The Role of Microglia in Diabetic Retinopathy: Inflammation, Microvasculature Defects and Neurodegeneration
Abstract
:1. Introduction
2. The Retina
3. Diabetic Retinopathy
3.1. Hyperglycemia in Diabetic Retinopathy
3.2. Leukostasis in Diabetic Retinopathy
4. Microglia
4.1. Microglia in the Retina
4.2. Inflammation in Diabetic Retinopathy
4.3. Microglia Activation in Diabetic Retinopathy
5. Molecular Pathways of Inflammation in Diabetic Retinopathy
5.1. Cytokines
5.2. VEGF
5.3. TNFα
5.4. Chemokines
5.5. Novel Molecular Targets in Diabetic Retinopathy
6. Neurodegeneration in Diabetic Retinopathy
7. The Influence of Microglia on Neurodegeneration in Diabetic Retinopathy
8. Microvascular Pathology and Defective BRB Integrity
9. Angiogenesis and Inflammation
10. Interaction of Microglia with Macroglia in the Retina
11. Treatment of Diabetic Retinopathy by Altering Microglia
11.1. Photocoagulation
11.2. VEGF Blocking
11.3. Steroid Therapy
11.4. Direct Prevention of Microglia Activation
12. Conclusions and Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AMPA | α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate |
| C5aR | anaphylatoxin C5a receptor |
| BRB | blood-retina barrier |
| CCL | CC chemokine ligands |
| CNS | central nervous system |
| CX3CL | chemokine (C-X3-C motif) ligand |
| COX | cyclooxygenase |
| DME | diabetic macular edema |
| DR | diabetic retinopathy |
| ERG | electroretinogram |
| ERK | extracellular signal–regulated kinase |
| HIF | hypoxia-inducible factor |
| IL | interleukins |
| ICAM | intracellular adhesion molecule |
| IRBP | iron-responsive element-binding proteins |
| MMP | matrix metalloproteases |
| MCP | membrane cofactor protein |
| NMDA | N-methyl-d-aspartame |
| NOX | nicotinamide adenine dinucleotide phosphate (NADPH) oxidases |
| NGF | nerve growth factor |
| NO | nitric oxide |
| AGE | advanced glycation end products |
| LEP | leptin |
| NPDR | non-proliferating diabetic retinopathy |
| NF | nuclear factor |
| PEDF | pigment epithelium-derived factor |
| PDR | proliferating diabetic retinopathy |
| ROS | reactive oxygen species |
| RBP | retinol-binding protein |
| STAT | signal transducer and activator of transcription |
| TNF | tumor necrosis factor |
| VCAM | vascular cell adhesion molecule |
| VEGF | vascular endothelial growth factor |
| ZO | zonula occludens |
References
- Bourne, R.R.; Stevens, G.A.; White, R.A.; Smith, J.L.; Flaxman, S.R.; Price, H.; Jonas, J.B.; Keeffe, J.; Leasher, J.; Naidoo, K.; et al. Causes of Vision Loss Worldwide, 1990–2010: A Systematic Analysis. Lancet Glob. Health 2013, 1, e339–e349. [Google Scholar] [CrossRef]
- Fong, D.S.; Aiello, L.; Gardner, T.W.; King, G.L.; Blankenship, G.; Cavallerano, J.D.; Ferris, F.L., 3rd; Klein, R. Diabetic Retinopathy. Diabetes Care 2003, 26, S99–S102. [Google Scholar] [CrossRef] [PubMed]
- Banati, R.B.; Gehrmann, J.; Schubert, P.; Kreutzberg, G.W. Cytotoxicity of Microglia. Glia 1993, 7, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Fong, D.S.; Aiello, L.P.; Ferris, F.L., 3rd; Klein, R. Diabetic Retinopathy. Diabetes Care 2004, 27, 2540–2553. [Google Scholar] [CrossRef] [PubMed]
- Nentwich, M.M.; Ulbig, M.W. Diabetic Retinopathy—Ocular Complications of Diabetes Mellitus. World J. Diabetes 2015, 6, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Cheung, N.; Mitchell, P.; Wong, T.Y. Diabetic Retinopathy. Lancet 2010, 376, 124–136. [Google Scholar] [CrossRef]
- Rathmann, W.; Giani, G. Global Prevalence of Diabetes: Estimates for the Year 2000 and Projections for 2030. Diabetes Care 2004, 27, 2568–2569. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A.; Chan, P.-S. Oxidative Stress and Diabetic Retinopathy. Exp. Diabetes Res. 2007, 2007, 43603. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, C.P.; Ferris, F.L., 3rd; Klein, R.E.; Lee, P.P.; Agardh, C.D.; Davis, M.; Dills, D.; Kampik, A.; Pararajasegaram, R.; Verdaguer, J.T. Proposed International Clinical Diabetic Retinopathy and Diabetic Macular Edema Disease Severity Scales. Ophthalmology 2003, 110, 1677–1682. [Google Scholar] [CrossRef]
- Arroba, A.I.; Valverde, A.M. Modulation of Microglia in the Retina: New Insights into Diabetic Retinopathy. Acta Diabetol. 2017, 54, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Stroud, S.; Mehta, A.; Rangasamy, S. New Treatments for Diabetic Retinopathy. Diabetes Obes. Metab. 2015, 17, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Stitt, A.W.; Lois, N.; Medina, R.J.; Adamson, P.; Curtis, T.M. Advances in Our Understanding of Diabetic Retinopathy. Clin. Sci. 2013, 125, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Ford, J.A.; Lois, N.; Royle, P.; Clar, C.; Shyangdan, D.; Waugh, N. Current Treatments in Diabetic Macular Oedema: Systematic Review and Meta-Analysis. BMJ Open 2013, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.; Chen, H.; Su, S.B. Neuroinflammatory Responses in Diabetic Retinopathy. J. Neuroinflamm. 2015, 12, 141. [Google Scholar] [CrossRef] [PubMed]
- Aiello, L.P. Diabetic Retinopathy and Other Ocular Findings in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study. Diabetes Care 2014, 37, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Semeraro, F.; Cancarini, A.; dell’Omo, R.; Rezzola, S.; Romano, M.R.; Costagliola, C. Diabetic Retinopathy: Vascular and Inflammatory Disease. J. Diabetes Res. 2015, 2015, 582060. [Google Scholar] [CrossRef] [PubMed]
- Santiago, A.R.; Cristovao, A.J.; Santos, P.F.; Carvalho, C.M.; Ambrosio, A.F. High Glucose Induces Caspase-Independent Cell Death in Retinal Neural Cells. Neurobiol. Dis. 2007, 25, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Barber, A.J.; Lieth, E.; Khin, S.A.; Antonetti, D.A.; Buchanan, A.G.; Gardner, T.W. Neural Apoptosis in the Retina During Experimental and Human Diabetes. Early Onset and Effect of Insulin. J. Clin. Investig. 1998, 102, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Barber, A.J. A New View of Diabetic Retinopathy: A Neurodegenerative Disease of the Eye. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2003, 27, 283–290. [Google Scholar] [CrossRef]
- Ramirez, A.I.; de Hoz, R.; Salobrar-Garcia, E.; Salazar, J.J.; Rojas, B.; Ajoy, D.; Lopez-Cuenca, I.; Rojas, P.; Trivino, A.; Ramirez, J.M. The Role of Microglia in Retinal Neurodegeneration: Alzheimer’s Disease, Parkinson, and Glaucoma. Front. Aging Neurosci. 2017, 9, 214. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.M.; Yang, S.; Huang, S.S.; Tang, B.S.; Guo, J.F. Microglial Activation in the Pathogenesis of Huntington's Disease. Front. Aging Neurosci. 2017, 9, 193. [Google Scholar] [CrossRef] [PubMed]
- Lieth, E.; Gardner, T.W.; Barber, A.J.; Antonetti, D.A. Retinal Neurodegeneration: Early Pathology in Diabetes. Clin. Exp. Ophthalmol. 2000, 28, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Jackson, G.R.; Barber, A.J. Visual Dysfunction Associated with Diabetic Retinopathy. Curr. Diabetes Rep. 2010, 10, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Tyrberg, M.; Lindblad, U.; Melander, A.; Lovestam-Adrian, M.; Ponjavic, V.; Andreasson, S. Electrophysiological Studies in Newly Onset Type 2 Diabetes without Visible Vascular Retinopathy. Doc. Ophthalmol. 2011, 123, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Juen, S.; Kieselbach, G.F. Electrophysiological Changes in Juvenile Diabetics without Retinopathy. Arch. Ophthalmol. 1990, 108, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.X.; Ng, Y.K.; Ling, E.A. Neuronal and Microglial Response in the Retina of Streptozotocin-Induced Diabetic Rats. Vis. Neurosci. 2000, 17, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, M.; Hirose, G. Origin of the Retina from Both Sides of the Embryonic Brain: A Contribution to the Problem of Crossing at the Optic Chiasma. Science 1978, 202, 637–639. [Google Scholar] [CrossRef] [PubMed]
- Krady, J.K.; Basu, A.; Allen, C.M.; Xu, Y.; LaNoue, K.F.; Gardner, T.W.; Levison, S.W. Minocycline Reduces Proinflammatory Cytokine Expression, Microglial Activation, and Caspase-3 Activation in a Rodent Model of Diabetic Retinopathy. Diabetes 2005, 54, 1559–1565. [Google Scholar] [CrossRef] [PubMed]
- Pournaras, C.J. Retinal Oxygen Distribution. Its Role in the Physiopathology of Vasoproliferative Microangiopathies. Retina 1995, 15, 332–347. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, J.; Braun, R.D.; Dunn, R., Jr.; Linsenmeier, R.A. Oxygen Distribution in the Macaque Retina. Investig. Ophthalmol. Vis. Sci. 1993, 34, 516–521. [Google Scholar]
- Reichenbach, A.; Stolzenburg, J.U.; Eberhardt, W.; Chao, T.I.; Dettmer, D.; Hertz, L. What Do Retinal Muller (Glial) Cells Do for Their Neuronal ‘Small Siblings’? J. Chem. Neuroanat. 1993, 6, 201–213. [Google Scholar] [CrossRef]
- Ames, A., 3rd. Energy Requirements of Cns Cells as Related to Their Function and to Their Vulnerability to Ischemia: A Commentary Based on Studies on Retina. Can. J. Physiol. Pharmacol. 1992, 70, S158–S164. [Google Scholar] [CrossRef] [PubMed]
- Kolko, M.; Vosborg, F.; Henriksen, U.L.; Hasan-Olive, M.M.; Diget, E.H.; Vohra, R.; Gurubaran, I.R.S.; Gjedde, A.; Mariga, S.T.; Skytt, D.M.; et al. Lactate Transport and Receptor Actions in Retina: Potential Roles in Retinal Function and Disease. Neurochem. Res. 2016, 41, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- Hurley, J.B.; Lindsay, K.J.; Du, J. Glucose, Lactate, and Shuttling of Metabolites in Vertebrate Retinas. J. Neurosci. Res. 2015, 93, 1079–1092. [Google Scholar] [CrossRef] [PubMed]
- Winkler, B.S.; Arnold, M.J.; Brassell, M.A.; Sliter, D.R. Glucose Dependence of Glycolysis, Hexose Monophosphate Shunt Activity, Energy Status, and the Polyol Pathway in Retinas Isolated from Normal (Nondiabetic) Rats. Investig. Ophthalmol. Vis. Sci. 1997, 38, 62–71. [Google Scholar]
- Poitry-Yamate, C.L.; Poitry, S.; Tsacopoulos, M. Lactate Released by Muller Glial Cells Is Metabolized by Photoreceptors from Mammalian Retina. J. Neurosci. 1995, 15, 5179–5191. [Google Scholar] [PubMed]
