gradation between Zn- and Cu-thioneins. Metallomics 2009, 1, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Kowald, G.R.; Stürzenbaum, S.R.; Blindauer, C.A. Earthworm Lumbricus rubellus MT-2: Metal binding and protein folding of a true cadmium-MT. Int. J. Mol. Sci. 2016, 17, 16. [Google Scholar] [CrossRef] [PubMed]
- Zeitoun-Ghandour, S.; Charnock, J.M.; Hodson, M.E.; Leszczyszyn, O.I.; Blindauer, C.A.; Sturzenbaum, S.R. The two caenorhabditis elegans metallothioneins (CeMT-1 and CeMT-2) discriminate between essential zinc and toxic cadmium. FEBS J. 2010, 277, 2531–2542. [Google Scholar] [CrossRef] [PubMed]
- Kille, P.; Lees, W.E.; Darke, B.M.; Winge, D.R.; Dameron, C.T.; Stephens, P.E.; Kay, J. Sequestration of cadmium and copper by recombinant rainbow-trout and human metallothioneins and by chimeric (mermaid and fishman) proteins with interchanged domains. J. Biol. Chem. 1992, 267, 8042–8049. [Google Scholar] [PubMed]
- Vergani, L.; Grattarola, M.; Borghi, C.; Dondero, F.; Viarengo, A. Fish and molluscan metallothioneins—A structural and functional comparison. FEBS J. 2005, 272, 6014–6023. [Google Scholar] [CrossRef] [PubMed]
- Wingfield, P.T. N-terminal methionine processing. Curr. Protoc. Protein Sci. 2017, 88. [Google Scholar] [CrossRef]
- Spector, S.; Flynn, J.M.; Tidor, B.; Baker, T.A.; Sauer, R.T. Expression of N-formylated proteins in Escherichia coli. Protein Expr. Purif. 2003, 32, 317–322. [Google Scholar] [CrossRef] [PubMed]
- D’Auria, S.; Carginale, V.; Scudiero, R.; Crescenzi, O.; Di Maro, D.; Temussi, P.A.; Parisi, E.; Capasso, C. Structural characterization and thermal stability of Notothenia coriiceps metallothionein. Biochem. J. 2001, 354, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Infante, H.G.; Cuyckens, F.; Van Campenhout, K.; Blust, R.; Claeys, M.; Van Vaeck, L.; Adams, F.C. Characterization of metal complexes with metallothioneins in the liver of the carp Cyprinus carpio by reversed-phase, HPLC with ICP-MS and electrospray ionization (ESI)-MS. J. Anal. At. Spectrom. 2004, 19, 159–166. [Google Scholar] [CrossRef]
