Expression and Activity of COX-1 and COX-2 in Acanthamoeba sp.-Infected Lungs According to the Host Immunological Status
Abstract
:1. Introduction
2. Results
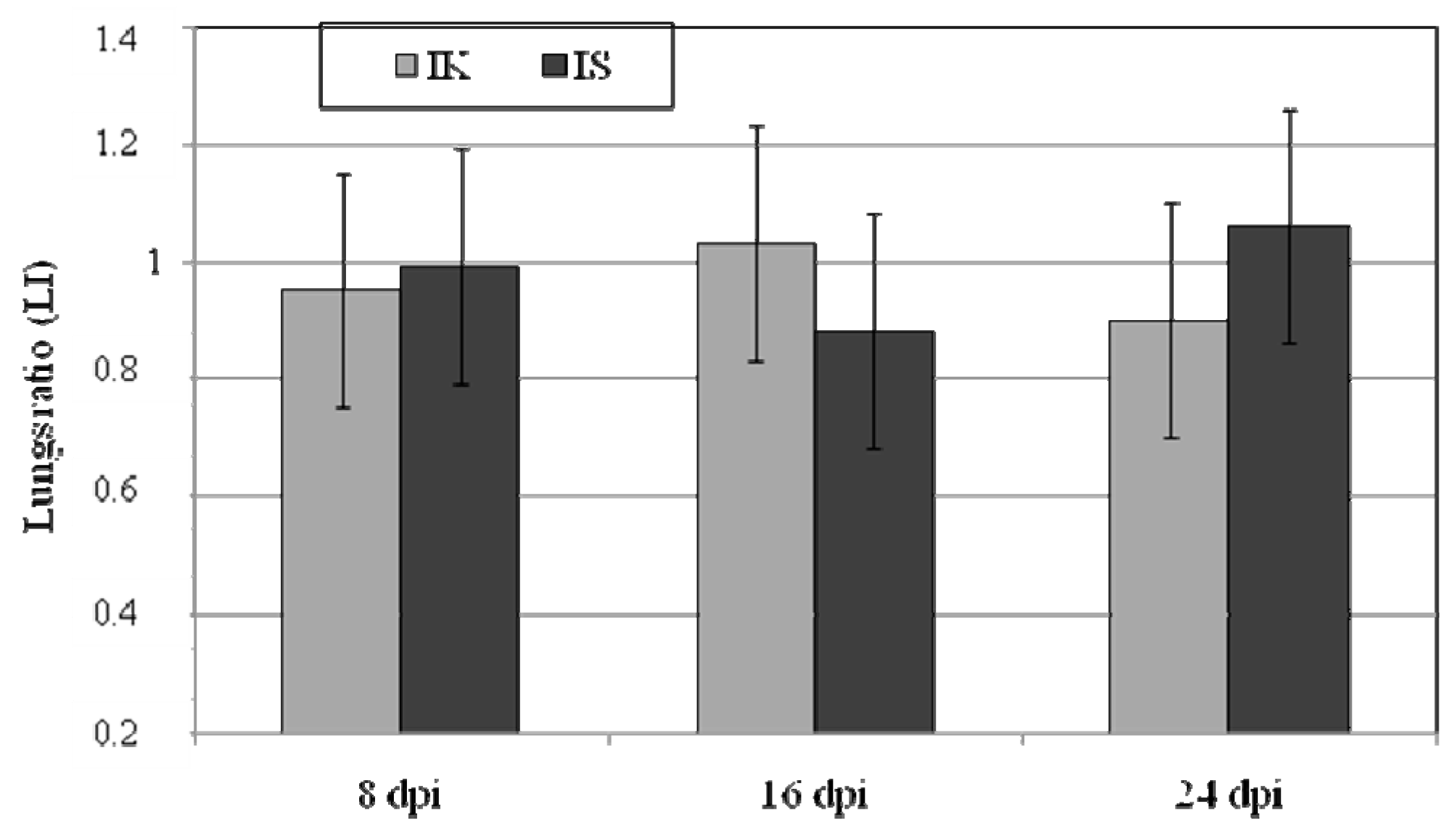
2.1. Body Weight, Lung Weight and Lung Weight Ratio
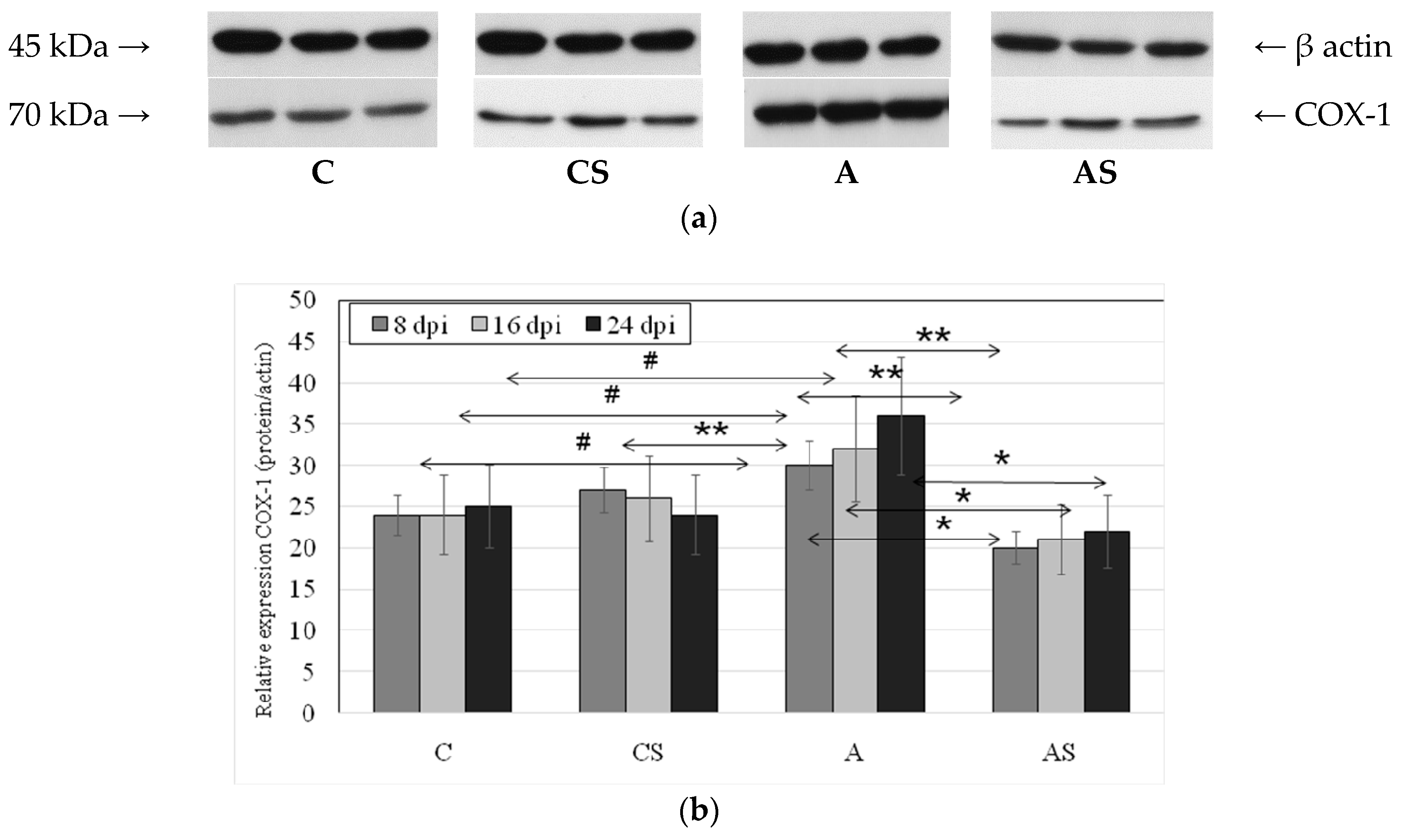
