Dynamic Changes of Pectin Epitopes in Cell Walls during the Development of the Procambium–Cambium Continuum in Poplar
Abstract
:1. Introduction
2. Results
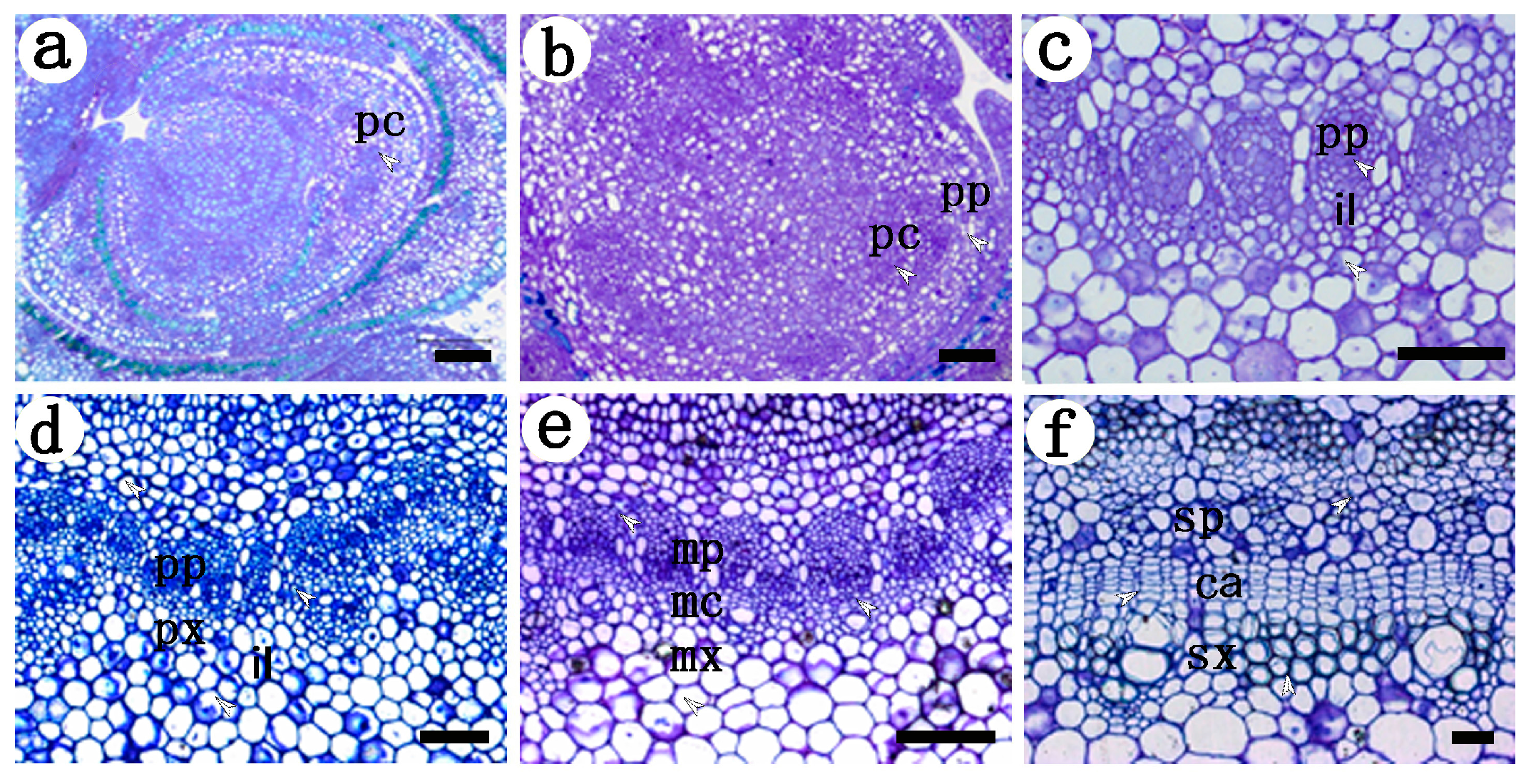
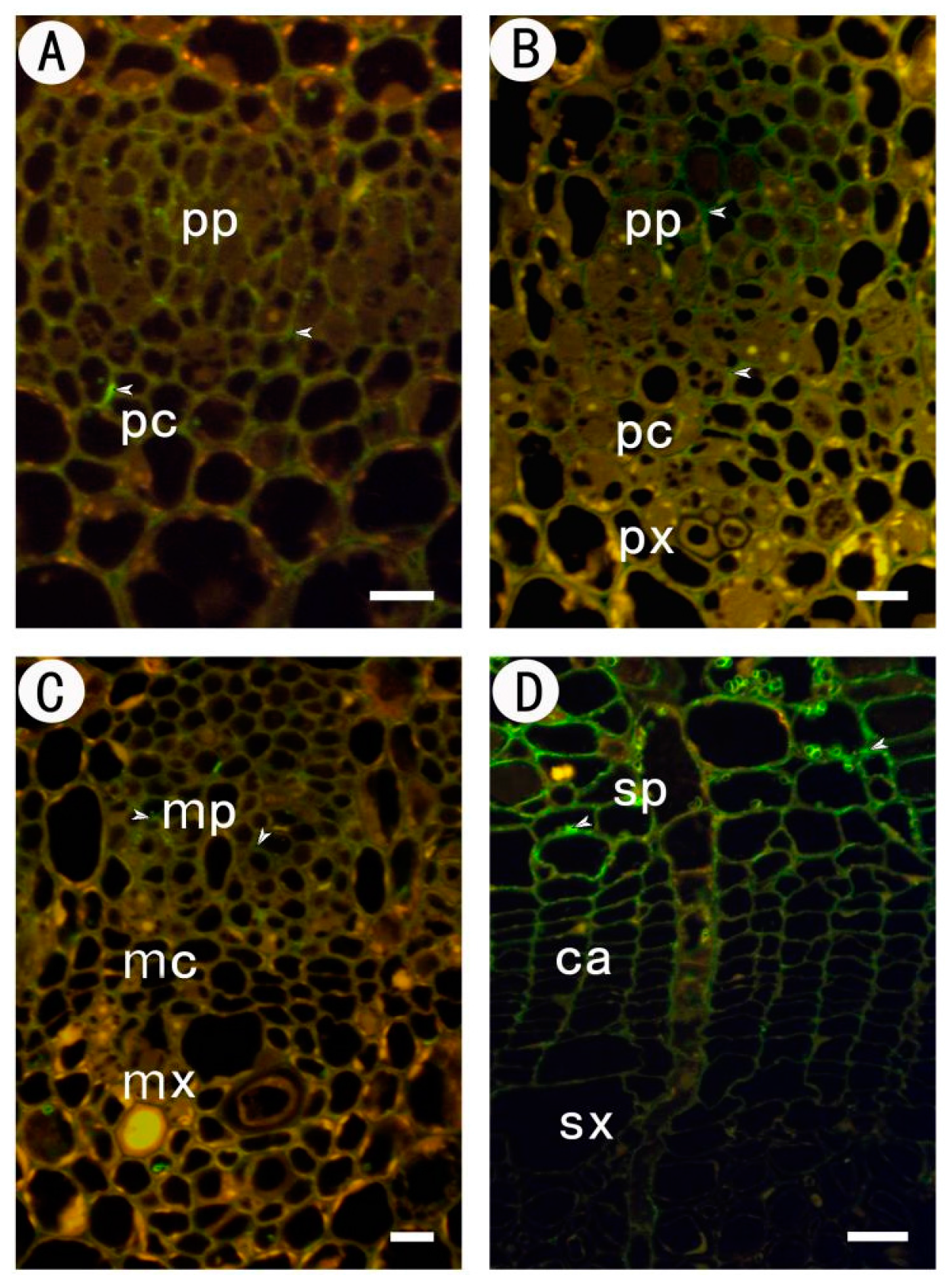
2.1. Development of the Procambium–Cambium Continuum in P. tomentosa
2.2. Distribution of Pectin Epitopes in Procambium–Cambium Continuum Development
2.2.1. Identification of Low Methyl HGA by Monoclonal Antibody JIM5
2.2.2. Identification of High Methyl HGA by Monoclonal Antibody JIM7
2.3.3. Identification of (1→4)-β-d-galactan in Rhamnogalacturonan-I Side Chains by LM5
2.3.4. Identification of (1→5)-a-l-arabinan in Rhamnogalacturonan-I Side Chains by LM6
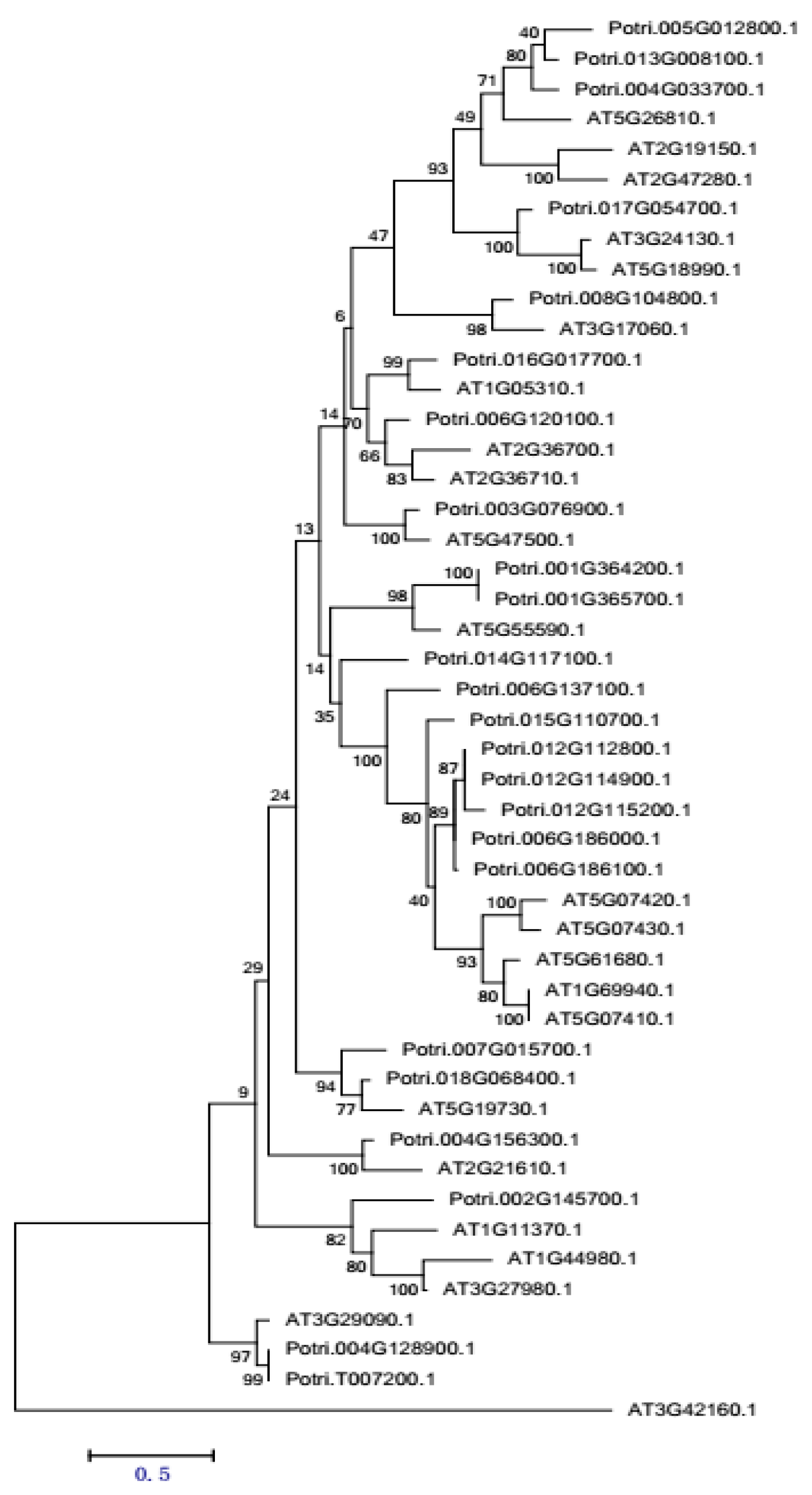
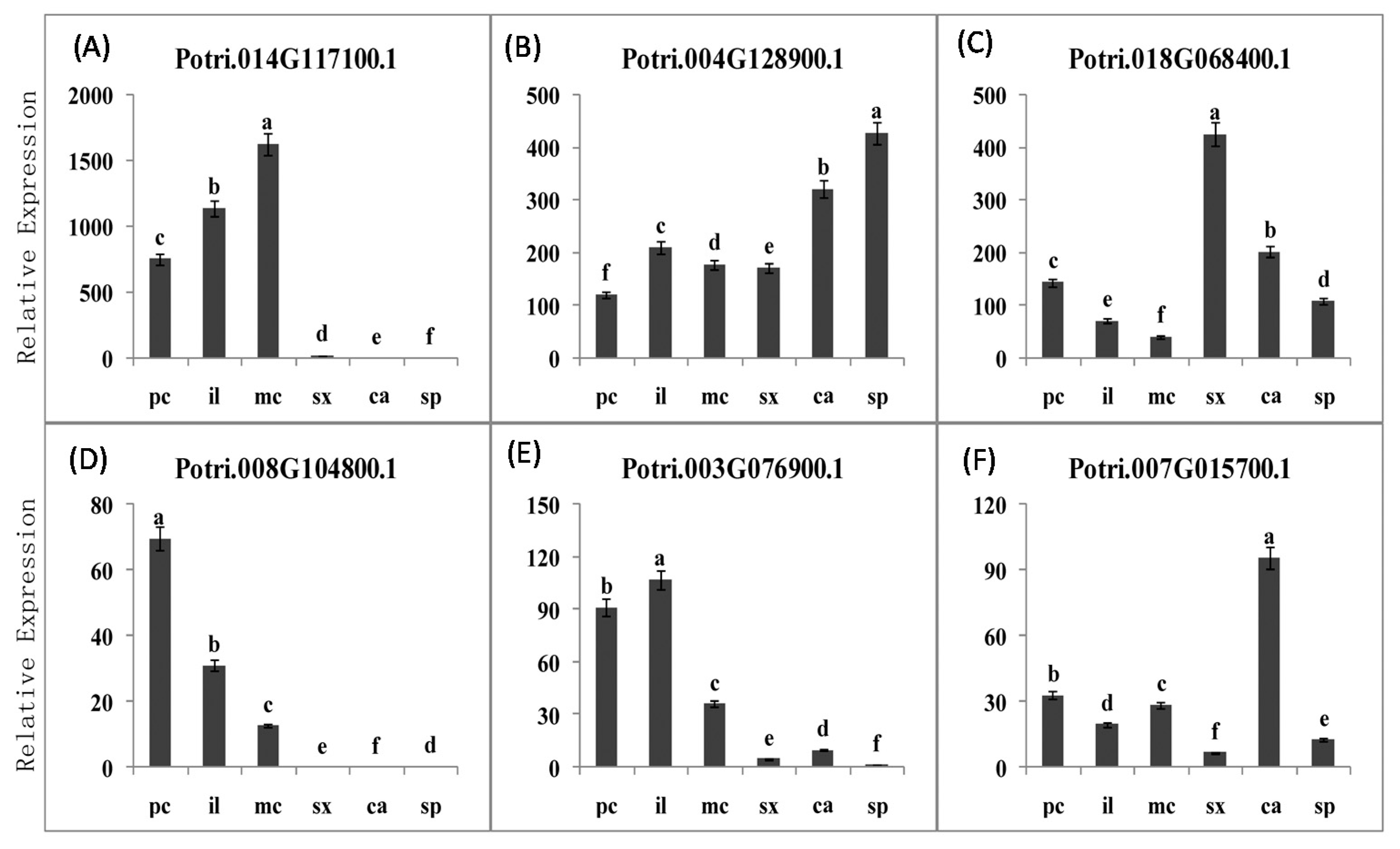
2.3.5. PME Gene Family Sequence Analysis and Expression in Procambium–Cambium Continuum Development
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Immunofluorescent Labelling
4.3. Tangential Cryo-Sectioning, RNA Isolation and RNA Sequencing
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hongo, S.; Sato, K.; Yokoyama, R.; Nishitani, K. Demethylesterification of the Primary Wall by PECTIN METHYLESTERASE35 Provides Mechanical Support to the Arabidopsis Stem. Plant Cell 2012, 24, 2624–2634. [Google Scholar] [CrossRef] [PubMed]
- Schrader, J.; Nilsson, J.; Mellerowicz, E.; Berglund, A.; Nilsson, P.; Hertzberg, M.; Sandberg, G. A high-resolution transcript profile across the wood-forming meristem of poplar identifies potential regulators of cambial stem cell identity. Plant Cell 2004, 16, 2278–2292. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Wang, H. Molecular Mechanisms for Vascular Development and Secondary Cell Wall Formation. Front. Plant Sci. 2016, 7, 356. [Google Scholar] [CrossRef] [PubMed]