- Palacios, O.; Atrian, S.; Capdevila, M. Zn- and Cu-thioneins: A functional classification for metallothioneins? J. Biol. Inorg. Chem. 2011, 16, 991–1009. [Google Scholar] [CrossRef] [PubMed]
- Artells, E.; Palacios, O.; Capdevila, M.; Atrian, S. Mammalian MT1 and MT2 metallothioneins differ in their metal binding abilities. Metallomics 2013, 5, 1397–1410. [Google Scholar] [CrossRef] [PubMed]
- Kelley, L.A.; Mezulis, S.; Yates, C.M.; Wass, M.N.; Sternberg, M.J.E. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 2015, 10, 845–858. [Google Scholar] [CrossRef] [PubMed]
- Braun, W.; Vašák, M.; Robbins, A.H.; Stout, C.D.; Wagner, G.; Kägi, J.H.R.; Wüthrich, K. Comparison of the NMR solution structure and the X-ray crystal-structure of rat metallothionein-2. Proc. Natl. Acad. Sci. USA 1992, 89, 10124–10128. [Google Scholar] [CrossRef] [PubMed]
- Loska, K.; Wiechuła, D.; Korus, I. Metal contamination of farming soils affected by industry. Environ. Int. 2004, 30, 159–165. [Google Scholar] [CrossRef]
- Gaspic, Z.K.; Zvonaric, T.; Vrgoc, N.; Odzak, N.; Baric, A. Cadmium and lead in selected tissues of two commercially important fish species from the Adriatic Sea. Water Res. 2002, 36, 5023–5028. [Google Scholar] [PubMed]
- Rajkowska, M.; Protasowicki, M. Distribution of metals (Fe, Mn, Zn, Cu) in fish tissues in two lakes of different trophy in Northwestern Poland. Environ. Monit. Assess. 2013, 185, 3493–3502. [Google Scholar] [CrossRef] [PubMed]
