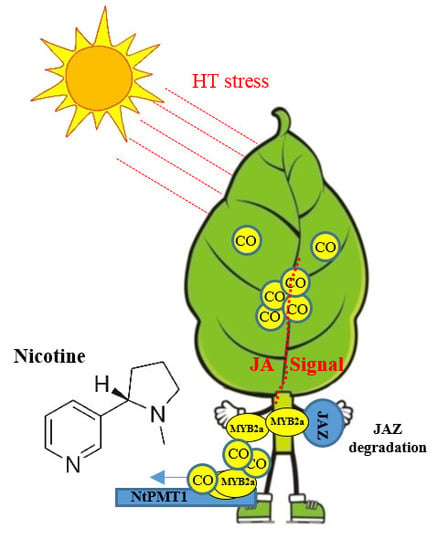
Carbon Monoxide Potentiates High Temperature-Induced Nicotine Biosynthesis in Tobacco
Abstract
:1. Introduction
2. Results
2.1. High Temperature Induces Nicotine Biosynthesis
2.2. High Temperature Induces CO and JA Biosynthesis
2.3. CO Signaling Potentiates HT-Induced Nicotine
2.4. CO Signaling Increases the Transcriptional Activity of MYC2a under HT Stress
3. Discussion
4. Materials and Methods
4.1. Plant Growth and High Temperature Treatment
4.2. Nicotine Content Analysis
4.3. Jasmonic Acid Content Analysis
4.4. RNA Extraction and Quantitative Real-Time RT-PCR (qRT-PCR)
4.5. Protein Isolation and Immunoblot Analysis
4.6. CO and Heme Oxygenase (HO) Activity Analysis
4.7. Transient Protoplast Transformation
4.8. Chip Assay
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bilban, M.; Haschemi, A.; Wegiel, B.; Chin, B.Y.; Wagner, O.; Otterbein, L.E. Heme oxygenase and carbon monoxide initiate homeostatic signaling. J. Mol. Med. 2008, 86, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Ryter, S.W.; Otterbein, L.E.; Morse, D.; Choi, A.M. Heme oxygenase/carbon monoxide signaling pathways: Regulation and functional significance. Mol. Cell. Biochem. 2002, 234–235, 249–263. [Google Scholar] [CrossRef] [PubMed]
- Kaczorowski, D.J.; Zuckerbraun, B.S. Carbon monoxide: Medicinal chemistry and biological effects. Curr. Med. Chem. 2007, 14, 2720–2725. [Google Scholar] [CrossRef] [PubMed]
- Coceani, F. Carbon monoxide in vasoregulation: The promise and the challenge. Circ. Res. 2000, 86, 1184–1186. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, S.; Ling, T.; Xu, L.; Shen, W. Heme oxygenase/carbon monoxide system participates in regulating wheat seed germination under osmotic stress involving the nitric oxide pathway. J. Plant Physiol. 2010, 167, 1371–1379. [Google Scholar] [CrossRef] [PubMed]
- Xuan, W.; Zhu, F.Y.; Xu, S.; Huang, B.K.; Ling, T.F.; Qi, J.Y.; Ye, M.B.; Shen, W.B. The heme oxygenase/carbon monoxide system is involved in the auxin-induced cucumber adventitious rooting process. Plant Physiol. 2008, 148, 881–893. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.J.; Mao, Y.; Duan, X.L.; Zhou, H.; Lai, D.W.; Zhang, Y.H.; Shen, W.B. Arabidopsis HY1-Modulated Stomatal Movement: An Integrative Hub Is Functionally Associated with ABI4 in Dehydration-Induced ABA Responsiveness. Plant Physiol. 2016, 170, 1699–1713. [Google Scholar] [PubMed]
- Duan, X.; Dai, C.; Li, Z.; Zhou, H.; Xiao, T.; Xie, Y.; Shen, W. Ectopic over-expression of BoHO1, a cabbage heme oxygenase gene, improved salt tolerance in Arabidopsis: A case study on proteomic analysis. J. Plant Physiol. 2016, 196–197, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Yang, Z.; Xie, Y.; Nie, L.; Cui, J.; Shen, W. Arabidopsis HY1 confers cadmium tolerance by decreasing nitric oxide production and improving iron homeostasis. Mol. Plant 2014, 7, 388–403. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Liao, W.B. Carbon Monoxide as a Signaling Molecule in Plants. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Shekhawat, G.S.; Verma, K. Haem oxygenase (HO): An overlooked enzyme of plant metabolism and defence. J. Exp. Bot. 2010, 61, 2255–2270. [Google Scholar] [CrossRef] [PubMed]
- Emborg, T.J.; Walker, J.M.; Noh, B.; Vierstra, R.D. Multiple heme oxygenase family members contribute to the biosynthesis of the phytochrome chromophore in Arabidopsis. Plant Physiol. 2006, 140, 856–868. [Google Scholar] [CrossRef] [PubMed]