- Snegireva, A.; Chernova, T.; Ageeva, M.; Lev-Yadun, S.; Gorshkova, T. Intrusive growth of primary and secondary phloem fibres in hemp stem determines fibre-bundle formation and structure. AoB Plants 2015, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Larson, P.R. The Vascular Cambium: Development and Structure; Springer: Berlin/Heidelberg, Germany, 1994. [Google Scholar]
- Larson, P.R. Development and organization of the primary vascular system in populus deltoides according to Phyllotaxy. Am. J. Bot. 1975, 62, 1084–1099. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, G.; Chen, J.J.; Taylor, G.; Cui, K.M.; He, X.Q. Molecular features of secondary vascular tissue regeneration after bark girdling in Populus. New Phytol. 2011, 192, 869–884. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.M.; Han, J.J.; Cui, K.M.; He, X.Q. Modification of cambial cell wall architecture during cambium periodicity in Populus. Trees 2010, 24, 533–540. [Google Scholar] [CrossRef]
- Kakegawa, K.; Edashige, Y.; Ishii, T. Metabolism of cell wall polysaccharides in cell suspension cultures of Populus alba in relation to cell growth. Physiol. Plant. 2000, 108, 420–425. [Google Scholar] [CrossRef]
- Fry, S.C. Primary cell wall metabolism: Tracking the careers of wall polymers in living plant cells. New Phytol. 2004, 161, 641–675. [Google Scholar] [CrossRef]
- Sørensen, I.; Pettolino, F.A.; Bacic, A.; Ralph, J.; Lu, F. The charophycean green algae provide insights into the early origins of plant cell walls. Plant J. 2011, 68, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Chaffey, N. Cambium: Old challenges-new opportunities. Trees 1999, 13, 138–151. [Google Scholar] [CrossRef]