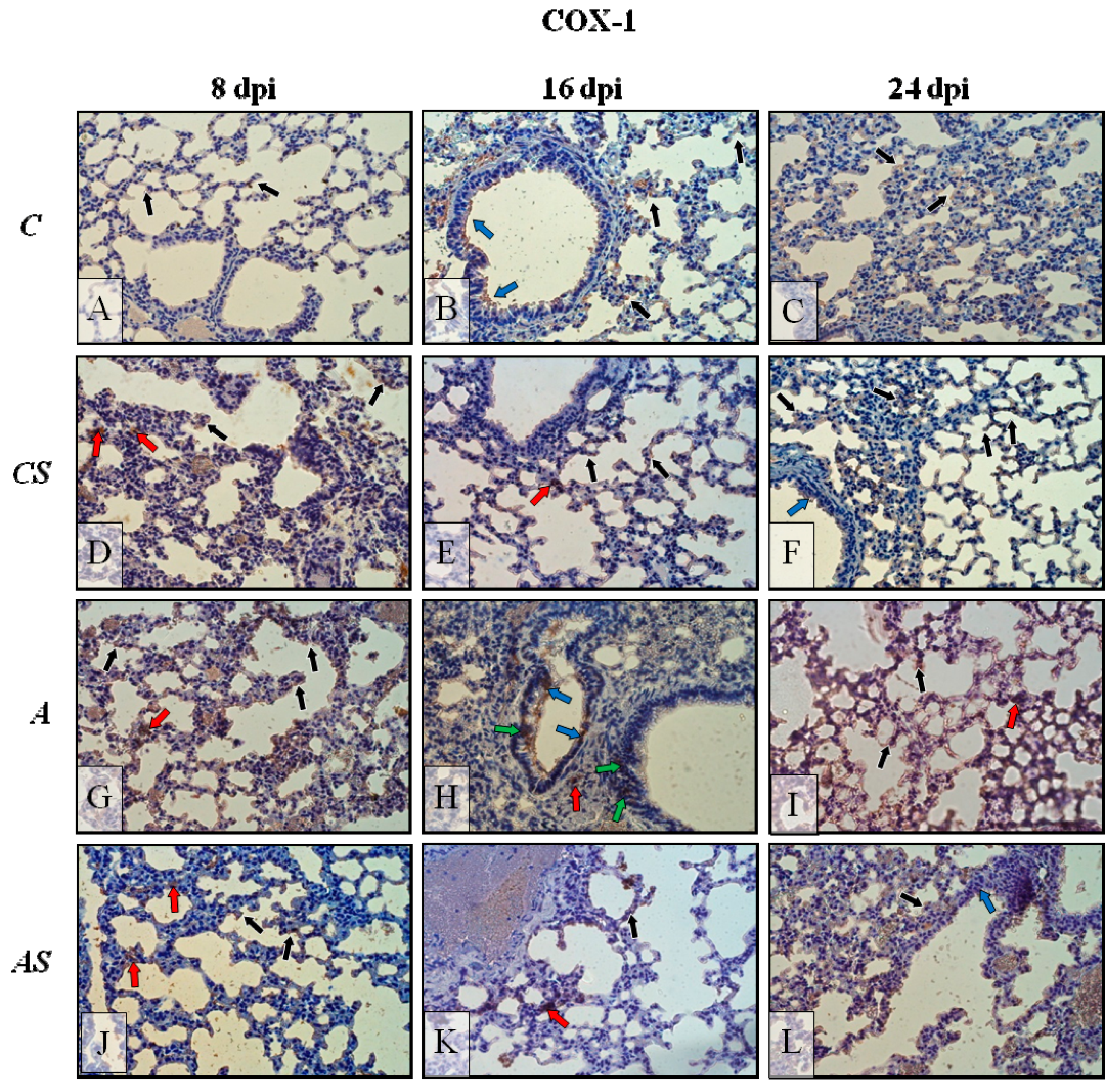
2.2. Acanthamoeba sp. COX-1 Expression in Lungs During Infection
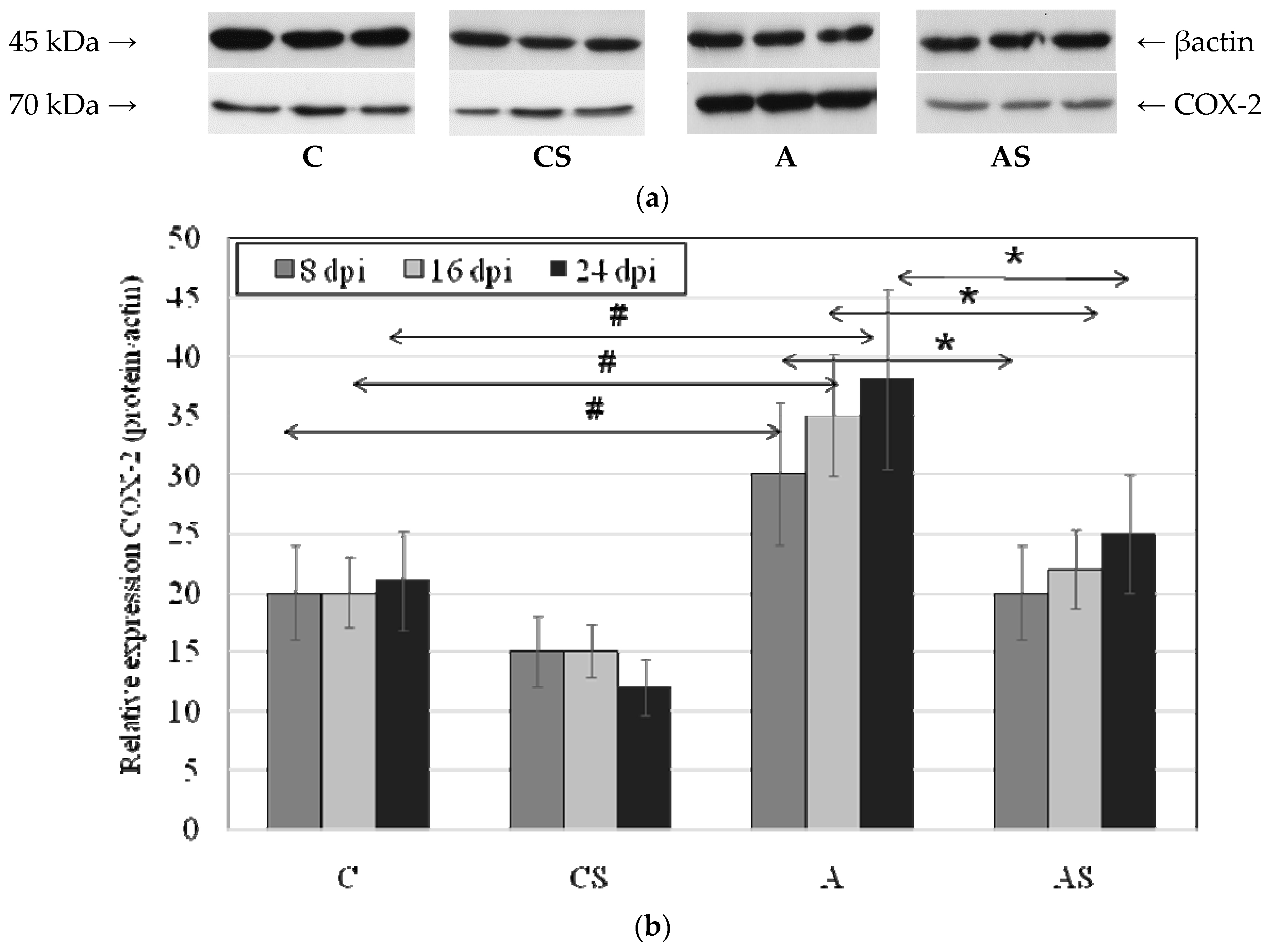
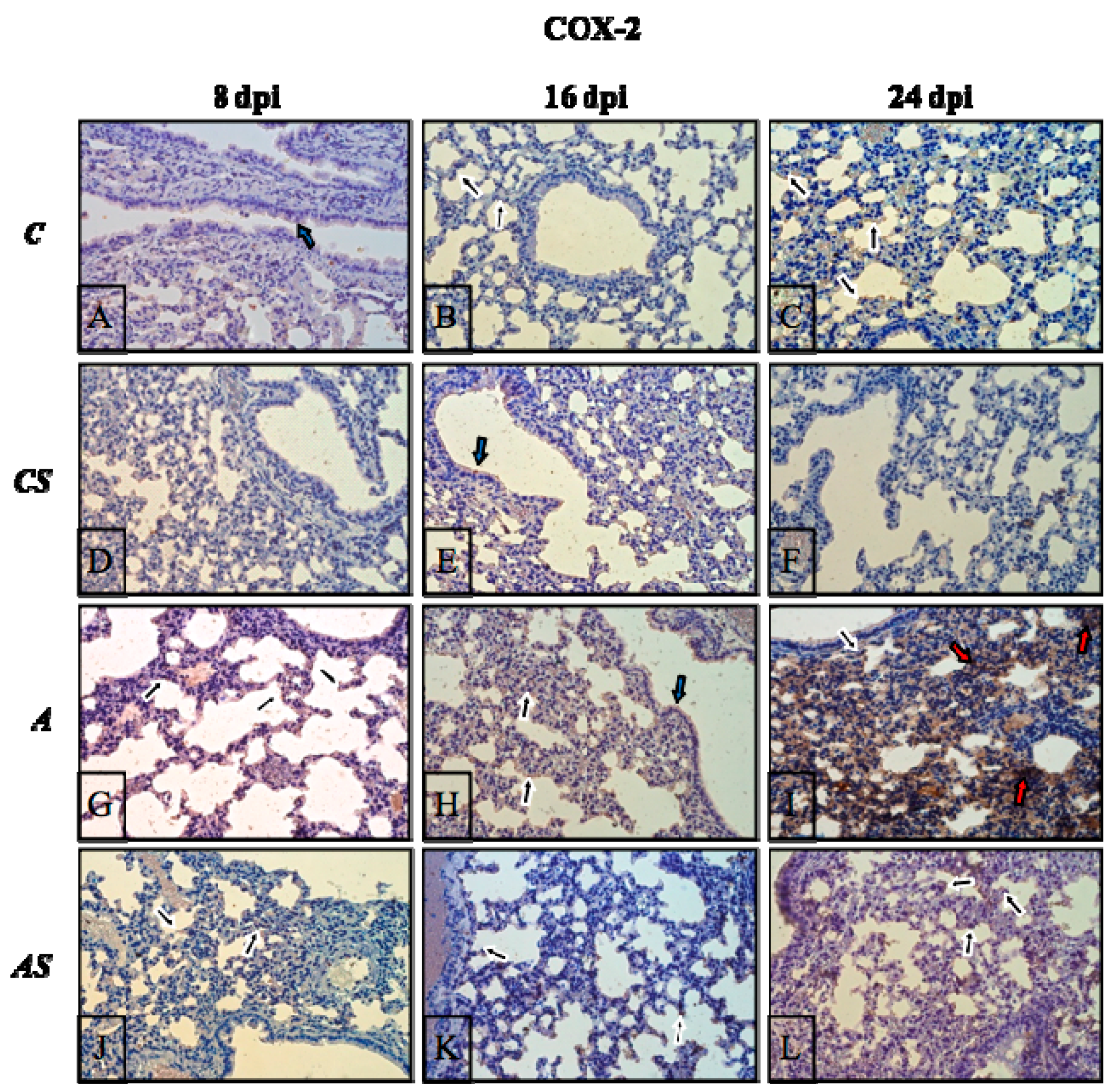
2.3. Acanthamoeba sp. COX-2 Expression in Lungs During Infection