- Philp, N.J.; Yoon, H.; Grollman, E.F. Monocarboxylate Transporter Mct1 Is Located in the Apical Membrane and Mct3 in the Basal Membrane of Rat Rpe. Am. J. Physiol. 1998, 274, R1824–R1828. [Google Scholar] [CrossRef] [PubMed]
- Castillo, X.; Rosafio, K.; Wyss, M.T.; Drandarov, K.; Buck, A.; Pellerin, L.; Weber, B.; Hirt, L. A Probable Dual Mode of Action for Both l- and d-Lactate Neuroprotection in Cerebral Ischemia. J. Cereb. Blood Flow Metab. 2015, 35, 1561–1569. [Google Scholar] [CrossRef] [PubMed]
- Horn, T.; Klein, J. Neuroprotective Effects of Lactate in Brain Ischemia: Dependence on Anesthetic Drugs. Neurochem. Int. 2013, 62, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Bouzat, P.; Sala, N.; Suys, T.; Zerlauth, J.B.; Marques-Vidal, P.; Feihl, F.; Bloch, J.; Messerer, M.; Levivier, M.; Meuli, R.; et al. Cerebral Metabolic Effects of Exogenous Lactate Supplementation on the Injured Human Brain. Intensive Care Med. 2014, 40, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Ichai, C.; Armando, G.; Orban, J.C.; Berthier, F.; Rami, L.; Samat-Long, C.; Grimaud, D.; Leverve, X. Sodium Lactate Versus Mannitol in the Treatment of Intracranial Hypertensive Episodes in Severe Traumatic Brain-Injured Patients. Intensive Care Med. 2009, 35, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Morrison, B.M.; Li, Y.; Lengacher, S.; Farah, M.H.; Hoffman, P.N.; Liu, Y.; Tsingalia, A.; Jin, L.; Zhang, P.W.; et al. Oligodendroglia Metabolically Support Axons and Contribute to Neurodegeneration. Nature 2012, 487, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Ballabh, P.; Braun, A.; Nedergaard, M. The Blood-Brain Barrier: An Overview: Structure, Regulation, and Clinical Implications. Neurobiol. Dis. 2004, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Antonetti, D.A.; Lieth, E.; Barber, A.J.; Gardner, T.W. Molecular Mechanisms of Vascular Permeability in Diabetic Retinopathy. Semin. Ophthalmol. 1999, 14, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Gardner, T.W.; Lieth, E.; Khin, S.A.; Barber, A.J.; Bonsall, D.J.; Lesher, T.; Rice, K.; Brennan, W.A., Jr. Astrocytes Increase Barrier Properties and ZO-1 Expression in Retinal Vascular Endothelial Cells. Investig. Ophthalmol. Vis. Sci. 1997, 38, 2423–2427. [Google Scholar]
- Abbott, N.J.; Revest, P.A.; Romero, I.A. Astrocyte-Endothelial Interaction: Physiology and Pathology. Neuropathol. Appl. Neurobiol. 1992, 18, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Nathan, D.M.; Genuth, S.; Lachin, J.; Cleary, P.; Crofford, O.; Davis, M.; Rand, L.; Siebert, C. The Effect of Intensive Treatment of Diabetes on the Development and Progression of Long-Term Complications in Insulin-Dependent Diabetes Mellitus. N. Engl. J. Med. 1993, 329, 977–986. [Google Scholar] [PubMed]
- UK Prospective Diabetes Study (Ukpds) Group. Intensive Blood-Glucose Control with Sulphonylureas or Insulin Compared with Conventional Treatment and Risk of Complications in Patients with Type 2 Diabetes (Ukpds 33). Lancet 1998, 352, 837–853. [Google Scholar]
- Hudson, B.I.; Schmidt, A.M. Rage: A Novel Target for Drug Intervention in Diabetic Vascular Disease. Pharm. Res. 2004, 21, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Stitt, A.W. The Role of Advanced Glycation in the Pathogenesis of Diabetic Retinopathy. Exp. Mol. Pathol. 2003, 75, 95–108. [Google Scholar] [CrossRef]
- Barile, G.R.; Pachydaki, S.I.; Tari, S.R.; Lee, S.E.; Donmoyer, C.M.; Ma, W.; Rong, L.L.; Buciarelli, L.G.; Wendt, T.; Horig, H.; et al. The Rage Axis in Early Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2005, 46, 2916–2924. [Google Scholar] [CrossRef] [PubMed]
- Aiello, L.P. The Potential Role of PKCβ in Diabetic Retinopathy and Macular Edema. Surv. Ophthalmol. 2002, 47, S263–S269. [Google Scholar] [CrossRef]
- Caldwell, R.B.; Bartoli, M.; Behzadian, M.A.; El-Remessy, A.E.; Al-Shabrawey, M.; Platt, D.H.; Liou, G.I.; Caldwell, R.W. Vascular Endothelial Growth Factor and Diabetic Retinopathy: Role of Oxidative Stress. Curr. Drug Targets 2005, 6, 511–524. [Google Scholar] [CrossRef] [PubMed]
- Soufi, F.G.; Vardyani, M.; Sheervalilou, R.; Mohammadi, M.; Somi, M.H. Long-Term Treatment with Resveratrol Attenuates Oxidative Stress Pro-Inflammatory Mediators and Apoptosis in Streptozotocin-Nicotinamide-Induced Diabetic Rats. Gen. Physiol. Biophys. 2012, 31, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Rains, J.L.; Jain, S.K. Oxidative Stress, Insulin Signaling, and Diabetes. Free Radic. Biol. Med. 2011, 50, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Du, X.L.; Edelstein, D.; Rossetti, L.; Fantus, I.G.; Goldberg, H.; Ziyadeh, F.; Wu, J.; Brownlee, M. Hyperglycemia-Induced Mitochondrial Superoxide Overproduction Activates the Hexosamine Pathway and Induces Plasminogen Activator Inhibitor-1 Expression by Increasing Sp1 Glycosylation. Proc. Natl. Acad. Sci. USA 2000, 97, 12222–12226. [Google Scholar] [CrossRef] [PubMed]
- Milne, R.; Brownstein, S. Advanced Glycation End Products and Diabetic Retinopathy. Amino Acids 2013, 44, 1397–1407. [Google Scholar] [CrossRef] [PubMed]
- Zong, H.; Ward, M.; Stitt, A.W. Ages, Rage, and Diabetic Retinopathy. Curr. Diabetes Rep. 2011, 11, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Smith, M.A.; Miller, C.M.; Kern, T.S. Diabetes-Induced Nitrative Stress in the Retina, and Correction by Aminoguanidine. J. Neurochem. 2002, 80, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Stern, D.M.; Yan, S.D.; Yan, S.F.; Schmidt, A.M. Receptor for Advanced Glycation Endproducts (Rage) and the Complications of Diabetes. Ageing Res. Rev. 2002, 1, 1–15. [Google Scholar] [CrossRef]
- Nishikawa, T.; Edelstein, D.; Du, X.L.; Yamagishi, S.; Matsumura, T.; Kaneda, Y.; Yorek, M.A.; Beebe, D.; Oates, P.J.; Hammes, H.P.; et al. Normalizing Mitochondrial Superoxide Production Blocks Three Pathways of Hyperglycaemic Damage. Nature 2000, 404, 787–790. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.K.; Lee, C.M.; Lee, D.S.; Na, G.; Lee, D.Y.; Choi, I.; Park, S.G.; Seo, S.K.; Yang, J.W.; Choi, J.S.; et al. The 15-Deoxy-Delta12,14-Prostaglandin J2 Inhibits Lpsstimulated Inflammation Via Enhancement of the Plateletactivating Factor Acetylhydrolase Activity in Human Retinal Pigment Epithelial Cells. Int. J. Mol. Med. 2014, 33, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.; Chang, L.; Wang, B.; Chu, L. Retinal Neuronal Mcp-1 Induced by Ages Stimulates TNFα Expression in Rat Microglia Via P38, Erk, and Nf-κB Pathways. Mol. Vis. 2014, 20, 616–628. [Google Scholar] [PubMed]
- Palsamy, P.; Subramanian, S. Ameliorative Potential of Resveratrol on Proinflammatory Cytokines, Hyperglycemia Mediated Oxidative Stress, and Pancreatic β-Cell Dysfunction in Streptozotocin-Nicotinamide-Induced Diabetic Rats. J. Cell. Physiol. 2010, 224, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, A.; Yoshida, S.; Ishibashi, T.; Kuwano, M.; Inomata, H. Suppression of Retinal Neovascularization by the Nf-κB Inhibitor Pyrrolidine Dithiocarbamate in Mice. Investig. Ophthalmol. Vis. Sci. 1999, 40, 1624–1629. [Google Scholar]
- Wang, A.L.; Yu, A.C.; He, Q.H.; Zhu, X.; Tso, M.O. Ages Mediated Expression and Secretion of TNFα in Rat Retinal Microglia. Exp. Eye Res. 2007, 84, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, M.; Kern, T.S.; Lorenzi, M. Accelerated Death of Retinal Microvascular Cells in Human and Experimental Diabetic Retinopathy. J. Clin. Investig. 1996, 97, 2883–2890. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, H.; Sugiyama, T.; Wu, D.M.; Kobayashi, M.; Yamanishi, S.; Katsumura, K.; Puro, D.G. Atp: A Vasoactive Signal in the Pericyte-Containing Microvasculature of the Rat Retina. J. Physiol. 2003, 551, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Platania, C.B.M.; Giurdanella, G.; di Paola, L.; Leggio, G.M.; Drago, F.; Salomone, S.; Bucolo, C. P2X7 Receptor Antagonism: Implications in Diabetic Retinopathy. Biochem. Pharmacol. 2017, 138, 130–139. [Google Scholar] [CrossRef] [PubMed]
- McLeod, D.S.; Lefer, D.J.; Merges, C.; Lutty, G.A. Enhanced Expression of Intracellular Adhesion Molecule-1 and P-Selectin in the Diabetic Human Retina and Choroid. Am. J. Pathol. 1995, 147, 642–653. [Google Scholar] [PubMed]
- Valle, A.; Giamporcaro, G.M.; Scavini, M.; Stabilini, A.; Grogan, P.; Bianconi, E.; Sebastiani, G.; Masini, M.; Maugeri, N.; Porretti, L.; et al. Reduction of Circulating Neutrophils Precedes and Accompanies Type 1 Diabetes. Diabetes 2013, 62, 2072–2077. [Google Scholar] [CrossRef] [PubMed]
- Chibber, R.; Ben-Mahmud, B.M.; Chibber, S.; Kohner, E.M. Leukocytes in Diabetic Retinopathy. Curr. Diabetes Rev. 2007, 3, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Joussen, A.M.; Poulaki, V.; Le, M.L.; Koizumi, K.; Esser, C.; Janicki, H.; Schraermeyer, U.; Kociok, N.; Fauser, S.; Kirchhof, B.; et al. A Central Role for Inflammation in the Pathogenesis of Diabetic Retinopathy. FASEB J. 2004, 18, 1450–1452. [Google Scholar] [CrossRef] [PubMed]
- Leal, E.C.; Manivannan, A.; Hosoya, K.; Terasaki, T.; Cunha-Vaz, J.; Ambrosio, A.F.; Forrester, J.V. Inducible Nitric Oxide Synthase Isoform Is a Key Mediator of Leukostasis and Blood-Retinal Barrier Breakdown in Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2007, 48, 5257–5265. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, K.; Khosrof, S.; Bursell, S.E.; Rohan, R.; Murata, T.; Clermont, A.C.; Aiello, L.P.; Ogura, Y.; Adamis, A.P. Prevention of Leukostasis and Vascular Leakage in Streptozotocin-Induced Diabetic Retinopathy Via Intercellular Adhesion Molecule-1 Inhibition. Proc. Natl. Acad. Sci. USA 1999, 96, 10836–10841. [Google Scholar] [CrossRef] [PubMed]
- Long, E.O. Intercellular Adhesion Molecule 1 (Icam-1): Getting a Grip on Leukocyte Adhesion. J. Immunol. 2011, 186. [Google Scholar] [CrossRef] [PubMed]
- Rothlein, R.; Dustin, M.L.; Marlin, S.D.; Springer, T.A. A Human Intercellular Adhesion Molecule (Icam-1) Distinct from Lfa-1. J. Immunol. 1986, 137, 1270–1274. [Google Scholar] [PubMed]