- Bird, D.J.; Rotchell, J.M.; Hesp, S.A.; Newton, L.C.; Hall, N.G.; Potter, I.C. To what extent are hepatic concentrations of heavy metals in Anguilla anguilla at a site in a contaminated estuary related to body size and age and reflected in the metallothionein concentrations? Environ. Pollut. 2008, 151, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Tarras-Wahlberg, N.H.; Flachier, A.; Lane, S.N.; Sangfors, O. Environmental impacts and metal exposure of aquatic ecosystems in rivers contaminated by small scale gold mining: The Puyango River basin, southern Ecuador. Sci. Total Environ. 2001, 278, 239–261. [Google Scholar] [CrossRef]
- Olsson, P.E.; Haux, C.; Förlin, L. Variations in hepatic metallothionein, zinc and copper levels during an annual reproductive cycle in rainbow trout, Salmo gairdneri. Fish Phys. Biochem. 1987, 3, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Yacoub, A.M.; Gad, N.S. Accumulation of some heavy metals and biochemical alterations in muscles of Oreochromis niloticus from the River Nile in Upper Egypt. Int. J. Environ. Sci. 2012, 3, 1–10. [Google Scholar]
- Abdel-Satar, A.M. Environmental studies on the impact of the drains effluent upon the southern sector of Lake Manzalh, Egypt. Egypt. J. Aquat. Biol. Fish. 2001, 3, 17–30. [Google Scholar]
- Khaled, A. Heavy metals concentrations in certain tissues of five commercially important fishes from El-Mex Bay, Alexandria, Egypt. Egypt. J. Aquat. Biol. Fish. 2004, 8, 51–64. [Google Scholar]
- Ali, M.H.; Fishar, M.R. Accumulation of trace metals in some benthic invertebrate and fish species relevant to their concentration in water and sediment of lake Qarun, Egypt. Egypt. J. Aquat. Res. 2005, 31, 289–301. [Google Scholar]
- Nussey, G.; van Vuren, J.H.J.; du Preez, H.H. Bioaccumulation of chromium, manganese, nickel and lead in the tissues of the moggel, Labeo umbratus (cyprinidae), from Witbank dam, Mpumalanga. Water SA 2000, 26, 269–284. [Google Scholar]