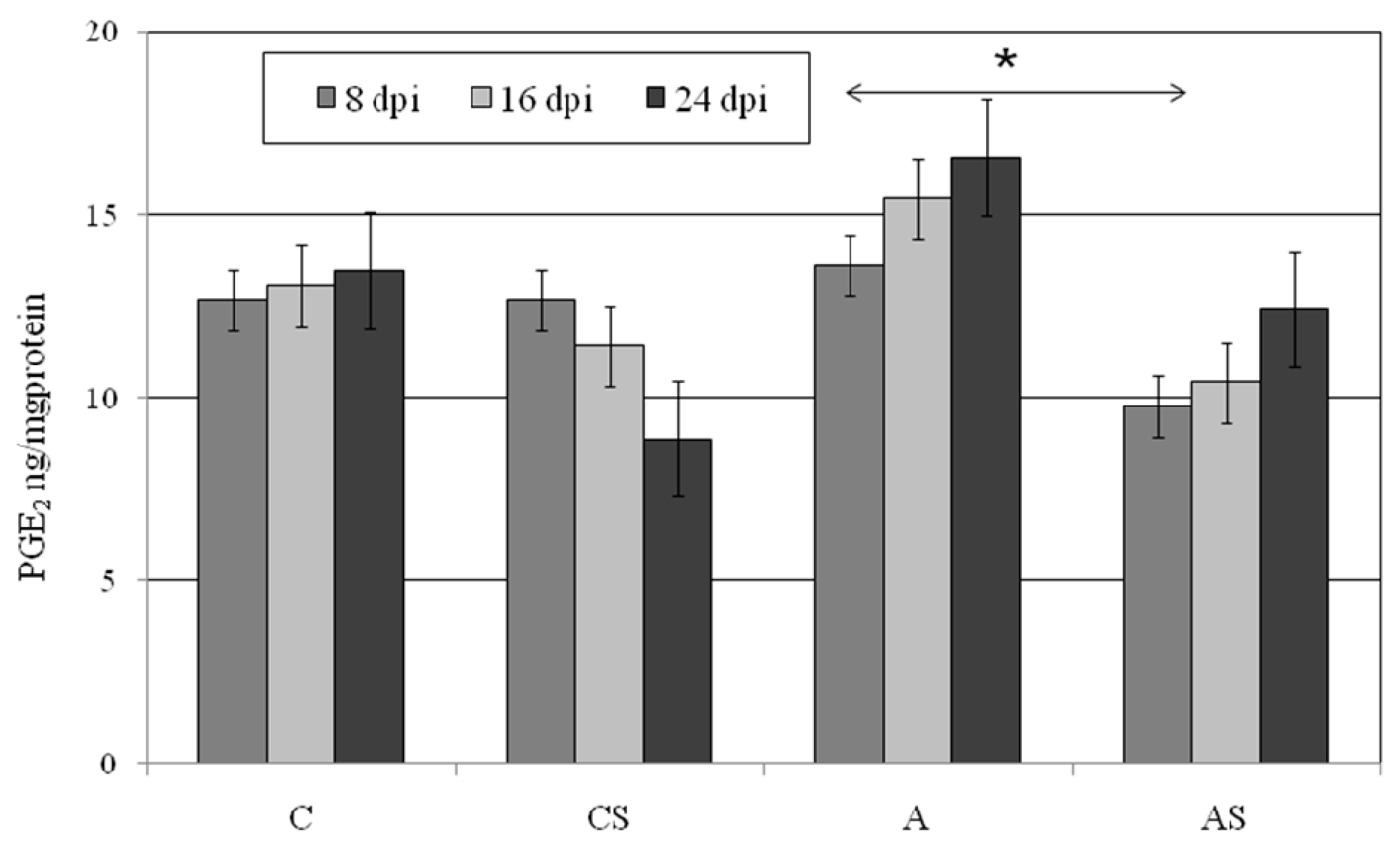
2.4. PGE2 in Lungs During Acanthamoeba sp. Infection
2.5. TXB2 in Lungs during Acanthamoeba sp. Infection
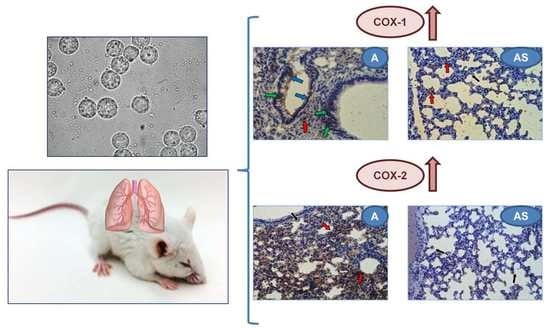
2.6. Immunohistochemistry
3. Discussion
4. Materials and Methods
4.1. Acanthamoeba sp. Isolate and Cultivation
4.2. Animals and Ethics Statement
- immunocompetent control group (C, n = 18) untreated mice.
- immunocompetent (A, n = 30) Acanthamoeba sp.-infected mice.
- immunosuppressed(AS, n = 30) Acanthamoeba sp.-infected mice.
- immunosuppressed(CS, n = 18) uninfected mice.