- Dustin, M.L.; Rothlein, R.; Bhan, A.K.; Dinarello, C.A.; Springer, T.A. Induction by Il 1 and Interferon-γ: Tissue Distribution, Biochemistry, and Function of a Natural Adherence Molecule (Icam-1). J. Immunol. 1986, 137, 245–254. [Google Scholar] [PubMed]
- Huang, H.; Gandhi, J.K.; Zhong, X.; Wei, Y.; Gong, J.; Duh, E.J.; Vinores, S.A. TNFα Is Required for Late Brb Breakdown in Diabetic Retinopathy, and Its Inhibition Prevents Leukostasis and Protects Vessels and Neurons from Apoptosis. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1336–1344. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.Y.; Kohsaka, S.; Rezaie, P. The Origin and Cell Lineage of Microglia: New Concepts. Brain Res. Rev. 2007, 53, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Saijo, K.; Glass, C.K. Microglial Cell Origin and Phenotypes in Health and Disease. Nat. Rev. Immunol. 2011, 11, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Kettenmann, H.; Hanisch, U.K.; Noda, M.; Verkhratsky, A. Physiology of Microglia. Physiol. Rev. 2011, 91, 461–553. [Google Scholar] [CrossRef] [PubMed]
- Polazzi, E.; Monti, B. Microglia and Neuroprotection: From in Vitro Studies to Therapeutic Applications. Prog. Neurobiol. 2010, 92, 293–315. [Google Scholar] [CrossRef] [PubMed]
- Schuetz, E.; Thanos, S. Microglia-Targeted Pharmacotherapy in Retinal Neurodegenerative Diseases. Curr. Drug Targets 2004, 5, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Gehrmann, J.; Banati, R.B.; Kreutzberg, G.W. Microglia in the Immune Surveillance of the Brain: Human Microglia Constitutively Express Hla-Dr Molecules. J. Neuroimmunol. 1993, 48, 189–198. [Google Scholar] [CrossRef]
- Provis, J.M.; Diaz, C.M.; Penfold, P.L. Microglia in Human Retina: A Heterogeneous Population with Distinct Ontogenies. Perspect. Dev. Neurobiol. 1996, 3, 213–222. [Google Scholar] [PubMed]
- Santiago, A.R.; Baptista, F.I.; Santos, P.F.; Cristóvão, G.; Ambrósio, A.F.; Cunha, R.A.; Gomes, C.A. Role of Microglia Adenosine A2A Receptors in Retinal and Brain Neurodegenerative Diseases. Mediat. Inflamm. 2014, 2014, 465694. [Google Scholar] [CrossRef] [PubMed]
- Ajami, B.; Bennett, J.L.; Krieger, C.; Tetzlaff, W.; Rossi, F.M. Local Self-Renewal Can Sustain CNS Microglia Maintenance and Function Throughout Adult Life. Nat. Neurosci. 2007, 10, 1538–1543. [Google Scholar] [CrossRef] [PubMed]
- Derecki, N.C.; Cronk, J.C.; Kipnis, J. The Role of Microglia in Brain Maintenance: Implications for Rett Syndrome. Trends Immunol. 2013, 34, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Perry, V.H.; Teeling, J. Microglia and Macrophages of the Central Nervous System: The Contribution of Microglia Priming and Systemic Inflammation to Chronic Neurodegeneration. Semin. Immunopathol. 2013, 35, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Kierdorf, K.; Prinz, M. Microglia in Steady State. J. Clin. Investig. 2017, 127, 3201–3209. [Google Scholar] [CrossRef] [PubMed]
- Wake, H.; Moorhouse, A.J.; Jinno, S.; Kohsaka, S.; Nabekura, J. Resting Microglia Directly Monitor the Functional State of Synapses in Vivo and Determine the Fate of Ischemic Terminals. J. Neurosci. 2009, 29, 3974–3980. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, L.; Zhang, J.; Fariss, R.N.; Ma, W.; Kretschmer, F.; Wang, M.; Qian, H.H.; Badea, T.C.; Diamond, J.S.; et al. Requirement for Microglia for the Maintenance of Synaptic Function and Integrity in the Mature Retina. J. Neurosci. 2016, 36, 2827–2842. [Google Scholar] [CrossRef] [PubMed]
- Nimmerjahn, A.; Kirchhoff, F.; Helmchen, F. Resting Microglial Cells Are Highly Dynamic Surveillants of Brain Parenchyma in Vivo. Science 2005, 308, 1314–1318. [Google Scholar] [CrossRef] [PubMed]
- Davalos, D.; Grutzendler, J.; Yang, G.; Kim, J.V.; Zuo, Y.; Jung, S.; Littman, D.R.; Dustin, M.L.; Gan, W.B. Atp Mediates Rapid Microglial Response to Local Brain Injury in Vivo. Nat. Neurosci. 2005, 8, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Dheen, S.T.; Kaur, C.; Ling, E.A. Microglial Activation and Its Implications in the Brain Diseases. Curr. Med. Chem. 2007, 14, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Li, C.H.; Liu, Y.L. Toll-Like Receptor (TLR)-2/4 Expression in Retinal Ganglion Cells in a High-Glucose Environment and Its Implications. Genet. Mol. Res. 2016, 15. [Google Scholar] [CrossRef] [PubMed]
- Streit, W.J.; Graeber, M.B.; Kreutzberg, G.W. Peripheral Nerve Lesion Produces Increased Levels of Major Histocompatibility Complex Antigens in the Central Nervous System. J. Neuroimmunol. 1989, 21, 117–123. [Google Scholar] [CrossRef]
- Chen, L.; Yang, P.; Kijlstra, A. Distribution, Markers, and Functions of Retinal Microglia. Ocul. Immunol. Inflamm. 2002, 10, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Kreutzberg, G.W. Microglia: A Sensor for Pathological Events in the Cns. Trends Neurosci. 1996, 19, 312–318. [Google Scholar] [CrossRef]
- Tambuyzer, B.R.; Ponsaerts, P.; Nouwen, E.J. Microglia: Gatekeepers of Central Nervous System Immunology. J. Leukoc. Biol. 2009, 85, 352–370. [Google Scholar] [CrossRef] [PubMed]
- Langmann, T. Microglia Activation in Retinal Degeneration. J. Leukoc. Biol. 2007, 81, 1345–1351. [Google Scholar] [CrossRef] [PubMed]
- Streit, W.J.; Kreutzberg, G.W. Response of Endogenous Glial Cells to Motor Neuron Degeneration Induced by Toxic Ricin. J. Comp. Neurol. 1988, 268, 248–263. [Google Scholar] [CrossRef] [PubMed]
- Arroba, A.I.; Alcalde-Estevez, E.; Garcia-Ramirez, M.; Cazzoni, D.; de la Villa, P.; Sanchez-Fernandez, E.M.; Mellet, C.O.; Fernandez, J.M.G.; Hernandez, C.; Simo, R.; et al. Modulation of Microglia Polarization Dynamics During Diabetic Retinopathy in db/db Mice. Biochim. Biophys. Acta 2016, 1862, 1663–1674. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Yi, H.; Xu, L.; Zhang, Z. Fluoxetine and S-Citalopram Inhibit M1 Activation and Promote M2 Activation of Microglia in Vitro. Neuroscience 2015, 294, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Helming, L.; Gordon, S. Alternative Activation of Macrophages: An Immunologic Functional Perspective. Annu. Rev. Immunol. 2009, 27, 451–483. [Google Scholar] [CrossRef] [PubMed]
- Lampron, A.; Elali, A.; Rivest, S. Innate Immunity in the Cns: Redefining the Relationship between the CNS and Its Environment. Neuron 2013, 78, 214–232. [Google Scholar] [CrossRef] [PubMed]
- Graeber, M.B.; Li, W.; Rodriguez, M.L. Role of Microglia in CNS Inflammation. FEBS Lett. 2011, 585, 3798–3805. [Google Scholar] [CrossRef] [PubMed]
- Raivich, G.; Bohatschek, M.; Kloss, C.U.; Werner, A.; Jones, L.L.; Kreutzberg, G.W. Neuroglial Activation Repertoire in the Injured Brain: Graded Response, Molecular Mechanisms and Cues to Physiological Function. Brain Res. Brain Res. Rev. 1999, 30, 77–105. [Google Scholar] [CrossRef]
- Colton, C.; Wilcock, D.M. Assessing Activation States in Microglia. CNS Neurol. Disord. Drug Targets 2010, 9, 174–191. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.P.; Sun, H.L.; Wu, L.M.; Guo, X.J.; Dou, H.L.; Tso, M.O.; Zhao, L.; Li, S.M. Baicalein Reduces Inflammatory Process in a Rodent Model of Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2319–2327. [Google Scholar] [CrossRef] [PubMed]
- Frick, L.; Pittenger, C. Microglial Dysregulation in Ocd, Tourette Syndrome, and Pandas. J. Immunol. Res. 2016, 2016, 8606057. [Google Scholar] [CrossRef] [PubMed]
- Von Bernhardi, R.; Bernhardi, L.E.; Eugenín, J. Microglial Cell Dysregulation in Brain Aging and Neurodegeneration. Front. Aging Neurosci. 2015, 7, 124. [Google Scholar] [CrossRef] [PubMed]
- Salter, M.W.; Beggs, S. Sublime Microglia: Expanding Roles for the Guardians of the CNS. Cell 2014, 158, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Hume, D.A.; Perry, V.H.; Gordon, S. Immunohistochemical Localization of a Macrophage-Specific Antigen in Developing Mouse Retina: Phagocytosis of Dying Neurons and Differentiation of Microglial Cells to Form a Regular Array in the Plexiform Layers. J. Cell Biol. 1983, 97, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.M.; Martin-Oliva, D.; Ferrer-Martin, R.M.; Tassi, M.; Calvente, R.; Sierra, A.; Carrasco, M.C.; Marin-Teva, J.L.; Navascues, J.; Cuadros, M.A. Microglial Response to Light-Induced Photoreceptor Degeneration in the Mouse Retina. J. Comp. Neurol. 2010, 518, 477–492. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Cui, J.; Li, L.; Hitchcock, P.F.; Li, Y. The Role of Microglia in the Neurogenesis of Zebrafish Retina. Biochem. Biophys. Res. Commun. 2012, 421, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.Y.; Green, W.R.; Tso, M.O. Microglial Activation in Human Diabetic Retinopathy. Arch. Ophthalmol. 2008, 126, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Jiao, H.; Natoli, R.; Valter, K.; Provis, J.M.; Rutar, M. Spatiotemporal Cadence of Macrophage Polarisation in a Model of Light-Induced Retinal Degeneration. PLoS ONE 2015, 10, e0143952. [Google Scholar] [CrossRef] [PubMed]
- Arroba, A.I.; Alvarez-Lindo, N.; van Rooijen, N.; de la Rosa, E.J. Microglia-Mediated Igf-I Neuroprotection in the Rd10 Mouse Model of Retinitis Pigmentosa. Investig. Ophthalmol. Vis. Sci. 2011, 52, 9124–9130. [Google Scholar] [CrossRef] [PubMed]
- Bilimoria, P.M.; Stevens, B. Microglia Function during Brain Development: New Insights from Animal Models. Brain Res. 2015, 1617, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Kettenmann, H.; Kirchhoff, F.; Verkhratsky, A. Microglia: New Roles for the Synaptic Stripper. Neuron 2013, 77, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Checchin, D.; Sennlaub, F.; Levavasseur, E.; Leduc, M.; Chemtob, S. Potential Role of Microglia in Retinal Blood Vessel Formation. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3595–3602. [Google Scholar] [CrossRef] [PubMed]