- Harholt, J.; Suttangkakul, A.; Vibe, S.H. Biosynthesis of pectin. Plant Physiol. 2010, 153, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Willats, W.G.; McCartney, L.; Mackie, W.; Knox, J.P. Pectin: Cell biology and prospects for functional analysis. Plant Mol. Biol. 2001, 47, 9–27. [Google Scholar] [CrossRef] [PubMed]
- Sujkowska, R.M.; Borucki, W. Pectins esterification in the apoplast of aluminum-treated pea root nodules. J. Plant Physiol. 2015, 184, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Lu, L.; Wu, X.; Li, Y.; Lin, J. Boron influences pollen germination and pollen tube growth in Picea meyeri. Tree Physiol. 2003, 23, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Willats, W.G.; Orfila, C.; Limberg, G.; Buchholt, H.C.; van Alebeek, G.J.; Voragen, A.G.; Marcus, S.E.; Christensen, T.M.; Mikkelsen, J.D.; Murray, B.S.; et al. Modulation of the degree and pattern of methyl-esterification of pectic homogalacturonan in plant cell walls. Implications for pectin methyl esterase action, matrix properties, and cell adhesion. J. Biol. Chem. 2001, 2769, 19404–19413. [Google Scholar] [CrossRef] [PubMed]
- Bosch, M.; Cheung, A.Y.; Hepler, P.K. Pectin methylesterase, a regulator of pollen tube growth. Plant Physiol. 2005, 138, 1334–1346. [Google Scholar] [CrossRef] [PubMed]
- Arend, M. Immunolocalization of (1,4)-β-galactan in tension wood fibers of poplar. Tree Physiol. 2011, 28, 1263–1267. [Google Scholar] [CrossRef]