4.3. Immunosuppression
4.4. Intranasal Inoculation and Pathogenic Test
4.5. Clinical Evaluation of the Mice
4.6. Western Blotting Analysis of COX-1 and COX-2 Expression
4.7. Measurements of Prostaglandin E2 and Thromboxane B2 Concentrations
4.8. Immunohistochemistry of COX-1 and COX-2 Expression
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| A | immunocompetent Acanthamoeba sp.-infected mice |
| AK | Acanthamoeba keratitis |
| AM | arithmetic mean |
| AM 22 | amoebic strain no. 22 |
| Med | medians |
| AML | acute myeloid leukemia |
| AS | immunosuppressed Acanthamoeba sp.-infected mice |
| C | immunocompetent uninfected control group mice |
| CA | cutaneous acanthamoebiasis |
| cAMP | cyclic adenosine monophosphate |
| COX-1 | cyclooxygenase-1 |
| COX-2 | cyclooxygenase-2 |
| CS | immunosuppressed uninfected mice |
| dpi | days post infection |
| ERK 1/2 | extracellular signal-regulated kinase |
| PGA | prostaglandin |
| PGE2 | prostaglandin E2 |
| GAE | granulomatous amebic encephalitis |
| IK | immunocompetent |
| IL-4 | interkeukin 4 |
| IL-13 | Interleukin 13 |
| IL-β | interleukin 1 beta |
| iNOS | inducible nitric oxide synthase |
| IS | immunosuppressed |
| JNK | c-Jun N-terminal kinases |
| K-W test | Kruskal-Wallis test |
| LI | relative weight ratio of lungs |
| LPS | lipopolysaccharide |
| MPS | methylprednisolone sodium succinate |
| MRP4 | multidrug resistance protein 4 |
| NF-κB | nuclear transcription factor |
| NSAIDs | non-steroidal anti-inflammatory drugs |
| p38 | mitogen-activated protein kinase |
| rs | correlation coefficient |
| SD | standard deviation |
| TLR2 | Toll-like receptor 2 |
| TLR4 | Toll-like receptor 4 |
| TXs | thromboxanes |
| TXA2 | thromboxane A2 |
| TXB2 | thromboxane B2 |
References
- Siddiqui, R.; Khan, N.A. Biology and pathogenesis of Acanthamoeba. Parasites Vectors 2012, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Lanocha, N.; Kosik-Bogacka, D.; Maciejewska, A.; Sawczuk, M.; Wilk, A.; Kuźna-Grygiel, W. The occurrence Acanthamoeba (free living amoeba) in environmental and respiratory samples in Poland. Acta Protozool. 2009, 48, 271–279. [Google Scholar]
- Lorenzo-Morales, J.; Khan, N.A.; Walochnik, J. An update on Acanthamoeba keratitis: Diagnosis, pathogenesis and treatment. Parasite 2015, 22, 10. [Google Scholar] [CrossRef] [PubMed]
- Park, M.K.; Cho, M.K.; Kang, S.A.; Park, H.K.; Kim, D.H.; Yu, H.S. Acanthamoeba protease activity promotes allergic airway inflammation via protease-activated receptor 2. PLoS ONE 2014, 9, e92726. [Google Scholar] [CrossRef]
- Im, K.; Kim, D.S. Acanthamoebiasis in Korea: Two new cases with clinical cases review. Yonsei Med. J. 1998, 39, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Vernon, S.E.; Acar, B.C.; Pham, S.M.; Fertel, D. Acanthamoeba infection in lung transplantation: Report of a case and review of the literature. Transpl. Infect. Dis. 2005, 7, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Girón, R.; Esteban, J.G.; Ribas, A.; Doganci, L. Protozoa in respiratory pathology: A review. Eur. Respir. J. 2008, 32, 1354–1370. [Google Scholar] [CrossRef] [PubMed]
- Górnik, K.; Kuźna-Grygiel, W. Histological studies of selected organs of mice experimentally infected with Acanthamoeba spp. Folia Morphol. 2005, 64, 161–167. [Google Scholar]
- Lee, I.T.; Yang, C.M. Inflammatory signalings involved in airway and pulmonary diseases. Mediat. Inflamm. 2013, 2013, 791231. [Google Scholar] [CrossRef] [PubMed]
- Szymanski, K.V.; Toennies, M.; Becher, A.; Fatykhova, D.; N’Guessan, P.D.; Gutbier, B.; Klauschen, F.; Neuschaefer-Rube, F.; Schneider, P.; Rueckert, J.; et al. Streptococcus pneumoniae-induced regulation of cyclooxygenase-2 in human lung tissue. Eur. Respir. J. 2012, 40, 1458–1467. [Google Scholar] [CrossRef] [PubMed]
- Dannhardt, G.; Kiefer, W. Cyclooxygenase inhibitors-current status and future prospects. Eur. J. Med. Chem. 2001, 36, 109–126. [Google Scholar] [CrossRef]
- Zarghi, A.; Arfaei, S. Selective COX-2 inhibitors: A review of their structure-activity relationships. Iran. J. Pharm. Res. 2011, 10, 655–683. [Google Scholar] [PubMed]
- Hartert, T.V.; Dworski, R.T.; Mellen, B.G.; Oates, J.A.; Murray, J.J.; Sheller, J.R. Prostaglandin E2 decreases allergen-stimulated release of prostaglandin D2 in airways of subjects with asthma. Am. J. Respir. Crit. Care Med. 2000, 162, 637–640. [Google Scholar] [CrossRef] [PubMed]
- Lundström, S.L.; Levänen, B.; Nording, M.; Klepczynska-Nyström, A.; Sköld, M.; Haeggström, J.Z.; Grunewald, J.; Svartengren, M.; Hammock, B.D.; Larsson, B.M.; et al. Asthmatics exhibit altered oxylipin profiles compared to healthy individuals after subway air exposure. PLoS ONE 2011, 6, e23864. [Google Scholar] [CrossRef] [PubMed]