- Powell, E.D.; Field, R.A. Diabetic Retinopathy and Rheumatoid Arthritis. Lancet 1964, 2, 17–18. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, H.; Al-Shabrawey, M.; Caldwell, R.W.; Caldwell, R.B. Inflammation and Diabetic Retinal Microvascular Complications. J. Cardiovasc. Dis. Res. 2011, 2, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Kern, T.S. Inflammation in Diabetic Retinopathy. Prog. Retin. Eye Res. 2011, 30, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Wellen, K.E.; Hotamisligil, G.S. Inflammation, Stress, and Diabetes. J. Clin. Investig. 2005, 115, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, S.; Perego, C.; Pischiutta, F.; Zanier, E.R.; de Simoni, M.G. The Ischemic Environment Drives Microglia and Macrophage Function. Front. Neurol. 2015, 6, 81. [Google Scholar] [CrossRef] [PubMed]
- Rajamani, U.; Jialal, I. Hyperglycemia Induces Toll-Like Receptor-2 and -4 Expression and Activity in Human Microvascular Retinal Endothelial Cells: Implications for Diabetic Retinopathy. J. Diabetes Res. 2014, 2014, 790902. [Google Scholar] [CrossRef] [PubMed]
- Pittala, V.; Fidilio, A.; Lazzara, F.; Platania, C.B.M.; Salerno, L.; Foresti, R.; Drago, F.; Bucolo, C. Effects of Novel Nitric Oxide-Releasing Molecules against Oxidative Stress on Retinal Pigmented Epithelial Cells. Oxid. Med. Cell. Longev. 2017, 2017, 1420892. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.E.; Gu, J.; Schau, M.; Bunn, H.F. Regulation of Hypoxia-Inducible Factor 1α Is Mediated by an O2-Dependent Degradation Domain Via the Ubiquitin-Proteasome Pathway. Proc. Natl. Acad. Sci. USA 1998, 95, 7987–7992. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.L.; Jiang, B.H.; Rue, E.A.; Semenza, G.L. Hypoxia-Inducible Factor 1 Is a Basic-Helix-Loop-Helix-Pas Heterodimer Regulated by Cellular O2 Tension. Proc. Natl. Acad. Sci. USA 1995, 92, 5510–5514. [Google Scholar] [CrossRef] [PubMed]
- Vadlapatla, R.K.; Vadlapudi, A.D.; Mitra, A.K. Hypoxia-Inducible Factor-1 (HIF-1): A Potential Target for Intervention in Ocular Neovascular Diseases. Curr. Drug Targets 2013, 14, 919–935. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.A.; Giaccia, A.J. Hypoxia, Gene Expression, and Metastasis. Cancer Metastasis Rev. 2007, 26, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Lukashev, D.; Ohta, A.; Sitkovsky, M. Hypoxia-Dependent Anti-Inflammatory Pathways in Protection of Cancerous Tissues. Cancer Metastasis Rev. 2007, 26, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, R.; Graham, C.H. Hypoxia-Driven Selection of the Metastatic Phenotype. Cancer Metastasis Rev. 2007, 26, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.M.; Groot, A.J.; van der Groep, P.; Sersansie, R.; Vooijs, M.; van Diest, P.J.; van Noorden, C.J.F.; Schlingemann, R.O.; Klaassen, I. Active Hif-1 in the Normal Human Retina. J. Histochem. Cytochem. 2010, 58, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Grimm, C.; Wenzel, A.; Groszer, M.; Mayser, H.; Seeliger, M.; Samardzija, M.; Bauer, C.; Gassmann, M.; Reme, C.E. HIF-1-Induced Erythropoietin in the Hypoxic Retina Protects against Light-Induced Retinal Degeneration. Nat. Med. 2002, 8, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Foresti, R.; Bucolo, C.; Platania, C.M.; Drago, F.; Dubois-Rande, J.L.; Motterlini, R. Nrf2 Activators Modulate Oxidative Stress Responses and Bioenergetic Profiles of Human Retinal Epithelial Cells Cultured in Normal or High Glucose Conditions. Pharmacol. Res. 2015, 99, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Cho, C.L.; Liang, C.L.; Chen, S.D.; Liliang, P.C.; Wang, S.Y.; Chen, H.J. Inhibition of the MEK/ERK Pathway Reduces Microglial Activation and Interleukin-1-β Expression in Spinal Cord Ischemia/Reperfusion Injury in Rats. J. Thorac. Cardiovasc. Surg. 2007, 133, 934–941. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, M.E.; Wang, W.; Chen, X.; Caberoy, N.B.; Guo, F.; Shen, C.; Ji, Y.; Tian, H.; Wang, H.; Chen, R.; et al. Secretogranin III as a Disease-Associated Ligand for Antiangiogenic Therapy of Diabetic Retinopathy. J. Exp. Med. 2017, 214, 1029–1047. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Zhang, X.M.; Chen, B.Y.; Yang, X.J. Vegf Activates Divergent Intracellular Signaling Components to Regulate Retinal Progenitor Cell Proliferation and Neuronal Differentiation. Development 2006, 133, 2201–2210. [Google Scholar] [CrossRef] [PubMed]
- Carter, A.B.; Monick, M.M.; Hunninghake, G.W. Both Erk and P38 Kinases Are Necessary for Cytokine Gene Transcription. Am. J. Respir. Cell Mol. Biol. 1999, 20, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.S.; Huang, Q.; Gurel, Z.; Sorenson, C.M.; Sheibani, N. High Glucose Alters Retinal Astrocytes Phenotype through Increased Production of Inflammatory Cytokines and Oxidative Stress. PLoS ONE 2014, 9, e103148. [Google Scholar] [CrossRef] [PubMed]
- Al-Hussaini, H.; Kilarkaje, N. Effects of Diabetes on Retinal Pigment Epithelial Cell Proliferation and Mitogen-Activated Protein Kinase Signaling in Dark Agouti Rats. Exp. Toxicol. Pathol. 2015, 67, 117–124. [Google Scholar] [CrossRef] [PubMed]
- McVicar, C.M.; Hamilton, R.; Colhoun, L.M.; Gardiner, T.A.; Brines, M.; Cerami, A.; Stitt, A.W. Intervention with an Erythropoietin-Derived Peptide Protects against Neuroglial and Vascular Degeneration During Diabetic Retinopathy. Diabetes 2011, 60, 2995–3005. [Google Scholar] [CrossRef] [PubMed]
- Rungger-Brandle, E.; Dosso, A.A.; Leuenberger, P.M. Glial Reactivity, an Early Feature of Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1971–1980. [Google Scholar]
- Chen, X.; Zhou, H.; Gong, Y.; Wei, S.; Zhang, M. Early Spatiotemporal Characterization of Microglial Activation in the Retinas of Rats with Streptozotocin-Induced Diabetes. Graefe’s Arch. Clin. Exp. Ophthalmol. 2015, 253, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Lai, A.Y.; Todd, K.G. Differential Regulation of Trophic and Proinflammatory Microglial Effectors Is Dependent on Severity of Neuronal Injury. Glia 2008, 56, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Roque, R.S.; Imperial, C.J.; Caldwell, R.B. Microglial Cells Invade the Outer Retina as Photoreceptors Degenerate in Royal College of Surgeons Rats. Investig. Ophthalmol. Vis. Sci. 1996, 37, 196–203. [Google Scholar]
- Hughes, E.H.; Schlichtenbrede, F.C.; Murphy, C.C.; Sarra, G.M.; Luthert, P.J.; Ali, R.R.; Dick, A.D. Generation of Activated Sialoadhesin-Positive Microglia During Retinal Degeneration. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2229–2234. [Google Scholar] [CrossRef]
- Zeng, H.Y.; Zhu, X.A.; Zhang, C.; Yang, L.P.; Wu, L.M.; Tso, M.O. Identification of Sequential Events and Factors Associated with Microglial Activation, Migration, and Cytotoxicity in Retinal Degeneration in Rd Mice. Investig. Ophthalmol. Vis. Sci. 2005, 46, 2992–2999. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.F.; Streilein, J.W. Light-Induced Migration of Retinal Microglia into the Subretinal Space. Investig. Ophthalmol. Vis. Sci. 2001, 42, 3301–3310. [Google Scholar]
- Karlstetter, M.; Scholz, R.; Rutar, M.; Wong, W.T.; Provis, J.M.; Langmann, T. Retinal Microglia: Just Bystander or Target for Therapy? Prog. Retin. Eye Res. 2015, 45, 30–57. [Google Scholar] [CrossRef] [PubMed]
- Ascaso, F.J.; Huerva, V.; Grzybowski, A. The Role of Inflammation in the Pathogenesis of Macular Edema Secondary to Retinal Vascular Diseases. Mediat. Inflamm. 2014, 2014, 432685. [Google Scholar] [CrossRef] [PubMed]
- Khalfaoui, T.; Lizard, G.; Ouertani-Meddeb, A. Adhesion Molecules (ICAM-1 and VCAM-1) and Diabetic Retinopathy in Type 2 Diabetes. J. Mol. Histol. 2008, 39, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Grant, M.B.; Afzal, A.; Spoerri, P.; Pan, H.; Shaw, L.C.; Mames, R.N. The Role of Growth Factors in the Pathogenesis of Diabetic Retinopathy. Expert Opin. Investig. Drugs 2004, 13, 1275–1293. [Google Scholar] [CrossRef] [PubMed]
- Schroder, S.; Palinski, W.; Schmid-Schonbein, G.W. Activated Monocytes and Granulocytes, Capillary Nonperfusion, and Neovascularization in Diabetic Retinopathy. Am. J. Pathol. 1991, 139, 81–100. [Google Scholar] [PubMed]
- Adamis, A.P. Is Diabetic Retinopathy an Inflammatory Disease? Br. J. Ophthalmol. 2002, 86, 363–365. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Gerhardinger, C.; Lorenzi, M. Early Complement Activation and Decreased Levels of Glycosylphosphatidylinositol-Anchored Complement Inhibitors in Human and Experimental Diabetic Retinopathy. Diabetes 2002, 51, 3499–3504. [Google Scholar] [CrossRef] [PubMed]
- Joussen, A.M.; Poulaki, V.; Mitsiades, N.; Cai, W.Y.; Suzuma, I.; Pak, J.; Ju, S.T.; Rook, S.L.; Esser, P.; Mitsiades, C.S.; et al. Suppression of Fas-Fasl-Induced Endothelial Cell Apoptosis Prevents Diabetic Blood-Retinal Barrier Breakdown in a Model of Streptozotocin-Induced Diabetes. FASEB J. 2003, 17, 76–78. [Google Scholar] [PubMed]
- Block, M.L.; Zecca, L.; Hong, J.S. Microglia-Mediated Neurotoxicity: Uncovering the Molecular Mechanisms. Nat. Rev. Neurosci. 2007, 8, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Kern, T.S. Contributions of Inflammatory Processes to the Development of the Early Stages of Diabetic Retinopathy. Exp. Diabetes Res. 2007, 2007, 95103. [Google Scholar] [CrossRef] [PubMed]
- Kaul, K.; Hodgkinson, A.; Tarr, J.M.; Kohner, E.M.; Chibber, R. Is Inflammation a Common Retinal-Renal-Nerve Pathogenic Link in Diabetes? Curr. Diabetes Rev. 2010, 6, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Kojima, S.; Yamada, T.; Tamai, M. Quantitative Analysis of Interleukin-6 in Vitreous from Patients with Proliferative Vitreoretinal Diseases. Jpn. J. Ophthalmol. 2001, 45, 40–45. [Google Scholar] [CrossRef]
- Ma, W.; Wong, W.T. Aging Changes in Retinal Microglia and Their Relevance to Age-Related Retinal Disease. Adv. Exp. Med. Biol. 2016, 854, 73–78. [Google Scholar] [PubMed]