- Bremner, I. Interactions between metallothionein and trace elements. Prog. Food Nutr. Sci. 1987, 11, 1–37. [Google Scholar] [PubMed]
- Chan, K.M.; Ku, L.L.; Chan, P.C.Y.; Cheuk, W.K. Metallothionein gene expression in zebrafish embryo-larvae and ZFL cell-line exposed to heavy metal ions. Mar. Environ. Res. 2006, 62, S83–S87. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Y.; Goyer, R.A.; Achanzar, W.; Waalkes, M.P. Metallothionein-I/II null mice are more sensitive than wild-type mice to the hepatotoxic and nephrotoxic effects of chronic oral or injected inorganic arsenicals. Toxicol. Sci. 2000, 55, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Hauser-Davis, R.A.; Silva, J.A.; Rocha, R.C.; Saint’Pierre, T.; Ziolli, R.L.; Arruda, M.A. Acute selenium selenite exposure effects on oxidative stress biomarkers and essential metals and trace-elements in the model organism zebrafish (Danio rerio). J. Trace Elem. Med. Biol. 2016, 33, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Klaassen, C.D.; Liu, J.; Diwan, B.A. Metallothionein protection of cadmium toxicity. Toxicol. Appl. Pharmacol. 2009, 238, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Hogstrand, C.; Lithner, G.; Haux, C. The importance of metallothionein for the accumulation of copper, zinc and cadmium in environmentally exposed perch, Perca fluviatilis. Pharmacol. Toxicol. 1991, 68, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Filipovic, V.; Raspor, B. Metallothionein and metal levels in cytosol of liver, kidney and brain in relation to growth parameters of Mullus surmuletus and Liza aurata from the eastern Adriatic sea. Water Res. 2003, 37, 3253–3262. [Google Scholar] [CrossRef]