- Kim, S.J.; Daniel, G. Immunocytochemical studies of axial resin canals. II. Localization of non-cellulosic polysaccharides in epithelium and subsidiary cells of Scots pine. IAWA 2014, 25, 253–269. [Google Scholar] [CrossRef]
- Jones, L.; Seymour, G.B.; Knox, J.P. Localization of pectic galactan in tomato cell walls using a monoclonal antibody specific to (1 [->] 4)-[beta]-D-galactan. Plant Physiol. 1997, 113, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Willats, W.G.; Marcus, S.E.; Knox, J.P. Generation of a monoclonal antibody specific to (1→5)-α-l-arabinan. Carbohydr. Res. 1998, 308, 149–152. [Google Scholar] [CrossRef]
- Knox, J.P. Revealing the structural and functional diversity of plant cell walls. Curr. Opin. Plant Biol. 2008, 11, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Ermel, F.F.; Follet-Gueye, M.L.; Cibert, C.; Vian, B.; Morvan, C.; Catesson, A.M.; Goldberg, R. Differential localization of arabinan and galactan side chains of rhamnogalacturonan 1 in cambial derivatives. Planta 2000, 210, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Herbette, S.; Bouchet, B.; Brunel, N.; Bonnin, E.; Cochard, H.; Guillon, F. Immunolabelling of intervessel pits for polysaccharides and lignin helps in understanding their hydraulic properties in Populus tremula × alba. Ann. Bot. 2015, 115, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Geisler-Lee, J.; Geisler, M.; Coutinho, P.M.; Segerman, B.; Nishikubo, N.; Takahashi, J.; Aspeborg, H.; Djerbi, S.; Master, E.; Andersson-Gunnerås, S.; et al. Poplar carbohydrate-active enzymes. Gene identification and expression analyses. Plant Physiol. 2006, 140, 946–962. [Google Scholar] [CrossRef] [PubMed]