- Zhang, L.; Nair, A.; Krady, K.; Corpe, C.; Bonneau, R.H.; Simpson, I.A.; Vannucci, S.J. Estrogen Stimulates Microglia and Brain Recovery from Hypoxia-Ischemia in Normoglycemic but Not Diabetic Female Mice. J. Clin. Investig. 2004, 113, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, S.; Xia, X. Role of Intravitreal Inflammatory Cytokines and Angiogenic Factors in Proliferative Diabetic Retinopathy. Curr. Eye Res. 2012, 37, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Carmo, A.; Cunha-Vaz, J.G.; Carvalho, A.P.; Lopes, M.C. l-Arginine Transport in Retinas from Streptozotocin Diabetic Rats: Correlation with the Level of Il-1β and No Synthase Activity. Vis. Res. 1999, 39, 3817–3823. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Odenbach, S. Role of Interleukin-1β in the Pathogenesis of Diabetic Retinopathy. Br. J. Ophthalmol. 2004, 88, 1343–1347. [Google Scholar] [CrossRef] [PubMed]
- Gerhardinger, C.; Costa, M.B.; Coulombe, M.C.; Toth, I.; Hoehn, T.; Grosu, P. Expression of Acute-Phase Response Proteins in Retinal Muller Cells in Diabetes. Investig. Ophthalmol. Vis. Sci. 2005, 46, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Gustavsson, C.; Agardh, C.D.; Agardh, E. Profile of Intraocular Tumour Necrosis Factor-α and Interleukin-6 in Diabetic Subjects with Different Degrees of Diabetic Retinopathy. Acta Ophthalmol. 2013, 91, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Sohn, H.J.; Han, D.H.; Kim, I.T.; Oh, I.K.; Kim, K.H.; Lee, D.Y.; Nam, D.H. Changes in Aqueous Concentrations of Various Cytokines after Intravitreal Triamcinolone Versus Bevacizumab for Diabetic Macular Edema. Am. J. Ophthalmol. 2011, 152, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Van Hoek, M.; Dehghan, A.; Zillikens, M.C.; Hofman, A.; Witteman, J.C.; Sijbrands, E.J. An RBP4 Promoter Polymorphism Increases Risk of Type 2 Diabetes. Diabetologia 2008, 51, 1423–1428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munkhtulga, L.; Nagashima, S.; Nakayama, K.; Utsumi, N.; Yanagisawa, Y.; Gotoh, T.; Omi, T.; Kumada, M.; Zolzaya, K.; Lkhagvasuren, T.; et al. Regulatory SNP in the RBP4 Gene Modified the Expression in Adipocytes and Associated with Bmi. Obesity 2010, 18, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Otalora, L.; Martin, A.A.; Moiseyev, G.; Vanlandingham, P.; Wang, Q.; Farjo, R.; Yeganeh, A.; Quiambao, A.; Farjo, K.M. Transgenic Mice Overexpressing Serum Retinol-Binding Protein Develop Progressive Retinal Degeneration through a Retinoid-Independent Mechanism. Mol. Cell. Biol. 2015, 35, 2771–2789. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Boulton, M. The Pathogenesis of Diabetic Retinopathy: Old Concepts and New Questions. Eye (Lond.) 2002, 16, 242–260. [Google Scholar] [CrossRef] [PubMed]
- Joussen, A.M.; Poulaki, V.; Mitsiades, N.; Kirchhof, B.; Koizumi, K.; Dohmen, S.; Adamis, A.P. Nonsteroidal Anti-Inflammatory Drugs Prevent Early Diabetic Retinopathy via TNFα Suppression. FASEB J. 2002, 16, 438–440. [Google Scholar] [PubMed]
- Behl, Y.; Krothapalli, P.; Desta, T.; DiPiazza, A.; Roy, S.; Graves, D.T. Diabetes-Enhanced Tumor Necrosis Factor-α Production Promotes Apoptosis and the Loss of Retinal Microvascular Cells in Type 1 and Type 2 Models of Diabetic Retinopathy. Am. J. Pathol. 2008, 172, 1411–1418. [Google Scholar] [CrossRef] [PubMed]
- Joussen, A.M.; Murata, T.; Tsujikawa, A.; Kirchhof, B.; Bursell, S.E.; Adamis, A.P. Leukocyte-Mediated Endothelial Cell Injury and Death in the Diabetic Retina. Am. J. Pathol. 2001, 158, 147–152. [Google Scholar] [CrossRef]
- Nawaz, M.I.; van Raemdonck, K.; Mohammad, G.; Kangave, D.; van Damme, J.; El-Asrar, A.M.A.; Struyf, S. Autocrine CCL2, CXCL4, CXCL9 and CXCL10 Signal in Retinal Endothelial Cells and Are Enhanced in Diabetic Retinopathy. Exp. Eye Res. 2013, 109, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, C.; Segura, R.M.; Fonollosa, A.; Carrasco, E.; Francisco, G.; Simo, R. Interleukin-8, Monocyte Chemoattractant Protein-1 and IL-10 in the Vitreous Fluid of Patients with Proliferative Diabetic Retinopathy. Diabet. Med. 2005, 22, 719–722. [Google Scholar] [CrossRef] [PubMed]
- Cardona, S.M.; Mendiola, A.S.; Yang, Y.C.; Adkins, S.L.; Torres, V.; Cardona, A.E. Disruption of Fractalkine Signaling Leads to Microglial Activation and Neuronal Damage in the Diabetic Retina. ASN Neuro 2015, 7. [Google Scholar] [CrossRef] [PubMed]
- Simo, R.; Hernandez, C. Novel Approaches for Treating Diabetic Retinopathy Based on Recent Pathogenic Evidence. Prog. Retin. Eye Res. 2015, 48, 160–180. [Google Scholar] [CrossRef] [PubMed]
- Simo, R.; Sundstrom, J.M.; Antonetti, D.A. Ocular Anti-VEGF Therapy for Diabetic Retinopathy: The Role of Vegf in the Pathogenesis of Diabetic Retinopathy. Diabetes Care 2014, 37, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.I.; Hykin, P.G.; Gregor, Z.J.; Boulton, M.; Cree, I.A. Angiopoietin Concentrations in Diabetic Retinopathy. Br. J. Ophthalmol. 2005, 89, 480–483. [Google Scholar] [CrossRef] [PubMed]
- Rangasamy, S.; Srinivasan, R.; Maestas, J.; McGuire, P.G.; Das, A. A Potential Role for Angiopoietin 2 in the Regulation of the Blood-Retinal Barrier in Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3784–3791. [Google Scholar] [CrossRef] [PubMed]
- Deliyanti, D.; Wilkinson-Berka, J.L. Inhibition of Nox1/4 with Gkt137831: A Potential Novel Treatment to Attenuate Neuroglial Cell Inflammation in the Retina. J. Neuroinflamm. 2015, 12, 136. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, G.; Kowluru, R.A. Novel Role of Mitochondrial Matrix Metalloproteinase-2 in the Development of Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3832–3841. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, G.; Kowluru, R.A. Matrix Metalloproteinase-2 in the Development of Diabetic Retinopathy and Mitochondrial Dysfunction. Lab. Investig. 2010, 90, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, G.; Kowluru, R.A. Diabetic Retinopathy and Signaling Mechanism for Activation of Matrix Metalloproteinase-9. J. Cell. Physiol. 2012, 227, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
- Giebel, S.J.; Menicucci, G.; McGuire, P.G.; Das, A. Matrix Metalloproteinases in Early Diabetic Retinopathy and Their Role in Alteration of the Blood-Retinal Barrier. Lab. Investig. 2005, 85, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, L.K.; Addepalli, V. Attenuation of Diabetic Retinopathy by Enhanced Inhibition of MMP-2 and MMP-9 Using Aspirin and Minocycline in Streptozotocin-Diabetic Rats. Am. J. Transl. Res. 2010, 2, 181–189. [Google Scholar] [PubMed]
- Kowluru, R.A.; Kanwar, M. Oxidative Stress and the Development of Diabetic Retinopathy: Contributory Role of Matrix Metalloproteinase-2. Free Radic. Biol. Med. 2009, 46, 1677–1685. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.H.; Park, S.W.; Kim, K.J.; Bae, J.S.; Lee, E.H.; Paek, S.H.; Kim, S.U.; Ye, S.; Kim, J.H.; Cho, C.H. Endothelial STAT3 Activation Increases Vascular Leakage through Downregulating Tight Junction Proteins: Implications for Diabetic Retinopathy. J. Cell. Physiol. 2017, 232, 1123–1134. [Google Scholar] [CrossRef] [PubMed]
- Ye, E.A.; Steinle, J.J. miR-146a Suppresses STAT3/VEGF Pathways and Reduces Apoptosis through IL-6 Signaling in Primary Human Retinal Microvascular Endothelial Cells in High Glucose Conditions. Vis. Res. 2017, 139, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hu, Y.; Zhou, T.; Zhou, K.K.; Mott, R.; Wu, M.; Boulton, M.; Lyons, T.J.; Gao, G.; Ma, J.X. Activation of the Wnt Pathway Plays a Pathogenic Role in Diabetic Retinopathy in Humans and Animal Models. Am. J. Pathol. 2009, 175, 2676–2685. [Google Scholar] [CrossRef] [PubMed]
- Abu el Asrar, A.M.; Maimone, D.; Morse, P.H.; Gregory, S.; Reder, A.T. Cytokines in the Vitreous of Patients with Proliferative Diabetic Retinopathy. Am. J. Ophthalmol. 1992, 114, 731–736. [Google Scholar] [CrossRef]
- Patel, J.I.; Saleh, G.M.; Hykin, P.G.; Gregor, Z.J.; Cree, I.A. Concentration of Haemodynamic and Inflammatory Related Cytokines in Diabetic Retinopathy. Eye 2008, 22, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Yuuki, T.; Kanda, T.; Kimura, Y.; Kotajima, N.; Tamura, J.; Kobayashi, I.; Kishi, S. Inflammatory Cytokines in Vitreous Fluid and Serum of Patients with Diabetic Vitreoretinopathy. J. Diabetes Complicat. 2001, 15, 257–259. [Google Scholar] [CrossRef]
- Doganay, S.; Evereklioglu, C.; Er, H.; Turkoz, Y.; Sevinc, A.; Mehmet, N.; Savli, H. Comparison of Serum No, TNFα, IL-1β, SIL-2r, IL-6 and IL-8 Levels with Grades of Retinopathy in Patients with Diabetes Mellitus. Eye 2002, 16, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Schram, M.T.; Chaturvedi, N.; Schalkwijk, C.G.; Fuller, J.H.; Stehouwer, C.D. Markers of Inflammation Are Cross-Sectionally Associated with Microvascular Complications and Cardiovascular Disease in Type 1 Diabetes—The Eurodiab Prospective Complications Study. Diabetologia 2005, 48, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Demircan, N.; Safran, B.G.; Soylu, M.; Ozcan, A.A.; Sizmaz, S. Determination of Vitreous Interleukin-1 (IL-1) and Tumour Necrosis Factor (TNF) Levels in Proliferative Diabetic Retinopathy. Eye 2006, 20, 1366–1369. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, P.; Corraliza, L.; Villena, J.A.; Carvalho, A.R.; Garcia-Arumi, J.; Ramos, D.; Ruberte, J.; Simo, R.; Hernandez, C. The db/db Mouse: A Useful Model for the Study of Diabetic Retinal Neurodegeneration. PLoS ONE 2014, 9, e97302. [Google Scholar] [CrossRef] [PubMed]
- Funatsu, H.; Yamashita, H.; Noma, H.; Mimura, T.; Yamashita, T.; Hori, S. Increased Levels of Vascular Endothelial Growth Factor and Interleukin-6 in the Aqueous Humor of Diabetics with Macular Edema. Am. J. Ophthalmol. 2002, 133, 70–77. [Google Scholar] [CrossRef]
- Jin, K.L.; Mao, X.O.; Greenberg, D.A. Vascular Endothelial Growth Factor: Direct Neuroprotective Effect in in Vitro Ischemia. Proc. Natl. Acad. Sci. USA 2000, 97, 10242–10247. [Google Scholar] [CrossRef] [PubMed]
- Sondell, M.; Lundborg, G.; Kanje, M. Vascular Endothelial Growth Factor Has Neurotrophic Activity and Stimulates Axonal Outgrowth, Enhancing Cell Survival and Schwann Cell Proliferation in the Peripheral Nervous System. J. Neurosci. 1999, 19, 5731–5740. [Google Scholar] [PubMed]