- Bervoets, L.; Knapen, D.; De Jonge, M.; Van Campenhout, K.; Blust, R. Differential hepatic metal and metallothionein levels in three feral fish species along a metal pollution gradient. PLoS ONE 2013, 8, e60805. [Google Scholar] [CrossRef] [PubMed]
- Steinbrenner, H.; Speckmann, B.; Klotz, L.O. Selenoproteins: Antioxidant selenoenzymes and beyond. Arch. Biochem. Biophys. 2016, 595, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Navarro, A.; Quiros, L.; Casado, M.; Faria, M.; Carrasco, L.; Benejam, L.; Benito, J.; Diez, S.; Raldua, D.; Barata, C.; et al. Physiological responses to mercury in feral carp populations inhabiting the low Ebro river (NE Spain), a historically contaminated site. Aquat. Toxicol. 2009, 93, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, D.A.; Rantin, F.T.; Kalinin, A.L. Inorganic mercury exposure: Toxicological effects, oxidative stress biomarkers and bioaccumulation in the tropical freshwater fish matrinx, Brycon amazonicus (Spix and Agassiz, 1829). Ecotoxicology 2010, 19, 105–123. [Google Scholar] [CrossRef] [PubMed]
- Sinaie, M.; Bastami, K.D.; Ghorbanpour, M.; Najafzadeh, H.; Shekari, M.; Haghparast, S. Metallothionein biosynthesis as a detoxification mechanism in mercury exposure in fish, spotted scat (Scatophagus argus). Fish Physiol. Biochem. 2010, 36, 1235–1242. [Google Scholar] [CrossRef] [PubMed]
- Banni, M.; Jebali, J.; Guerbej, H.; Dondero, F.; Boussetta, H.; Viarengo, A. Mixture toxicity assessment of nickel and chlorpyrifos in the sea bass Dicentrarchus labrax. Arch. Environ. Contam. Toxicol. 2011, 60, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Velma, V.; Tchounwou, P.B. Hexavalent chromium-induced multiple biomarker responses in liver and kidney of goldfish, Carassius auratus. Environ. Toxicol. 