- Louvet, R.; Cavel, E.; Gutierrez, L.; Guénin, S.; Roger, D.; Gillet, F.; Guerineau, F.; Pelloux, J. Comprehensive expression profiling of the pectin methylesterase gene family during silique development in Arabidopsis thaliana. Planta 2006, 224, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Pelloux, J.; Rustérucci, C.; Mellerowicz, E.J. New insights into pectin methylesterase structure and function. Trends Plant Sci. 2007, 12, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yuan, D.; Gao, W.; Li, Y.; Tan, J.; Zhang, X. A comparative genome analysis of PME and PMEI families reveals the evolution of pectin metabolism in plant cell walls. PLoS ONE 2013, 8, e72082. [Google Scholar] [CrossRef] [PubMed]
- Keegstra, K. Plant cell walls. Plant Physiol. 2010, 154, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Peaucelle, A.; Braybrook, S.A.; Le, G.L.; Bron, E.; Kuhlemeier, C. Pectin-induced changes in cell wall mechanics underlie organ initiation in Arabidopsis. Curr. Biol. 2011, 21, 1720–1726. [Google Scholar] [CrossRef] [PubMed]
- Chateigner-Boutin, A.; Suliman, M.; Bouchet, B.; Alvarado, C.; Lollier, V.; Rogniaux, H.; Guillon, F.; Larré, C. Endomembrane proteomics reveals putative enzymes involved in cell wall metabolism in wheat grain outer layers. J. Exp. Bot. 2015, 66, 2649–2658. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, P.; Fernández, V.; García, M.L.; Khayet, M.; Fernández, A.; Gil, L. Localization of polysaccharides in isolated and intact cuticles of eucalypt, poplar and pear leaves by enzyme-gold labelling. Plant Physiol. Biochem. 2014, 76, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, Y.B.; Cosgrove, D.J. Xyloglucan and its Interactions with Other Components of the Growing Cell Wall. Plant Cell Physiol. 2014, 56, 180–194. [Google Scholar] [CrossRef] [PubMed]