- Horikiri, T.; Hara, H.; Saito, N.; Araya, J.; Takasaka, N.; Utsumi, H.; Yanagisawa, H.; Hashimoto, M.; Yoshii, Y.; Wakui, H.; et al. Increased levels of prostaglandin E-major urinary metabolite (PGE-MUM) in chronic fibrosing interstitial pneumonia. Respir. Med. 2017, 122, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Claar, D.; Hartert, T.V.; Peebles, R.S., Jr. The role of prostaglandins in allergic lung inflammation and asthma. Expert Rev. Respir. Med. 2015, 9, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, S.E.; Westcott, J.Y.; Larsen, G.L. Bronchoalveolar lavage fluid mediator levels 5 minutes after allergen challenge in atopic subjects with asthma: Relationship to the development of late asthmatic responses. J. Allergy Clin. Immunol. 1991, 87, 540–548. [Google Scholar] [CrossRef]
- Hasturk, S.; Kemp, B.; Kalapurakal, S.K.; Kurie, J.M.; Hong, W.K.; Lee, J.S. Expression of cyclooxygenase-1 and cyclooxygenase-2 in bronchial epithelium and nonsmall cell lung carcinoma. Cancer 2002, 94, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Radi, Z.A. Pathophysiology of cyclooxygenase inhibition in animal models. Toxicol. Pathol. 2009, 37, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Walenga, R.W.; Kester, M.; Coroneos, E.; Butcher, S.; Dwivedi, R.; Statt, C. Constitutive expression of prostaglandin endoperoxide G/H synthetase (PGHS)-2 but not PGHS-1 in human tracheal epithelial cells in vitro. Prostaglandins 1996, 52, 341–359. [Google Scholar] [CrossRef]
- Kosik-Bogacka, D.I.; Baranowska-Bosiacka, I.; Kolasa-Wołosiuk, A.; Lanocha-Arendarczyk, N.; Gutowska, I.; Korbecki, J.; Namięta, H.; Rotter, I. The inflammatory effect of infection with Hymenolepis diminuta via the increased expression and activity of COX-1 and COX-2 in the rat jejunum and colon. Exp. Parasitol. 2016, 169, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Asaad, N.Y.; Sadek, G.S. Pulmonary cryptosporidiosis: Role of COX2 and NF-κB. APMIS 2006, 114, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Lonardoni, M.V.; Russo, M.; Jancar, S. Essential role of platelet-activating factor in control of Leishmania (Leishmania) amazonensis infection. Infect. Immun. 2000, 68, 6355–6361. [Google Scholar] [CrossRef] [PubMed]
- Lejeune, M.; Moreau, F.; Chadee, K. Prostaglandin E2 produced by Entamoeba histolytica signals via EP4 receptor and alters claudin-4 to increase ion permeability of tight junctions. Am. J. Pathol. 2011, 179, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, A.; Majumder, S.; Das, S.; Ghosh, S.; Biswas, S.; Majumdar, S. Leishmania donovani-induced prostaglandin E2 generation is critically dependent on host toll-like receptor 2-cytosolic phospholipase A2 signaling. Infect. Immun. 2016, 84, 2963–2973. [Google Scholar] [CrossRef] [PubMed]
- Michelin, M.A.; Silva, J.S.; Cunha, F.Q. Inducible cyclooxygenase released prostaglandin mediates immunosuppression in acute phase of experimental Trypanosoma cruzi infection. Exp. Parasitol. 2005, 111, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, G.K.; Faria, G.E.; Silva, K.T.; Castro, E.C.; Reis, M.A.; Michelin, M.A. Trypanosoma cruzi: The role of PGE2 in immune response during the acute phase of experimental infection. Exp. Parasitol. 2008, 118, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Malvezi, A.D.; Panis, C.; da Silva, R.V.; de Freitas, R.C.; Lovo-Martins, M.I.; Tatakihara, V.L.; Zanluqui, N.G.; Neto, E.C.; Goldenberg, S.; Bordignon, J.; et al. Inhibition of cyclooxygenase-1 and cyclooxygenase-2 impairs Trypanosoma cruzi entry into cardiac cells and promotes differential modulation of the inflammatory response. Antimicrob. Agents Chemother. 2014, 58, 6157–6164. [Google Scholar] [CrossRef] [PubMed]
- Anyona, S.B.; Kempaiah, P.; Davenport, G.C.; Vulule, J.M.; Hittner, J.B.; Ong’echa, J.M.; Perkins, D.J. Suppressed circulating bicyclo-PGE2 levels and leukocyte COX-2 transcripts in children co-infected with P. falciparum malaria and HIV-1 or bacteremia. Biochem. Biophys. Res. Commun. 2013, 436, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Hadas, E.; Mazur, T. Biosynthesis of prostaglandins in pathogenic and nonpathogenic strains of Acanthamoeba spp. Parasitol. Res. 1997, 83, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Eida, A.M. Prostanoids and parasitic diseases. Parasitol. United J. 2015, 8, 38–51. [Google Scholar] [CrossRef]
- Radi, Z.A.; Meyerholz, D.K.; Ackermann, M.R. Pulmonary cyclooxygenase-1 (COX-1) and COX-2 cellular expression and distribution after respiratory syncytial virus and parainfluenza virus infection. Viral Immunol. 2010, 23, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, S.; Mancassola, R.; Naciri, M.; Laurent, F. Cryptosporidium parvum-specific mucosal immune response in C57BL/6 neonatal and γ interferon-deficient mice: Role of tumor necrosis factor alpha in protection. Infect. Immun. 2001, 69, 1635–1642. [Google Scholar] [CrossRef] [PubMed]
- Basir, R.; Rahiman, S.F.; Hasballah, K.; Chong, W.; Talib, H.; Yam, M.; Jabbarzare, M.; Tie, T.; Othman, F.; Moklas, M.; et al. Plasmodium berghei ANKA infection in ICR mice as a model of cerebral malaria. Iran. J. Parasitol. 2012, 7, 62–74. [Google Scholar] [PubMed]