- Goldberg, M.A.; Schneider, T.J. Similarities between the Oxygen-Sensing Mechanisms Regulating the Expression of Vascular Endothelial Growth Factor and Erythropoietin. J. Biol. Chem. 1994, 269, 4355–4359. [Google Scholar] [PubMed]
- Aiello, L.P.; Bursell, S.E.; Clermont, A.; Duh, E.; Ishii, H.; Takagi, C.; Mori, F.; Ciulla, T.A.; Ways, K.; Jirousek, M.; et al. Vascular Endothelial Growth Factor-Induced Retinal Permeability Is Mediated by Protein Kinase C in Vivo and Suppressed by an Orally Effective β-Isoform-Selective Inhibitor. Diabetes 1997, 46, 1473–1480. [Google Scholar] [CrossRef] [PubMed]
- Aiello, L.P.; Wong, J.S. Role of Vascular Endothelial Growth Factor in Diabetic Vascular Complications. Kidney Int. Suppl. 2000, 77, S113–S119. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, X.; Elliott, M.H.; Zhu, M.; Le, Y.Z. Muller Cell-Derived VEGF Is Essential for Diabetes-Induced Retinal Inflammation and Vascular Leakage. Diabetes 2010, 59, 2297–2305. [Google Scholar] [CrossRef] [PubMed]
- Portillo, J.A.; Greene, J.A.; Okenka, G.; Miao, Y.; Sheibani, N.; Kern, T.S.; Subauste, C.S. Cd40 Promotes the Development of Early Diabetic Retinopathy in Mice. Diabetologia 2014, 57, 2222–2231. [Google Scholar] [CrossRef] [PubMed]
- Aveleira, C.A.; Lin, C.M.; Abcouwer, S.F.; Ambrosio, A.F.; Antonetti, D.A. TNFα Signals through Pkczeta/Nf-κb to Alter the Tight Junction Complex and Increase Retinal Endothelial Cell Permeability. Diabetes 2010, 59, 2872–2882. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.N.; Vindeirinho, J.; Cavadas, C.; Ambrosio, A.F.; Santos, P.F. Contribution of TNF Receptor 1 to Retinal Neural Cell Death Induced by Elevated Glucose. Mol. Cell. Neurosci. 2012, 50, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Gharaee-Kermani, M.; Denholm, E.M.; Phan, S.H. Costimulation of Fibroblast Collagen and Transforming Growth Factor β1 Gene Expression by Monocyte Chemoattractant Protein-1 Via Specific Receptors. J. Biol. Chem. 1996, 271, 17779–17784. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.; Li, X.; Xiao, L.; Yu, W.; Wang, B.; Chu, L. Upregulation of Retinal Neuronal MCP-1 in the Rodent Model of Diabetic Retinopathy and Its Function in Vitro. Investig. Ophthalmol. Vis. Sci. 2012, 53, 7567–7575. [Google Scholar] [CrossRef] [PubMed]
- Koistinaho, M.; Koistinaho, J. Role of P38 and P44/42 Mitogen-Activated Protein Kinases in Microglia. Glia 2002, 40, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.S.; El-Remessy, A.B.; Matragoon, S.; Zhang, W.; Patel, Y.; Khan, S.; Al-Gayyar, M.M.; El-Shishtawy, M.M.; Liou, G.I. Retinal Microglial Activation and Inflammation Induced by Amadori-Glycated Albumin in a Rat Model of Diabetes. Diabetes 2011, 60, 1122–1133. [Google Scholar] [CrossRef] [PubMed]
- Zabel, M.K.; Zhao, L.; Zhang, Y.; Gonzalez, S.R.; Ma, W.; Wang, X.; Fariss, R.N.; Wong, W.T. Microglial Phagocytosis and Activation Underlying Photoreceptor Degeneration Is Regulated by CX3CL1-CX3CR1 Signaling in a Mouse Model of Retinitis Pigmentosa. Glia 2016, 64, 1479–1491. [Google Scholar] [CrossRef] [PubMed]
- Beli, E.; Dominguez, J.M., 2nd; Hu, P.; Thinschmidt, J.S.; Caballero, S.; Calzi, S.L.; Luo, D.; Shanmugam, S.; Salazar, T.E.; Duan, Y.; et al. CX3CR1 Deficiency Accelerates the Development of Retinopathy in a Rodent Model of Type 1 Diabetes. J. Mol. Med. 2016, 94, 1255–1265. [Google Scholar] [CrossRef] [PubMed]
- Mendiola, A.S.; Garza, R.; Cardona, S.M.; Mythen, S.A.; Lira, S.A.; Akassoglou, K.; Cardona, A.E. Fractalkine Signaling Attenuates Perivascular Clustering of Microglia and Fibrinogen Leakage During Systemic Inflammation in Mouse Models of Diabetic Retinopathy. Front. Cell. Neurosci. 2016, 10, 303. [Google Scholar] [CrossRef] [PubMed]
- Chakravarthy, H.; Navitskaya, S.; O’Reilly, S.; Gallimore, J.; Mize, H.; Beli, E.; Wang, Q.; Kady, N.; Huang, C.; Blanchard, G.J.; et al. Role of Acid Sphingomyelinase in Shifting the Balance between Proinflammatory and Reparative Bone Marrow Cells in Diabetic Retinopathy. Stem Cells 2016, 34, 972–983. [Google Scholar] [CrossRef] [PubMed]
- Kady, N.; Yan, Y.; Salazar, T.; Wang, Q.; Chakravarthy, H.; Huang, C.; Beli, E.; Navitskaya, S.; Grant, M.; Busik, J. Increase in Acid Sphingomyelinase Level in Human Retinal Endothelial Cells and Cd34+ Circulating Angiogenic Cells Isolated from Diabetic Individuals Is Associated with Dysfunctional Retinal Vasculature and Vascular Repair Process in Diabetes. J. Clin. Lipidol. 2017, 11, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Noda, K.; Ishida, S.; Inoue, M.; Obata, K.; Oguchi, Y.; Okada, Y.; Ikeda, E. Production and Activation of Matrix Metalloproteinase-2 in Proliferative Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2163–2170. [Google Scholar] [CrossRef]
- Zhou, T.; Hu, Y.; Chen, Y.; Zhou, K.K.; Zhang, B.; Gao, G.; Ma, J.X. The Pathogenic Role of the Canonical Wnt Pathway in Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2010, 51, 4371–4379. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Lee, K.; Zhang, B.; Zhou, T.; He, X.; Gao, G.; Murray, A.R.; Ma, J.X. Identification of a Novel Inhibitor of the Canonical Wnt Pathway. Mol. Cell. Biol. 2011, 31, 3038–3051. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Hu, Y.; Ding, L.; Chen, Y.; Takahashi, Y.; Mott, R.; Ma, J.X. Therapeutic Potential of a Monoclonal Antibody Blocking the Wnt Pathway in Diabetic Retinopathy. Diabetes 2012, 61, 2948–2957. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cheng, R.; Lee, K.; Tyagi, P.; Ding, L.; Kompella, U.B.; Chen, J.; Xu, X.; Ma, J.X. Nanoparticle-Mediated Expression of a Wnt Pathway Inhibitor Ameliorates Ocular Neovascularization. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; He, J.; Zhou, Y.; Bai, X.; Wu, G.; Wang, X.; Liu, Z.; Chen, Y.; Ma, J.X.; Liu, Z. Plasma and Vitreous Fluid Levels of Dickkopf-1 in Patients with Diabetic Retinopathy. Eye 2014, 28, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Jiang, J.; Wang, S.; Xia, X. Dkk1 Inhibits Proliferation and Migration in Human Retinal Pigment Epithelial Cells Via the Wnt/Β-Catenin Signaling Pathway. Exp. Ther. Med. 2016, 12, 859–863. [Google Scholar] [CrossRef] [PubMed]
- Hammes, H.P.; Federoff, H.J.; Brownlee, M. Nerve Growth Factor Prevents Both Neuroretinal Programmed Cell Death and Capillary Pathology in Experimental Diabetes. Mol. Med. 1995, 1, 527–534. [Google Scholar] [PubMed]
- Kerrigan, L.A.; Zack, D.J.; Quigley, H.A.; Smith, S.D.; Pease, M.E. Tunel-Positive Ganglion Cells in Human Primary Open-Angle Glaucoma. Arch. Ophthalmol. 1997, 115, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, H.W.; Verbraak, F.D.; Kok, P.H.; Stehouwer, M.; Garvin, M.K.; Sonka, M.; DeVries, J.H.; Schlingemann, R.O.; Abramoff, M.D. Early Neurodegeneration in the Retina of Type 2 Diabetic Patients. Investig. Ophthalmol. Vis. Sci. 2012, 53, 2715–2719. [Google Scholar] [CrossRef] [PubMed]
- Simo, R.; Hernandez, C. Neurodegeneration in the Diabetic Eye: New Insights and Therapeutic Perspectives. Trends Endocrinol. Metab. 2014, 25, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Parisi, V.; Uccioli, L. Visual Electrophysiological Responses in Persons with Type 1 Diabetes. Diabetes Metab. Res. Rev. 2001, 17, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Ghirlanda, G.; di Leo, M.A.; Caputo, S.; Cercone, S.; Greco, A.V. From Functional to Microvascular Abnormalities in Early Diabetic Retinopathy. Diabetes Metab. Rev. 1997, 13, 15–35. [Google Scholar] [CrossRef]
- Bresnick, G.H. Diabetic Retinopathy Viewed as a Neurosensory Disorder. Arch. Ophthalmol. 1986, 104, 989–990. [Google Scholar] [CrossRef] [PubMed]
- Greenstein, V.C.; Shapiro, A.; Zaidi, Q.; Hood, D.C. Psychophysical Evidence for Post-Receptoral Sensitivity Loss in Diabetics. Investig. Ophthalmol. Vis. Sci. 1992, 33, 2781–2790. [Google Scholar]
- Bearse, M.A., Jr.; Han, Y.; Schneck, M.E.; Barez, S.; Jacobsen, C.; Adams, A.J. Local Multifocal Oscillatory Potential Abnormalities in Diabetes and Early Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2004, 45, 3259–3265. [Google Scholar] [CrossRef] [PubMed]
- Wolter, J.R. Diabetic Retinopathy. Am. J. Ophthalmol. 1961, 51, 1123–1141. [Google Scholar] [CrossRef]
- Bloodworth, J.M., Jr. Diabetic Retinopathy. Diabetes 1962, 11, 1–22. [Google Scholar] [PubMed]
- Araszkiewicz, A.; Zozulinska-Ziolkiewicz, D.; Meller, M.; Bernardczyk-Meller, J.; Pilacinski, S.; Rogowicz-Frontczak, A.; Naskret, D.; Wierusz-Wysocka, B. Neurodegeneration of the Retina in Type 1 Diabetic Patients. Polskie Archiwum Medycyny Wewnętrznej 2012, 122, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Kern, T.S.; Barber, A.J. Retinal Ganglion Cells in Diabetes. J. Physiol. 2008, 586, 4401–4408. [Google Scholar] [CrossRef] [PubMed]
- Gillies, M.C.; Su, T.; Stayt, J.; Simpson, J.M.; Naidoo, D.; Salonikas, C. Effect of High Glucose on Permeability of Retinal Capillary Endothelium in Vitro. Investig. Ophthalmol. Vis. Sci. 1997, 38, 635–642. [Google Scholar]
- Kusari, J.; Zhou, S.; Padillo, E.; Clarke, K.G.; Gil, D.W. Effect of Memantine on Neuroretinal Function and Retinal Vascular Changes of Streptozotocin-Induced Diabetic Rats. Investig. Ophthalmol. Vis. Sci. 2007, 48, 5152–5159. [Google Scholar] [CrossRef] [PubMed]
- Robinson, R.; Barathi, V.A.; Chaurasia, S.S.; Wong, T.Y.; Kern, T.S. Update on Animal Models of Diabetic Retinopathy: From Molecular Approaches to Mice and Higher Mammals. Dis. Model. Mech. 2012, 5, 444–456. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, E.; Hernandez, C.; de Torres, I.; Farres, J.; Simo, R. Lowered Cortistatin Expression Is an Early Event in the Human Diabetic Retina and Is Associated with Apoptosis and Glial Activation. Mol. Vis. 2008, 14, 1496–1502. [Google Scholar] [PubMed]
- Garcia-Ramirez, M.; Hernandez, C.; Villarroel, M.; Canals, F.; Alonso, M.A.; Fortuny, R.; Masmiquel, L.; Navarro, A.; Garcia-Arumi, J.; Simo, R. Interphotoreceptor Retinoid-Binding Protein (IRBP) Is Downregulated at Early Stages of Diabetic Retinopathy. Diabetologia 2009, 52, 2633–2641. [Google Scholar] [CrossRef] [PubMed]