2011, 26, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Ceyhun, S.B.; Aksakal, E.; Ekinci, D.; Erdogan, O.; Beydemir, S. Influence of cobalt and zinc exposure on mRNA expression profiles of metallothionein and cytocrome P450 in rainbow trout. Biol. Trace Elem. Res. 2011, 144, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Paul, J.F. Developing and applying an index of environmental integrity for the US Mid-Atlantic region. J. Environ. Manag. 2003, 67, 175–185. [Google Scholar] [CrossRef]
- Tomlinson, D.L.; Wilson, J.G.; Harris, C.R.; Jeffrey, D.W. Problems in the assessment of heavy-metal levels in estuaries and the formation of a pollution index. Helgol. Meeresunters. 1980, 33, 566–575. [Google Scholar] [CrossRef]
- Hakanson, L. An ecological risk index for aqwuatoc pollution control. A sedimentological approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Dallinger, R.; Berger, B.; Gruber, C.; Hunziker, P.; Stürzenbaum, S. Metallothioneins in terrestrial invertebrates: Structural aspects, biological significance and implications for their use as biomarkers. Cell. Mol. Biol. 2000, 46, 331–346. [Google Scholar] [PubMed]
- Höckner, M.; Dallinger, R.; Stürzenbaum, S.R. Nematode and snail metallothioneins. J. Biol. Inorg. Chem. 2011, 16, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
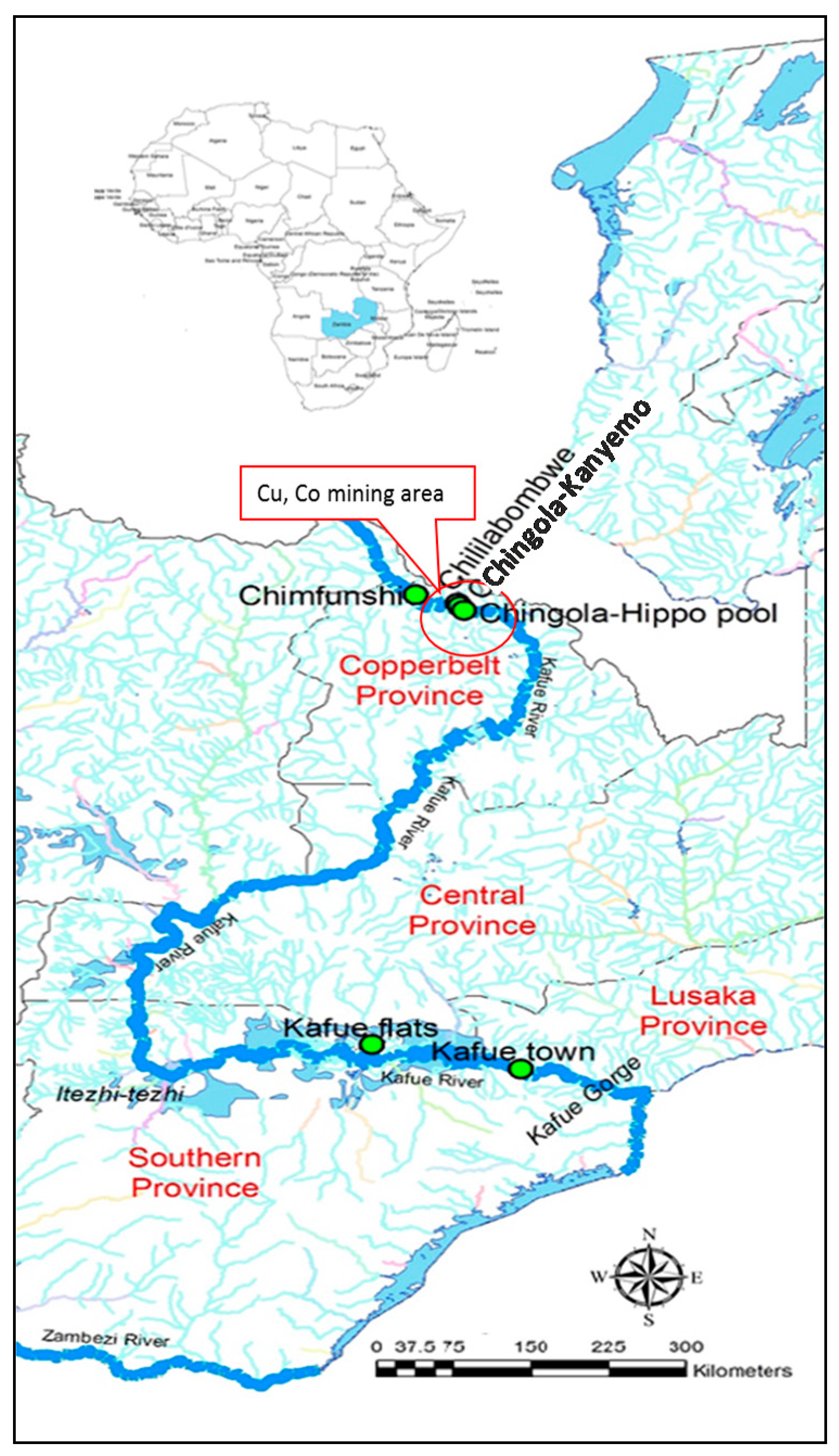
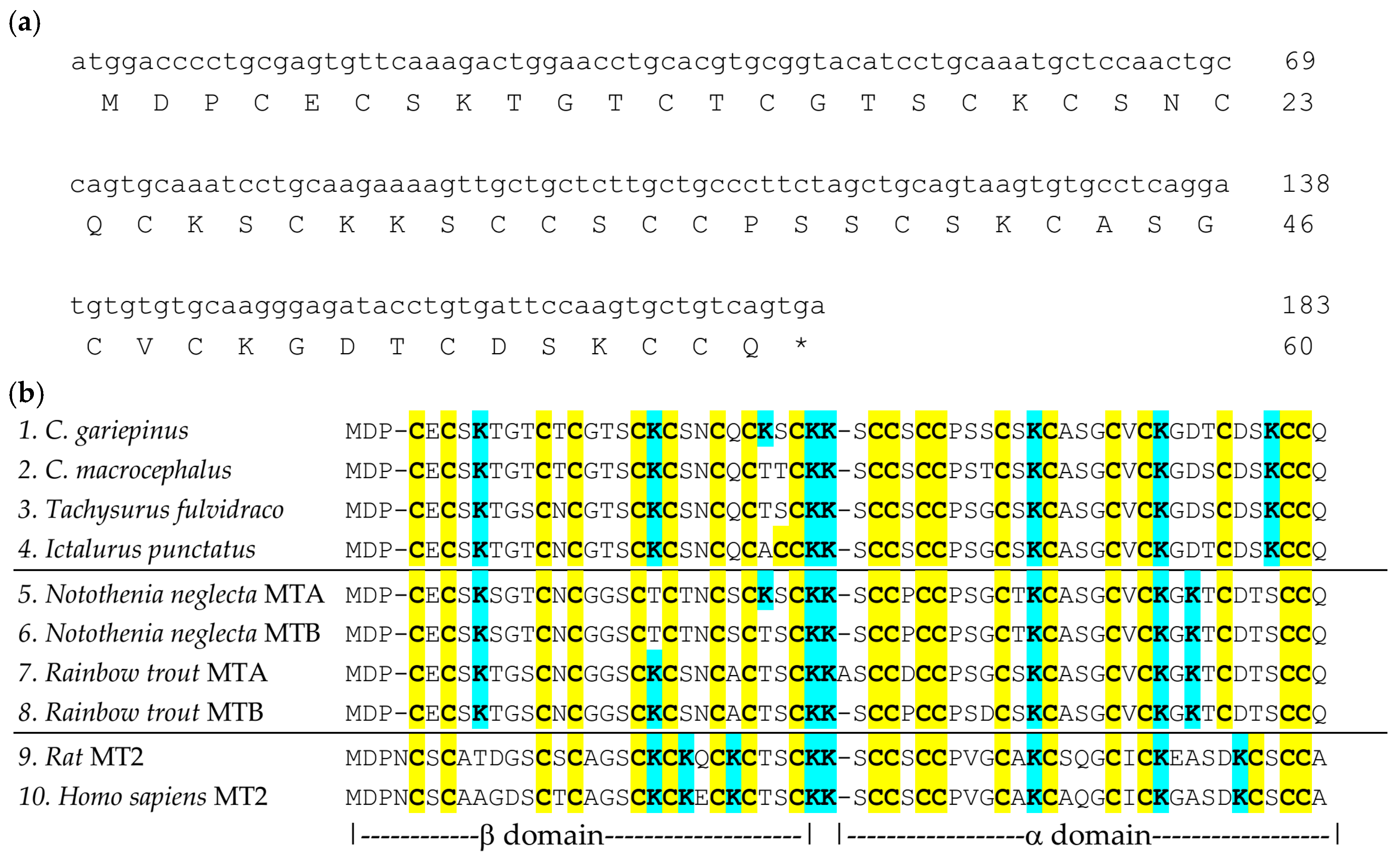
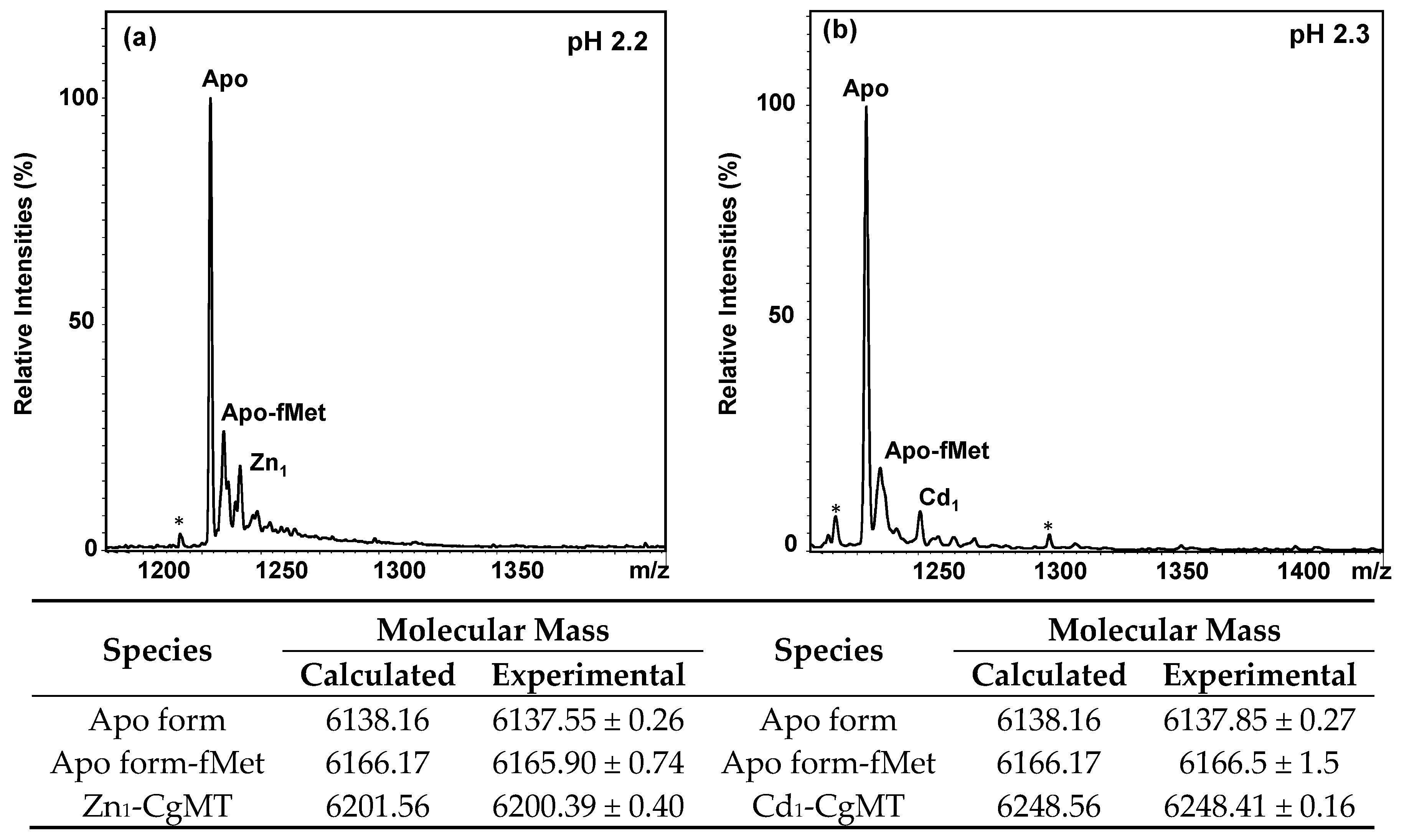

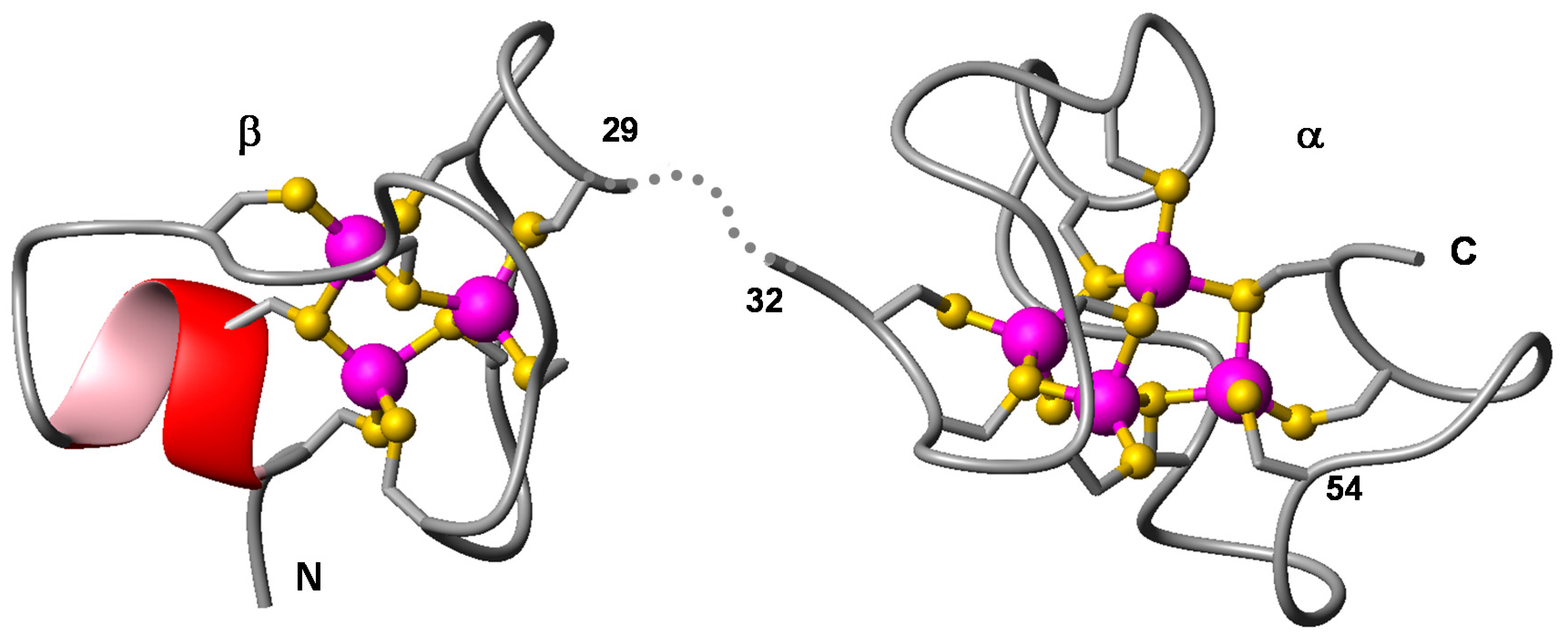
| Supplemented Ion | Sulphur (mg/L) | Sulphur (µM) | Protein (µM) | Metal | Metal (mg/L) | Metal (µM) | M: Protein Ratio |
|---|---|---|---|---|---|---|---|
| Zn(II) | 0.78 | 97.81 | 4.66 | Zn | 0.54 | 33.06 | 6.88 ± 0.89 |
| Cd(II) | 2.31 | 144.38 | 6.88 | Cd | 2.98 | 53.01 | 7.72 ± 0.83 |
| Al | Cr | Mn | Fe | Co | Cu | Zn | Se | Cd | Hg | Pb | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Al | - | (0.5719) | (0.0426 *) | (0.1794) | (0.6672) | (0.7432) | (0.2225) | (0.2097) | (0.0087 *) | (0.1707) | (0.3160) |