- Abdel-Hafeez, E.H.; Ahmed, A.K.; Abdellatif, M.Z.; Kamal, A.M.; Toni, M.D. The efficacy of pomegranate (Punicagranatum) peel extract on experimentally infected rats with Blastocystis spp. J. Infect. Dis. Prev. Med. 2016, 4, 131. [Google Scholar] [CrossRef]
- Wilson, B.D.; Mondloch, V.M.; Katzenstein, A.L.; Moore, V.L. Hypersensitivity pneumonitis in rabbits. Modulation of pulmonary inflammation by long-term aerosol challenge with antigen. J. Allergy Clin. Immunol. 1984, 74, 180–184. [Google Scholar] [CrossRef]
- Olszowski, T.; Gutowska, I.; Baranowska-Bosiacka, I.; Piotrowska, K.; Korbecki, J.; Kurzawski, M.; Chlubek, D. The Effect of cadmium on COX-1 and COX-2 gene, protein expression, and enzymatic activity in THP-1 macrophages. Biol. Trace Elem. Res. 2015, 165, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Carey, M.A.; Germolec, D.R.; Langenbach, R.; Zeldin, D.C. Cyclooxygenase enzymes in allergic inflammation and asthma. Prostaglandins Leukot. Essent. Fatty Acids 2003, 69, 157–162. [Google Scholar] [CrossRef]
- Kirkby, N.S.; Zaiss, A.K.; Wright, W.R.; Jiao, J.; Chan, M.V.; Warner, T.D.; Herschman, H.R.; Mitchell, J.A. Differential COX-2 induction by viral and bacterial PAMPs: Consequences for cytokine and interferon responses and implications for anti-viral COX-2 directed therapies. Biochem. Biophys. Res. Commun. 2013, 438, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Radi, Z.A.; Ackermann, M.R. Ontogeny of pulmonary cyclooxygenase-1 (COX-1) and -2 (COX-2). Pathophysiology 2011, 18, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Hoesel, B.; Schmid, J.A. The complexity of NF-κB signaling in inflammation and cancer. Mol. Cancer 2013, 212, 86. [Google Scholar] [CrossRef] [PubMed]
- Korbecki, J.; Baranowska-Bosiacka, I.; Gutowska, I.; Chlubek, D. Cyclooxygenase pathways. Acta Biochim. Pol. 2014, 61, 639–649. [Google Scholar]
- Fukata, M.; Chen, A.; Klepper, A.; Krishnareddy, S.; Vamadevan, A.S.; Thomas, L.S.; Xu, R.; Inoue, H.; Arditi, M.; Dannenberg, A.J.; et al. Cox-2 is regulated by Toll-like receptor-4 (TLR4) signaling: Role in proliferation and apoptosis in the intestine. Gastroenterology 2006, 131, 862–877. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, Y.; Sotolongo, J.; Breglio, K.; Conduah, D.; Chen, A.; Xu, R.; Hsu, D.; Ungaro, R.; Hayes, L.A.; Pastorini, C.; et al. The role of prostaglandin E2 (PGE2) in toll-like receptor 4 (TLR4)-mediated colitis-associated neoplasia. BMC Gastroenterol. 2010, 10, 82. [Google Scholar] [CrossRef] [PubMed]
- Derda, M.; Wojtkowiak-Giera, A.; Kolasa-Wołosiuk, A.; Kosik-Bogacka, D.; Hadaś, E.; Jagodziński, P.P.; Wandurska-Nowak, E. Acanthamoeba infection in lungs of mice expressed by toll-like receptors (TLR2 and TLR4). Exp. Parasitol. 2016, 165, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Peres-Buzalaf, C.; de Paula, L.; Frantz, F.G.; Soares, E.M.; Medeiros, A.I.; Peters-Golden, M.; Silva, C.L.; Faccioli, L.H. Control of experimental pulmonary tuberculosis depends more on immunostimulatory leukotrienes than on the absence of immunosuppressive prostaglandins. Prostaglandins Leukot. Essent. Fatty Acids 2011, 85, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Divangahi, M.; Gan, H.; Shin, D.S.; Hong, S.; Lee, D.M.; Serhan, C.N.; Behar, S.M.; Remold, H.G. Lipid mediators in innate immunity against tuberculosis: Opposing roles of PGE2 and LXA4 in the induction of macrophage death. J. Exp. Med. 2008, 205, 2791–2801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leonard, B.E. The immune system, depression and the action of antidepressants. Program Neuro Psychopharmcol. Biol. Psychiatry 2001, 25, 767–780. [Google Scholar] [CrossRef]
- Agard, M.; Asakrah, S.; Morici, L.A. PGE2 suppression of innate immunity during mucosal bacterial infection. Front. Cell. Infect. Microbiol. 2013, 3, 45. [Google Scholar] [CrossRef] [PubMed]
- Vancheri, C.; Mastruzzo, C.; Sortino, M.A.; Crimi, N. The lung as a privileged site for the beneficial actions of PGE2. Trends Immunol. 2004, 25, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Zhang, W.; Shi, X.M. Glucocorticoid-induced leucine zipper (GILZ) mediates glucocorticoid action and inhibits inflammatory cytokine-induced COX-2 expression. J. Cell. Biochem. 2008, 103, 1760–1771. [Google Scholar] [CrossRef] [PubMed]
- Hideko Tatakihara, V.L.; Cecchini, R.; Borges, C.L.; Malvezi, A.D.; Graça-de Souza, V.K.; Yamada-Ogatta, S.F.; Rizzo, L.V.; Pinge-Filho, P. Effects of cyclooxygenase inhibitors on parasite burden, anemia and oxidative stress in murine Trypanosoma cruzi infection. FEMS Immunol. Med. Microbiol. 2008, 52, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.F.; Newton, R.; Evans, T.W.; Barnes, P.J. Differential regulation of cyclo-oxygenase-1 and cyclo-oxygenase-2 gene expression by lipopolysaccharide treatment in vivo in the rat. Clin. Sci. 1996, 90, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Font-Nieves, M.; Sans-Fons, M.G.; Gorina, R.; Bonfill-Teixidor, E.; Salas-Pérdomo, A.; Márquez-Kisinousky, L.; Santalucia, T.; Planas, A.M. Induction of COX-2 enzyme and down-regulation of COX-1 expression by lipopolysaccharide (LPS) control prostaglandin E2 production in astrocytes. J. Biol. Chem. 2012, 287, 6454–6468. [Google Scholar] [CrossRef] [PubMed]