- Sohn, E.H.; van Dijk, H.W.; Jiao, C.; Kok, P.H.; Jeong, W.; Demirkaya, N.; Garmager, A.; Wit, F.; Kucukevcilioglu, M.; van Velthoven, M.E.; et al. Retinal Neurodegeneration May Precede Microvascular Changes Characteristic of Diabetic Retinopathy in Diabetes Mellitus. Proc. Natl. Acad. Sci. USA 2016, 113, E2655–E2664. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Ma, J.; Li, Y.; Zhang, Z. Association between Retinal Neuronal Degeneration and Visual Function Impairment in Type 2 Diabetic Patients without Diabetic Retinopathy. Sci. China Life Sci. 2015, 58, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Lopes de Faria, J.M.; Russ, H.; Costa, V.P. Retinal Nerve Fibre Layer Loss in Patients with Type 1 Diabetes Mellitus without Retinopathy. Br. J. Ophthalmol. 2002, 86, 725–728. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, S.E. Erg in Juvenile Diabetics: A Prognostic Study. In Symposium on the Treatment of Diabetic Retinopathy; Goldberg, M., Fine, S.L., Eds.; Department of Health, Education and Welfare: Arlington, VA, USA, 1969; pp. 681–689. [Google Scholar]
- Santiago, A.R.; Gaspar, J.M.; Baptista, F.I.; Cristovao, A.J.; Santos, P.F.; Kamphuis, W.; Ambrosio, A.F. Diabetes Changes the Levels of Ionotropic Glutamate Receptors in the Rat Retina. Mol. Vis. 2009, 15, 1620–1630. [Google Scholar] [PubMed]
- Ng, Y.K.; Zeng, X.X.; Ling, E.A. Expression of Glutamate Receptors and Calcium-Binding Proteins in the Retina of Streptozotocin-Induced Diabetic Rats. Brain Res. 2004, 1018, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. The Pathobiology of Diabetic Complications: A Unifying Mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Tonkiss, J.; Roy, S. Aging Increases Retinal Vascular Lesions Characteristic of Early Diabetic Retinopathy. Biogerontology 2010, 11, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yanoff, M.; Liu, X.; Ye, X. Retinal Capillary Pericyte Apoptosis in Early Human Diabetic Retinopathy. Chin. Med. J. 1997, 110, 659–663. [Google Scholar] [PubMed]
- Barouch, F.C.; Miyamoto, K.; Allport, J.R.; Fujita, K.; Bursell, S.E.; Aiello, L.P.; Luscinskas, F.W.; Adamis, A.P. Integrin-Mediated Neutrophil Adhesion and Retinal Leukostasis in Diabetes. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1153–1158. [Google Scholar]
- Oosthuyse, B.; Moons, L.; Storkebaum, E.; Beck, H.; Nuyens, D.; Brusselmans, K.; van Dorpe, J.; Hellings, P.; Gorselink, M.; Heymans, S.; et al. Deletion of the Hypoxia-Response Element in the Vascular Endothelial Growth Factor Promoter Causes Motor Neuron Degeneration. Nat. Genet. 2001, 28, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Bek, T. Glial Cell Involvement in Vascular Occlusion of Diabetic Retinopathy. Acta Ophthalmol. Scand. 1997, 75, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Barot, M.; Gokulgandhi, M.R.; Patel, S.; Mitra, A.K. Microvascular Complications and Diabetic Retinopathy: Recent Advances and Future Implications. Future Med. Chem. 2013, 5, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Antonetti, D.A.; Barber, A.J.; Khin, S.; Lieth, E.; Tarbell, J.M.; Gardner, T.W. Vascular Permeability in Experimental Diabetes Is Associated with Reduced Endothelial Occludin Content: Vascular Endothelial Growth Factor Decreases Occludin in Retinal Endothelial Cells. Penn State Retina Research Group. Diabetes 1998, 47, 1953–1959. [Google Scholar] [CrossRef] [PubMed]
- Barber, A.J.; Antonetti, D.A.; Gardner, T.W. Altered Expression of Retinal Occludin and Glial Fibrillary Acidic Protein in Experimental Diabetes. The Penn State Retina Research Group. Investig. Ophthalmol. Vis. Sci. 2000, 41, 3561–3568. [Google Scholar]
- Lupo, G.; Motta, C.; Giurdanella, G.; Anfuso, C.D.; Alberghina, M.; Drago, F.; Salomone, S.; Bucolo, C. Role of Phospholipases A2 in Diabetic Retinopathy: In Vitro and in Vivo Studies. Biochem. Pharmacol. 2013, 86, 1603–1613. [Google Scholar] [CrossRef] [PubMed]
- Alder, V.A.; Su, E.N.; Yu, D.Y.; Cringle, S.J.; Yu, P.K. Diabetic Retinopathy: Early Functional Changes. Clin. Exp. Pharmacol. Physiol. 1997, 24, 785–788. [Google Scholar] [CrossRef] [PubMed]
- Curtis, T.M.; Hamilton, R.; Yong, P.H.; McVicar, C.M.; Berner, A.; Pringle, R.; Uchida, K.; Nagai, R.; Brockbank, S.; Stitt, A.W. Müller Glial Dysfunction During Diabetic Retinopathy in Rats Is Linked to Accumulation of Advanced Glycation End-Products and Advanced Lipoxidation End-Products. Diabetologia 2011, 54, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Bhagat, N.; Grigorian, R.A.; Tutela, A.; Zarbin, M.A. Diabetic Macular Edema: Pathogenesis and Treatment. Surv. Ophthalmol. 2009, 54, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Durham, J.T.; Herman, I.M. Microvascular Modifications in Diabetic Retinopathy. Curr. Diabetes Rep. 2011, 11, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Legacy, J.; Hanea, S.; Theoret, J.; Smith, P.D. Granulocyte Macrophage Colony-Stimulating Factor Promotes Regeneration of Retinal Ganglion Cells in Vitro through a Mammalian Target of Rapamycin-Dependent Mechanism. J. Neurosci. Res. 2013, 91, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Sappington, R.M.; Chan, M.; Calkins, D.J. Interleukin-6 Protects Retinal Ganglion Cells from Pressure-Induced Death. Investig. Ophthalmol. Vis. Sci. 2006, 47, 2932–2942. [Google Scholar] [CrossRef] [PubMed]
- Kaur, C.; Foulds, W.S.; Ling, E.-A. Hypoxia-Ischemia and Retinal Ganglion Cell Damage. Clin. Ophthalmol. 2008, 2, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Heiduschka, P.; Alex, A.F.; Niekamper, D.; Eter, N. Behaviour of CD11B-Positive Cells in an Animal Model of Laser-Induced Choroidal Neovascularisation. Ophthalmologica 2017, 237, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Zijlstra, A.; Seandel, M.; Kupriyanova, T.A.; Partridge, J.J.; Madsen, M.A.; Hahn-Dantona, E.A.; Quigley, J.P.; Deryugina, E.I. Proangiogenic Role of Neutrophil-Like Inflammatory Heterophils during Neovascularization Induced by Growth Factors and Human Tumor Cells. Blood 2006, 107, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Voronov, E.; Shouval, D.S.; Krelin, Y.; Cagnano, E.; Benharroch, D.; Iwakura, Y.; Dinarello, C.A.; Apte, R.N. Il-1 Is Required for Tumor Invasiveness and Angiogenesis. Proc. Natl. Acad. Sci. USA 2003, 100, 2645–2650. [Google Scholar] [CrossRef] [PubMed]
- Naldini, A.; Leali, D.; Pucci, A.; Morena, E.; Carraro, F.; Nico, B.; Ribatti, D.; Presta, M. Cutting Edge: Il-1β Mediates the Proangiogenic Activity of Osteopontin-Activated Human Monocytes. J. Immunol. 2006, 177, 4267–4270. [Google Scholar] [CrossRef] [PubMed]
- Leali, D.; Dell’Era, P.; Stabile, H.; Sennino, B.; Chambers, A.F.; Naldini, A.; Sozzani, S.; Nico, B.; Ribatti, D.; Presta, M. Osteopontin (ETA-1) and Fibroblast Growth Factor-2 Cross-Talk in Angiogenesis. J. Immunol. 2003, 171, 1085–1093. [Google Scholar] [CrossRef] [PubMed]
- Speyer, C.L.; Ward, P.A. Role of Endothelial Chemokines and Their Receptors During Inflammation. J. Investig. Surg. 2011, 24, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Sprague, A.H.; Khalil, R.A. Inflammatory Cytokines in Vascular Dysfunction and Vascular Disease. Biochem. Pharmacol. 2009, 78, 539–552. [Google Scholar] [CrossRef] [PubMed]
- Dor, Y.; Porat, R.; Keshet, E. Vascular Endothelial Growth Factor and Vascular Adjustments to Perturbations in Oxygen Homeostasis. Am. J. Physiol. Cell Physiol. 2001, 280, C1367–C1374. [Google Scholar] [CrossRef] [PubMed]
- Boyer, D.S.; Hopkins, J.J.; Sorof, J.; Ehrlich, J.S. Anti-Vascular Endothelial Growth Factor Therapy for Diabetic Macular Edema. Ther. Adv. Endocrinol. Metab. 2013, 4, 151–169. [Google Scholar] [CrossRef] [PubMed]
- Aplin, A.C.; Gelati, M.; Fogel, E.; Carnevale, E.; Nicosia, R.F. Angiopoietin-1 and Vascular Endothelial Growth Factor Induce Expression of Inflammatory Cytokines before Angiogenesis. Physiol. Genom. 2006, 27, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Angelo, L.S.; Kurzrock, R. Vascular Endothelial Growth Factor and Its Relationship to Inflammatory Mediators. Clin. Cancer Res. 2007, 13, 2825–2830. [Google Scholar] [CrossRef] [PubMed]
- Lieth, E.; LaNoue, K.F.; Antonetti, D.A.; Ratz, M. Diabetes Reduces Glutamate Oxidation and Glutamine Synthesis in the Retina. The Penn State Retina Research Group. Exp. Eye Res. 2000, 70, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, M.; Gerhardinger, C.; Lorenzi, M. Muller Cell Changes in Human Diabetic Retinopathy. Diabetes 1998, 47, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Ma, W.; Zhao, L.; Fariss, R.N.; Wong, W.T. Adaptive Muller Cell Responses to Microglial Activation Mediate Neuroprotection and Coordinate Inflammation in the Retina. J. Neuroinflamm. 2011, 8, 173. [Google Scholar] [CrossRef] [PubMed]
- Nagayach, A.; Patro, N.; Patro, I. Astrocytic and Microglial Response in Experimentally Induced Diabetic Rat Brain. Metab. Brain Dis. 2014, 29, 747–761. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, X.; Zhao, L.; Ma, W.; Rodriguez, I.R.; Fariss, R.N.; Wong, W.T. Macroglia-Microglia Interactions Via Tspo Signaling Regulates Microglial Activation in the Mouse Retina. J. Neurosci. 2014, 34, 3793–3806. [Google Scholar] [CrossRef] [PubMed]
- Reme, C.E.; Rol, P.; Grothmann, K.; Kaase, H.; Terman, M. Bright Light Therapy in Focus: Lamp Emission Spectra and Ocular Safety. Technol. Health Care 1996, 4, 403–413. [Google Scholar] [PubMed]