- Siedlecka, A.; Wiklund, S.; Péronne, M.A.; Micheli, F.; Lesniewska, J.; Sethson, I.; Edlund, U.; Richard, L.; Sundberg, B.; Mellerowicz, E.J. Pectin methyl esterase in hibits intrusive and symplastic cell growth in developing wood cells of Populus. Plant Physiol. 2008, 146, 554–565. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Sun, J.; Li, L. Diverse roles of PtrDUF579 proteins in Populus and PtrDUF579-1 function in vascular cambium proliferation during secondary growth. Plant Mol. Biol. 2014, 85, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Egelund, J.; Skjøt, M.; Geshi, N.; Ulvskov, P.; Petersen, B.L. A complementary bioinformatics approach to identify potential plant cell wall glycosyltransferase-encoding genes. Plant Physiol. 2004, 136, 2609–2620. [Google Scholar] [CrossRef] [PubMed]
- Nishikubo, N.; Takahashi, J.; Roos, A.A.; Derba-Maceluch, M.; Piens, K.; Brumer, H.; Teeri, T.T.; Stålbrand, H.; Mellerowicz, E.J. Xyloglucan endo-Transglycosylase-Mediated xyloglucan rearrangements in developing wood of hybrid aspen. Plant J. 2011, 155, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Biswal, A.K.; Hao, Z.; Pattathil, S.; Yang, X.; Winkeler, K.; Collins, C.; Mohanty, S.S.; Richardson, E.A.; Gelineo-Albersheim, I.; Hunt, K.; et al. Downregulation of GAUT12 in Populus deltoides by RNA silencing results in reduced recalcitrance; increased growth and reduced xylan and pectin in a woody biofuel feedstock. Biotechnol. Biofuels 2015, 8, 41. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Larson, P.R. The concept of cambium. In New Perspectives in Wood Anatomy; Baas, P., Ed.; Springer: Haarlem, The Netherlands, 1982; pp. 85–92. [Google Scholar]