| Cr | −0.15 | - | (0.7745) | (0.5747) | (0.7506) | (0.4018) | (0.7898) | (0.5735) | (0.0380 *) | (0.8042 | (0.6222) |
| Mn | 0.18 | −0.08 | - | (0.0169 *) | (<0.00001 *) | (<0.00001 *) | (0.0032 *) | (0.2207) | (0.0285 *) | (0.0056 *) | (0.0010 *) |
| Fe | 0.12 | −0.15 | 0.22 | - | (<0.00001 *) | (<0.00001 *) | (<0.00001 *) | (0.1127) | (0.8114) | (0.0883) | (0.2067) |
| Co | −0.04 | −0.08 | 0.45 | 0.38 | - | (<0.00001 *) | (<0.00001 *) | (0.0005 *) | (<0.00001 *) | (0.0058 *) | (<0.00001 *) |
| Cu | 0.03 | 0.22 | 0.36 | 0.41 | 0.66 | - | (<0.00001 *) | (0.0019 *) | 0.0290 *) | (0.0344 *) | (<0.00001 *) |
| Zn | 0.11 | 0.07 | 0.26 | 0.60 | 0.41 | 0.66 | - | (0.0453 *) | (0.4079) | (0.0017 *) | (0.1168) |
| Se | −0.11 | 0.15 | 0.11 | 0.14 | 0.31 | 0.28 | 0.18 | - | 0.0003 *) | (0.8902) | (0.0319 *) |
| Cd | −0.29 | 0.54 | 0.24 | 0.03 | 0.56 | 0.24 | −0.09 | 0.39 | - | (0.6306) | (0.0109 *) |
| Hg | 0.13 | (0.07) | 0.25 | 0.15 | 0.25 | 0.19 | 0.28 | 0.01 | 0.05 | - | (0.9603) |
| Pb | 0.12 | 0.17 | 0.38 | 0.15 | 0.49 | 0.47 | 0.19 | 0.25 | 0.30 | 0.01 | - |
| Metal | Chimfunshi | Chililabombwe | Kafue Flats | Kafue Town | ||||
|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | |
| Al | 0.20 | 0.2896 | 0.06 | 0.7389 | 0.10 | 0.5186 | 0.15 | 0.2775 |
| Cr | - | - | −0.19 | 0.2840 | - | - | - | - |
| Mn | 0.29 | 0.1102 | 0.09 | 0.5927 | −0.09 | 0.5530 | −0.09 | 0.5326 |
| Fe | 0.05 | 0.7673 | −0.32 | 0.0608 | 0.17 | 0.2675 | 0.23 | 0.0892 |
| Co | 0.08 | 0.6564 | 0.20 | 0.2429 | −0.16 | 0.2867 | 0.04 | 0.7789 |
| Cu | 0.39 | 0.0270 * | 0.40 | 0.0162 * | 0.37 | 0.0135 * | 0.58 | <0.0001 * |
| Zn | 0.52 | 0.0024 * | −0.04 | 0.8096 | 0.45 | 0.0022 * | 0.47 | 0.0003 * |
| Se | −0.12 | 0.5094 | 0.57 | 0.0003 * | −0.03 | 0.8677 | 0.10 | 0.4710 |