- Rozanowska, M.; Sarna, T. Light-Induced Damage to the Retina: Role of Rhodopsin Chromophore Revisited. Photochem. Photobiol. 2005, 81, 1305–1330. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, A.; Grimm, C.; Samardzija, M.; Reme, C.E. Molecular Mechanisms of Light-Induced Photoreceptor Apoptosis and Neuroprotection for Retinal Degeneration. Prog. Retin. Eye Res. 2005, 24, 275–306. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Liang, K.J.; Fariss, R.N.; Wong, W.T. Ex Vivo Dynamic Imaging of Retinal Microglia Using Time-Lapse Confocal Microscopy. Investig. Ophthalmol. Vis. Sci. 2008, 49, 4169–4176. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Garcia, S.; Reichhart, N.; Hernandez-Matas, C.; Zabulis, X.; Kociok, N.; Brockmann, C.; Joussen, A.M.; Strauss, O. In Vivo Analysis of the Time and Spatial Activation Pattern of Microglia in the Retina Following Laser-Induced Choroidal Neovascularization. Exp. Eye Res. 2015, 139, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Shen, J.K.; Lam, T.T.; Zeng, H.Y.; Chiang, S.K.; Yang, F.; Tso, M.O. Activation of Microglia and Chemokines in Light-Induced Retinal Degeneration. Mol. Vis. 2005, 11, 887–895. [Google Scholar] [PubMed]
- Song, D.; Sulewski, M.E., Jr.; Wang, C.; Song, J.; Bhuyan, R.; Sterling, J.; Clark, E.; Song, W.C.; Dunaief, J.L. Complement C5a Receptor Knockout Has Diminished Light-Induced Microglia/Macrophage Retinal Migration. Mol. Vis. 2017, 23, 210–218. [Google Scholar] [PubMed]
- Roodhart, J.M.; Langenberg, M.H.; Witteveen, E.; Voest, E.E. The Molecular Basis of Class Side Effects Due to Treatment with Inhibitors of the VEGF/VEGFR Pathway. Curr. Clin. Pharmacol. 2008, 3, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Zapata, M.J.; Marti-Carvajal, A.J.; Sola, I.; Pijoan, J.I.; Buil-Calvo, J.A.; Cordero, J.A.; Evans, J.R. Anti-Vascular Endothelial Growth Factor for Proliferative Diabetic Retinopathy. Cochrane Database Syst. Rev. 2014, CD008721. [Google Scholar] [CrossRef] [PubMed]
- Arevalo, J.F. Intravitreal Bevacizumab as Anti-Vascular Endothelial Growth Factor in the Management of Complications of Proliferative Diabetic Retinopathy. Med. Hypothesis Discov. Innov. Ophthalmol. 2013, 2, 20–24. [Google Scholar] [PubMed]
- Huang, H.; Parlier, R.; Shen, J.K.; Lutty, G.A.; Vinores, S.A. VEGF Receptor Blockade Markedly Reduces Retinal Microglia/Macrophage Infiltration into Laser-Induced CNV. PLoS ONE 2013, 8, e71808. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yoshida, S.; Kubo, Y.; Yoshimura, T.; Kobayashi, Y.; Nakama, T.; Yamaguchi, M.; Ishikawa, K.; Oshima, Y.; Ishibashi, T. Different Distributions of M1 and M2 Macrophages in a Mouse Model of Laser-Induced Choroidal Neovascularization. Mol. Med. Rep. 2017, 15, 3949–3956. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Wang, H.; Liu, Y.; Li, W.; Kim, D.; Huang, H. Blockade of Vascular Endothelial Growth Factor Receptor 1 Prevents Inflammation and Vascular Leakage in Diabetic Retinopathy. J. Ophthalmol. 2015, 2015, 605946. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.S.; Sun, J.K.; Aiello, L.P. Role of Steroids in the Management of Diabetic Macular EDEMA and Proliferative Diabetic Retinopathy. Semin. Ophthalmol. 2009, 24, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Regillo, C.D.; Callanan, D.G.; Do, D.V.; Fine, H.F.; Holekamp, N.M.; Kuppermann, B.D.; Singer, M.A.; Singh, R.P. Use of Corticosteroids in the Treatment of Patients with Diabetic Macular Edema Who Have a Suboptimal Response to Anti-Vegf: Recommendations of an Expert Panel. Ophthalmic Surg. Lasers Imaging Retina 2017, 48, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, S.; Gomi, F.; Oshima, Y.; Tohyama, M.; Tano, Y. Vascular Endothelial Growth Factor Reduced and Connective Tissue Growth Factor Induced by Triamcinolone in Arpe19 Cells under Oxidative Stress. Investig. Ophthalmol. Vis. Sci. 2005, 46, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Yang, L.; Li, H.; Gong, H.; Cheng, L.; Zheng, H.; Zhang, L.M.; Lan, Y. Downregulation of VEGF mRNA Expression by Triamcinolone Acetonide Acetate-Loaded Chitosan Derivative Nanoparticles in Human Retinal Pigment Epithelial Cells. Int. J. Nanomed. 2012, 7, 4649–4660. [Google Scholar]
- Shen, W.; Lee, S.R.; Araujo, J.; Chung, S.H.; Zhu, L.; Gillies, M.C. Effect of Glucocorticoids on Neuronal and Vascular Pathology in a Transgenic Model of Selective Muller Cell Ablation. Glia 2014, 62, 1110–1124. [Google Scholar] [CrossRef] [PubMed]
- McColl, A.; Bournazos, S.; Franz, S.; Perretti, M.; Morgan, B.P.; Haslett, C.; Dransfield, I. Glucocorticoids Induce Protein S-Dependent Phagocytosis of Apoptotic Neutrophils by Human Macrophages. J. Immunol. 2009, 183, 2167–2175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heasman, S.J.; Giles, K.M.; Ward, C.; Rossi, A.G.; Haslett, C.; Dransfield, I. Glucocorticoid-Mediated Regulation of Granulocyte Apoptosis and Macrophage Phagocytosis of Apoptotic Cells: Implications for the Resolution of Inflammation. J. Endocrinol. 2003, 178, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Glezer, I.; Simard, A.R.; Rivest, S. Neuroprotective Role of the Innate Immune System by Microglia. Neuroscience 2007, 147, 867–883. [Google Scholar] [CrossRef] [PubMed]
- Lai, A.Y.; Todd, K.G. Hypoxia-Activated Microglial Mediators of Neuronal Survival Are Differentially Regulated by Tetracyclines. Glia 2006, 53, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Yrjanheikki, J.; Tikka, T.; Keinanen, R.; Goldsteins, G.; Chan, P.H.; Koistinaho, J. A Tetracycline Derivative, Minocycline, Reduces Inflammation and Protects against Focal Cerebral Ischemia with a Wide Therapeutic Window. Proc. Natl. Acad. Sci. USA 1999, 96, 13496–13500. [Google Scholar] [CrossRef] [PubMed]
- Abcouwer, S.F.; Lin, C.M.; Shanmugam, S.; Muthusamy, A.; Barber, A.J.; Antonetti, D.A. Minocycline Prevents Retinal Inflammation and Vascular Permeability Following Ischemia-Reperfusion Injury. J. Neuroinflamm. 2013, 10, 149. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.L.; Yu, A.C.; Lau, L.T.; Lee, C.; Le, M.W.; Zhu, X.; Tso, M.O. Minocycline Inhibits LPS-Induced Retinal Microglia Activation. Neurochem. Int. 2005, 47, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Cukras, C.A.; Petrou, P.; Chew, E.Y.; Meyerle, C.B.; Wong, W.T. Oral Minocycline for the Treatment of Diabetic Macular Edema (DME): Results of a Phase I/II Clinical Study. Investig. Ophthalol. Vis. Sci. 2012, 53, 3865–3874. [Google Scholar] [CrossRef] [PubMed]
- Douglas, Y.; Bhatwadekar, A.D.; Shaw, L.C.; Carnegie, D.; Caballero, S.; Li, Q.; Calzi, S.L.; Raizada, M.K.; Stitt, A.W.; Grant, M.B. Bone Marrow-CNS Connections: Implications in the Pathogenesis of Diabetic Retinopathy. Prog. Retin. Eye Res. 2012, 31, 481–494. [Google Scholar] [CrossRef] [PubMed]
- Jantzie, L.L.; Cheung, P.Y.; Todd, K.G. Doxycycline Reduces Cleaved Caspase-3 and Microglial Activation in an Animal Model of Neonatal Hypoxia-Ischemia. J. Cereb. Blood Flow Metab. 2005, 25, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Scott, I.U.; Jackson, G.R.; Quillen, D.A.; Larsen, M.; Klein, R.; Liao, J.; Holfort, S.; Munch, I.C.; Gardner, T.W. Effect of Doxycycline vs. Placebo on Retinal Function and Diabetic Retinopathy Progression in Patients with Severe Nonproliferative or Non-High-Risk Proliferative Diabetic Retinopathy: A Randomized Clinical Trial. JAMA Ophthalmol. 2014, 132, 535–543. [Google Scholar] [CrossRef] [PubMed]
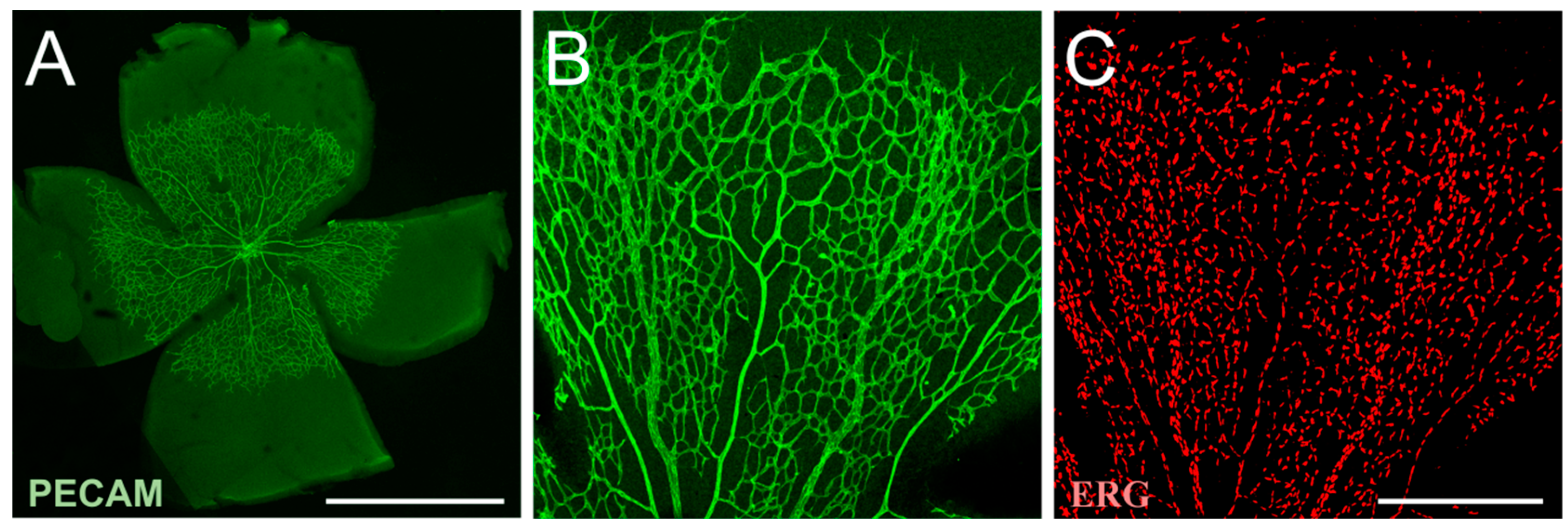
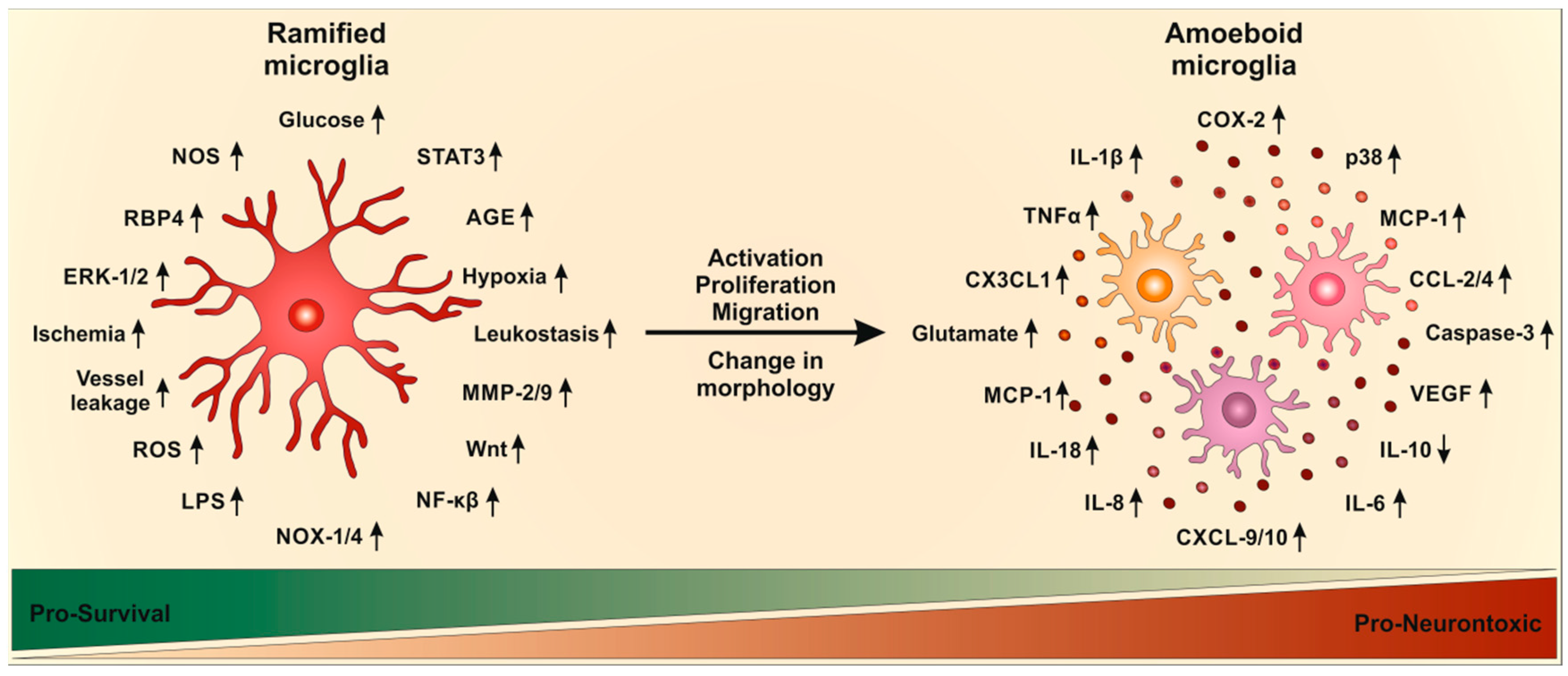
| Mediators | Regulation | Relevance | References |
|---|---|---|---|
| Cytokines | |||
| IL-1β | ↑ | Immuno-stimulation Increased ICAM-1 | [28,168,169,170,171] |
| IL-6 | ↑ | Immuno-stimulation | [172,173] |
| IL-8 | ↑ | Immuno-stimulation | [173] |
| IL-18 | ↑ | Immuno-stimulation | [174,175,176] |
| VEGF | ↑ | Immuno-stimulation Angio-stimulation Neuroprotective | [177] |
| TNFα | ↑ | Immuno-stimulation Increased ICAM-1 Increased leukostasis | [28,168,178,179,180] |
| COX-2 | ↑ | Immuno-stimulation | [180] |
| Chemokines | |||
| CCL-2 | ↑ | Immuno-stimulation | [181] |
| CCL-4 | ↑ | Immuno-stimulation | [181] |
| CXCL-9 | ↑ | Immuno-stimulation | [181] |
| CXCL-10 | ↑ | Immuno-stimulation | [181] |
| MCP-1 | ↑ | Immuno-stimulation Increased fibrosis Angio-stimulation | [28,173,182] |
| CX3CL1 | ↑ | Immuno-stimulation Neuroprotective | [183] |
| Growth factors | |||
| NGF | ↓ | Cellular toxicity | [184] |
| PEDF | ↓ | Cellular toxicity | [184] |
| IRBP | ↓ | Cellular toxicity | [184] |
| Somatostatin | ↓ | Cellular toxicity | [184] |
| Adhesion molecules | |||
| ICAM-1 | ↑ | Increased leukostasis | [64,65,66] |
| VCAM-1 | ↑ | Increased leukostasis | [64,65,66] |
| Neurotoxins | |||
| ROS | ↑ | Cellular toxicity | [185] |
| NO | ↑ | Cellular toxicity | [185] |
| Glutamate | ↑ | Cellular toxicity | [185] |
| Caspase-3 | ↑ | Cellular toxicity | [185] |
| Others | |||
| LPS | ↑ | Immuno-stimulation Cellular toxicity | [106] |
| AGE | ↑ | Immuno-stimulation Cellular toxicity | [49,50,51,52,53,54,55,56] |
| LEP | ↑ | Immuno-stimulation Cellular toxicity | [49,50,51,52,53,54,55,56] |
| Angiopoietin-2 | ↑ | Angio-stimulation | [186,187] |
| RBP4 | ↑ | Immuno-stimulation Neurotoxic Increased vascular leakage | [174,175,176] |
| NOX-1/4 | ↑ | Immuno-stimulation Increased leukostasis Increased ROS Increased vascular leakage | [188] |
| MMP-2/9 | ↑ | Immuno-stimulation Increased chemokines Neurotoxic | [189,190,191,192,193,194] |
| STAT3 | ↑ | Immuno-stimulation Increased vascular leakage | [195,196] |
| Wnt | ↑ | Immuno-stimulation Increased ROS | [197] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altmann, C.; Schmidt, M.H.H. The Role of Microglia in Diabetic Retinopathy: Inflammation, Microvasculature Defects and Neurodegeneration. Int. J. Mol. Sci. 2018, 19, 110. https://doi.org/10.3390/ijms19010110
Altmann C, Schmidt MHH. The Role of Microglia in Diabetic Retinopathy: Inflammation, Microvasculature Defects and Neurodegeneration. International Journal of Molecular Sciences. 2018; 19(1):110. https://doi.org/10.3390/ijms19010110
Chicago/Turabian StyleAltmann, Christine, and Mirko H. H. Schmidt. 2018. "The Role of Microglia in Diabetic Retinopathy: Inflammation, Microvasculature Defects and Neurodegeneration" International Journal of Molecular Sciences 19, no. 1: 110. https://doi.org/10.3390/ijms19010110