- Jin, F.; Li, J.; Ding, Q.; Wang, Q.S.; He, X.Q. Proteomic analysis provides insights into changes in the central metabolism of the cambium during dormancy release in poplar. J. Plant Physiol. 2017, 208, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Larson, P.R. Procambium vs. Cambium and Protoxylem vs. Metaxylem in Populus deltoides Seedlings. Am. J. Bot. 1976, 63, 1332–1348. [Google Scholar] [CrossRef]
- Lee, Y.; Ayeh, K.O.; Ambrose, M.; Hvoslef-Eide, A.K. Immunolocalization of pectic polysaccharides during abscission in pea seeds (Pisumsativum L.) and in abscission less def pea mutant seeds. BMC Res. Notes 2016, 9, 427. [Google Scholar] [CrossRef] [PubMed]
- Guillemin, F.; Guillon, F.; Bonnin, E.; Devaux, M.F.; Chevalier, T.; Knox, J.P.; Liners, F.; Thibault, J.F. Distribution of pectic epitopes in cell walls of the sugar beet root. Planta 2005, 222, 355–371. [Google Scholar] [CrossRef] [PubMed]
- McCartney, L.; Steele-King, C.G.; Jordan, E.; Knox, J.P. Cell wall pectic (1→4)-β-d-galactan marks the acceleration of cell elongation in the Arabidopsis seedling root meristem. Plant J. 2003, 33, 447–454. [Google Scholar] [CrossRef] [PubMed]
- McCartney, L.; Ormerod, A.P.; Gidley, M.J.; Knox, J.P. Temporal and spatial regulation of pectic (1→4)-β-d-galactan in cell walls of developing pea cotyledons: Implications for mechanical properties. Plant J. 2000, 22, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Vicré, M.; Jauneau, A.; Knox, J.P.; Driouich, A. Immunolocalization of β-(1→4) and β-(1→6)-d-galactan epitopes in the cell wall and Golgi stacks of developing flax root tissues. Protoplasma 1998, 203, 26–34. [Google Scholar] [CrossRef]