- Akundi, R.S.; Candelario-Jalil, E.; Hess, S.; Hüll, M.; Lieb, K.; Gebicke-Haerter, P.J.; Fiebich, B.L. Signal transduction pathways regulating cyclooxygenase-2 in lipopolysaccharide-activated primary rat microglia. Glia 2005, 51, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Luo, Y.; Kuang, S.; Yang, Y.; Tian, X.; Ma, J.; Mai, S.; Xue, L.; Yang, J. Cyclooxygenase-2 Signalling Pathway in the Cortex is Involved in thePathophysiological Mechanisms in the Rat Model of Depression. Sci. Rep. 2017, 7, 488. [Google Scholar] [CrossRef] [PubMed]
- Sumimoto, S.; Muramatsu, R.; Yamashita, T. Thromboxane A2 stimulates neurite outgrowth in cerebral cortical neurons via mitogen activated protein kinasesignaling. Brain Res. 2015, 1594, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Culbertson, C.G.; Ensminger, P.W.; Overton, W.M. The isolation of additional strains FF pathogenic Hartmanella sp. (Acanthamoeba): Proposed culture method for application to biological material. Am. J. Clin. Pathol. 1965, 43, 383–387. [Google Scholar] [PubMed]
- Markowitz, S.M.; Sobieski, T.; Martinez, A.J.; Duma, R.J. Experimental Acanthamoeba infections in mice pretreated with methylprednisolone or tetracycline. Am. J. Pathol. 1978, 92, 733–744. [Google Scholar] [PubMed]
- Pilarczyk, B.; Doligalska, M.J.; Donskow-Schmelter, K.; Balicka-Ramisz, A.; Ramisz, A. Selenium supplementation enhances the protective response to Toxocara canis larvae in mice. Parasite Immunol. 2008, 30, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Özkaya, H.; Akcan, A.B.; Aydemir, G.; Aydinöz, S.; Razia, Y.; Gammon, S.T.; McKinney, J. Salmonella typhimurium infections in BALB/c mice: A comparison of tissue bioluminescence, tissue cultures and mice clinical scores. New Microbiol. 2012, 35, 53–59. [Google Scholar] [PubMed]

| Parameter | n/g | Group | C | CS | A | AS | P (K-W Test) |
|---|---|---|---|---|---|---|---|
| Body mass | |||||||
| n | 18 | 18 | 30 | 30 | |||
| Total | g | AM ± SD | 26.30 ± 1.88 * | 29.28 ± 1.58 | 24.57 ± 4.19 *,# | 28.30 ± 3.29 # | 0.0001 |
| Median | 26.59 | 29.30 | 25.47 | 29.30 | |||
| Range | 21.34–30.13 | 27.20–32.80 | 13.41–29.50 | 20.04–33.01 | |||
| n | 6 | 6 | 10 | 10 | |||
| 8 dpi | g | AM ± SD | 25.47 ± 2.92 | 28.18 ± 0.93 | 24.15 ± 4.16 | 29.78 ± 1.25 | 0.41 |
| Median | 26.06 | 27.70 | 25.16 | 29.45 | |||
| Range | 21.34–30.13 | 27.30–29.05 | 13.41–27.26 | 28.41–32.40 | |||
| 16 dpi | g | AM ± SD | 26.55 ± 0.65 * | 29.7 ± 1.51 | 21.65 ± 3.91 *,# | 28.5 ± 4.45 # | 0.001 |
| Median | 26.62 | 29.70 | 22.04 | 29.05 | |||
| Range | 25.59–27.55 | 27.2–31.6 | 15.58–26.91 | 20.04–33.01 | |||
| 24 dpi | g | AM ± SD | 26.62 ± 1.60 | 29.86 ± 1.86 | 27.92 ± 1.42 | 28.13 ± 3.43 | 0.42 |
| Median | 27.06 | 29.80 | 28.17 | 28.90 | |||
| Range | 24.14–28.43 | 27.80–32.80 | 24.87–29.50 | 21.40–31.50 | |||
| Lung mass | |||||||
| n | 18 | 18 | 30 | 30 | |||
| Total | g | AM ± SD | 0.19 ± 0.03 * | 0.22 ± 0.03 | 0.21 ± 0.18 * | 0.20 ± 0.04 | 0.001 |
| Median | 0.19 | 0.20 | 0.17 | 0.20 | |||
| Range | 0.15–0.23 | 0.10–0.30 | 0.12–1.10 | 0.10–0.30 | |||
| n | 6 | 6 | 10 | 10 | |||
| 8 dpi | g | AM ± SD | 0.20 ± 0.04 | 0.22 ± 0.04 | 0.18 ± 0.05 | 0.21 ± 0.03 | 0.25 |
| Median | 0.21 | 0.20 | 0.17 | 0.20 | |||
| Range | 0.15–0.26 | 0.20–0.30 | 0.12–0.28 | 0.20–0.30 | |||
| 16 dpi | g | AM ± SD | 0.19 ± 0.02 | 0.23 ± 0.05 | 0.16 ± 0.03 | 0.2 ± 0.05 | 0.42 |
| Median | 0.19 | 0.20 | 0.16 | 0.20 | |||
| Range | 0.17–0.22 | 0.2–0.30 | 0.12–0.21 | 0.1–0.30 | |||
| 24 dpi | g | AM ± SD | 0.19 ± 0.03 | 0.20 ± 0.07 | 0.28 ± 0.29 | 0.20 ± 0.05 | 0.21 |
| Median | 0.19 | 0.20 | 0.19 | 0.20 | |||
| Range | 0.15–0.23 | 0.10–0.30 | 0.13–1.10 | 0.10–0.30 | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łanocha-Arendarczyk, N.; Baranowska-Bosiacka, I.; Kot, K.; Gutowska, I.; Kolasa-Wołosiuk, A.; Chlubek, D.; Kosik-Bogacka, D. Expression and Activity of COX-1 and COX-2 in Acanthamoeba sp.-Infected Lungs According to the Host Immunological Status. Int. J. Mol. Sci. 2018, 19, 121. https://doi.org/10.3390/ijms19010121
Łanocha-Arendarczyk N, Baranowska-Bosiacka I, Kot K, Gutowska I, Kolasa-Wołosiuk A, Chlubek D, Kosik-Bogacka D. Expression and Activity of COX-1 and COX-2 in Acanthamoeba sp.-Infected Lungs According to the Host Immunological Status. International Journal of Molecular Sciences. 2018; 19(1):121. https://doi.org/10.3390/ijms19010121
Chicago/Turabian StyleŁanocha-Arendarczyk, Natalia, Irena Baranowska-Bosiacka, Karolina Kot, Izabela Gutowska, Agnieszka Kolasa-Wołosiuk, Dariusz Chlubek, and Danuta Kosik-Bogacka. 2018. "Expression and Activity of COX-1 and COX-2 in Acanthamoeba sp.-Infected Lungs According to the Host Immunological Status" International Journal of Molecular Sciences 19, no. 1: 121. https://doi.org/10.3390/ijms19010121