| Cd | −0.34 | 0.0572 | 0.40 | 0.0172 * | 0.08 | 0.6277 | - | - |
| Hg | 0.22 | 0.2197 | −0.11 | 0.5482 | 0.38 | 0.0101 * | 0.35 | 0.0097 * |
| Pb | −0.26 | 0.1493 | 0.16 | 0.3699 | 0.18 | 0.2410 | - | - |
| Gene | Primer/Probe | Sequence |
|---|---|---|
| cgMT | Forward primer | 5′-ACTGCCAGTGCAAATCCTG-3′ |
| Reverse primer | 5′-TCCTGAGGCACACTTACTGC-3′ | |
| Probe | 5′-TGCTGCTC-3′ | |
| cgβ-actin | Forward primer | 5′-AGACACCAGGGTGTGATGGT-3′ |
| Reverse primer | 5′-GCTCTGAGCTTCATCACCA-3′ | |
| Probe | 5′-GACCCAGA-3′ |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
M’kandawire, E.; Mierek-Adamska, A.; Stürzenbaum, S.R.; Choongo, K.; Yabe, J.; Mwase, M.; Saasa, N.; Blindauer, C.A. Metallothionein from Wild Populations of the African Catfish Clarias gariepinus: From Sequence, Protein Expression and Metal Binding Properties to Transcriptional Biomarker of Metal Pollution. Int. J. Mol. Sci. 2017, 18, 1548. https://doi.org/10.3390/ijms18071548
M’kandawire E, Mierek-Adamska A, Stürzenbaum SR, Choongo K, Yabe J, Mwase M, Saasa N, Blindauer CA. Metallothionein from Wild Populations of the African Catfish Clarias gariepinus: From Sequence, Protein Expression and Metal Binding Properties to Transcriptional Biomarker of Metal Pollution. International Journal of Molecular Sciences. 2017; 18(7):1548. https://doi.org/10.3390/ijms18071548
Chicago/Turabian StyleM’kandawire, Ethel, Agnieszka Mierek-Adamska, Stephen R. Stürzenbaum, Kennedy Choongo, John Yabe, Maxwell Mwase, Ngonda Saasa, and Claudia A. Blindauer. 2017. "Metallothionein from Wild Populations of the African Catfish Clarias gariepinus: From Sequence, Protein Expression and Metal Binding Properties to Transcriptional Biomarker of Metal Pollution" International Journal of Molecular Sciences 18, no. 7: 1548. https://doi.org/10.3390/ijms18071548