- Uggla, C.; Sundberg, B.; Chaffey, N. Sampling of cambial region tissues for high resolution analysis. In Wood Formation in Trees; Taylor & Francis: Abingdon, UK, 2002; pp. 215–228. [Google Scholar]
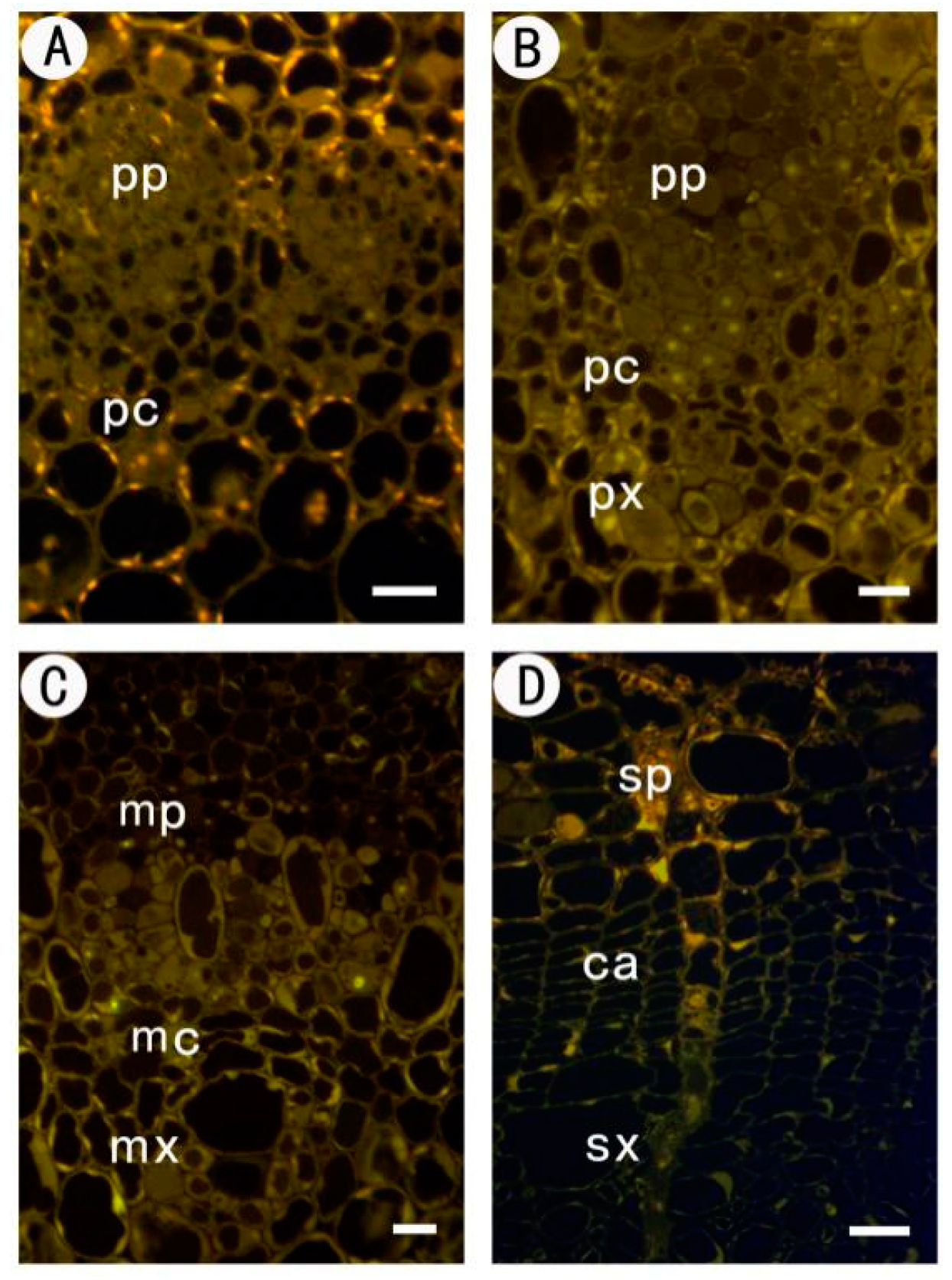
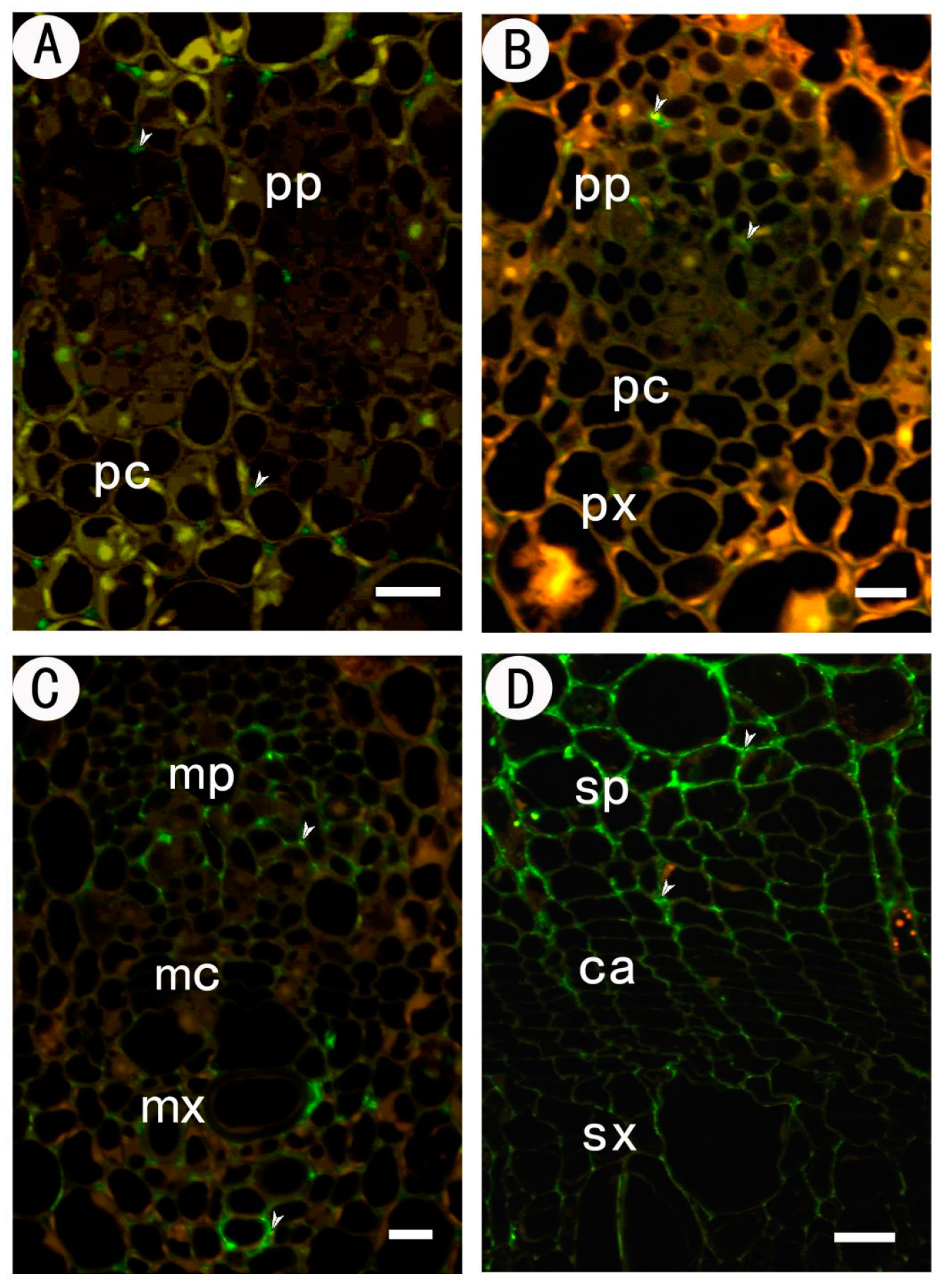

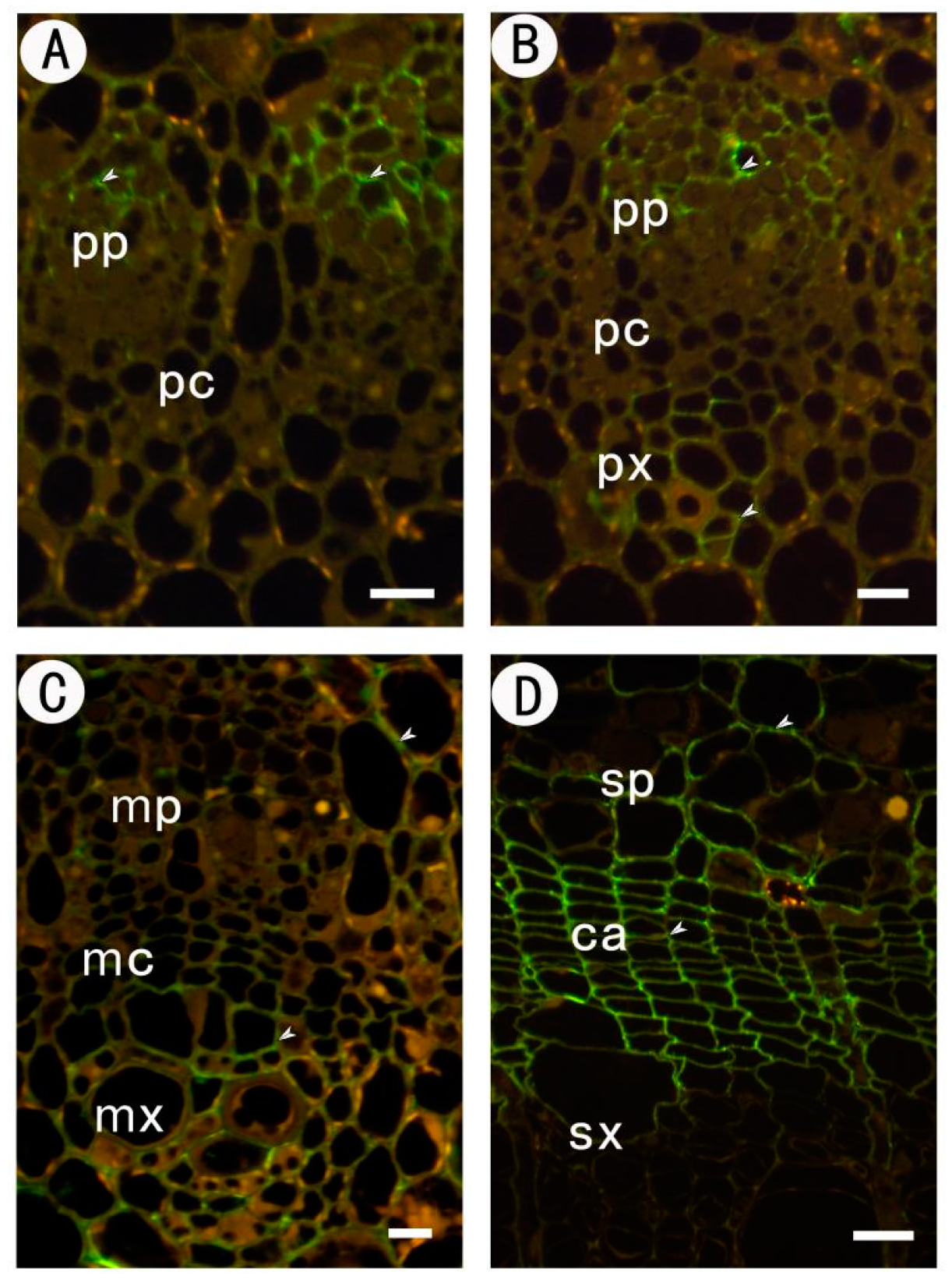
| Tissues | JIM5 | JIM7 | LM5 | LM6 |
|---|---|---|---|---|
| protophloem | ++ | + | + | + |
| procambium | − | + | − | + |
| protoxylem | + | + | + | + |
| metaphloem | +++ | + + | + | + |
| metacambium | + | + | + | − |
| metaxylem | ++ | + | + | − |
| secondary phloem | ++++ | +++ | ++ | +++ |
| cambium | ++ | +++ | +++ | ++ |
| secondary xylem | +++ | +++ | ++ | + |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Hou, J.; Chen, H.; Pei, K.; Li, Y.; He, X.-Q. Dynamic Changes of Pectin Epitopes in Cell Walls during the Development of the Procambium–Cambium Continuum in Poplar. Int. J. Mol. Sci. 2017, 18, 1716. https://doi.org/10.3390/ijms18081716
Liu J, Hou J, Chen H, Pei K, Li Y, He X-Q. Dynamic Changes of Pectin Epitopes in Cell Walls during the Development of the Procambium–Cambium Continuum in Poplar. International Journal of Molecular Sciences. 2017; 18(8):1716. https://doi.org/10.3390/ijms18081716
Chicago/Turabian StyleLiu, Jundi, Jie Hou, Huimin Chen, Keliang Pei, Yi Li, and Xin-Qiang He. 2017. "Dynamic Changes of Pectin Epitopes in Cell Walls during the Development of the Procambium–Cambium Continuum in Poplar" International Journal of Molecular Sciences 18, no. 8: 1716. https://doi.org/10.3390/ijms18081716