- Kessler, A.; Baldwin, I.T. Plant responses to insect herbivory: The emerging molecular analysis. Annu. Rev. Plant Biol. 2002, 53, 299–328. [Google Scholar] [CrossRef] [PubMed]
- Steppuhn, A.; Gase, K.; Krock, B.; Halitschke, R.; Baldwin, I.T. Nicotine’s defensive function in nature. PLoS Biol. 2004, 2, E217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kajikawa, M.; Sierro, N.; Kawaguchi, H.; Bakaher, N.; Ivanov, N.V.; Hashimoto, T.; Shoji, T. Genomic Insights into the Evolution of the Nicotine Biosynthesis Pathway in Tobacco. Plant Physiol. 2017, 174, 999–1011. [Google Scholar] [CrossRef] [PubMed]
- Chou, W.M.; Kutchan, T.M. Enzymatic oxidations in the biosynthesis of complex alkaloids. Plant J. 1998, 15, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Naconsie, M.; Kato, K.; Shoji, T.; Hashimoto, T. Molecular evolution of N-methylputrescine oxidase in tobacco. Plant Cell Physiol. 2014, 55, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Shoji, T.; Ogawa, T.; Hashimoto, T. Jasmonate-induced nicotine formation in tobacco is mediated by tobacco COI1 and JAZ genes. Plant Cell Physiol. 2008, 49, 1003–1012. [Google Scholar] [CrossRef] [PubMed]
- Paschold, A.; Halitschke, R.; Baldwin, I.T. Co(i)-ordinating defenses: NaCOI1 mediates herbivore-induced resistance in Nicotiana attenuata and reveals the role of herbivore movement in avoiding defenses. Plant J. 2007, 51, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Shoji, T.; Hashimoto, T. Tobacco MYC2 regulates jasmonate-inducible nicotine biosynthesis genes directly and by way of the NIC2-locus ERF genes. Plant Cell Physiol. 2011, 52, 1117–1130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.B.; Bokowiec, M.T.; Rushton, P.J.; Han, S.C.; Timko, M.P. Tobacco transcription factors NtMYC2a and NtMYC2b form nuclear complexes with the NtJAZ1 repressor and regulate multiple jasmonate-inducible steps in nicotine biosynthesis. Mol. Plant 2012, 5, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Ji, J.; Wang, H.; Harris-Shultz, K.R.; Abd Allah, E.F.; Luo, Y.; Guan, Y.; Hu, X. Carbon Monoxide Interacts with Auxin and Nitric Oxide to Cope with Iron Deficiency in Arabidopsis. Front. Plant Sci. 2016, 7, 112. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.F.; Timko, M.P. Methyl jasmonate induced expression of the tobacco putrescine N-methyltransferase genes requires both G-box and GCC-motif elements. Plant Mol. Biol. 2004, 55, 743–761. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.G.; Chen, J.H.; Kong, X.X.; Todd, C.D.; Yang, Y.P.; Hu, X.Y.; Li, D.Z. Carbon monoxide enhances the chilling tolerance of recalcitrant Baccaurea ramiflora seeds via nitric oxide-mediated glutathione homeostasis. Free Radic. Biol. Med. 2012, 53, 710–720. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.Q.; Xu, S.; Xie, Y.J.; Han, B.; Nie, L.; Shen, W.B.; Wang, R. Molecular cloning, characterization, and expression of an alfalfa (Medicago sativa L.) heme oxygenase-1 gene, MsHO1, which is pro-oxidants-regulated. Plant Physiol. Biochem. 2011, 49, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Balogun, E.; Foresti, R.; Green, C.J.; Motterlini, R. Changes in temperature modulate heme oxygenase-1 induction by curcumin in renal epithelial cells. Biochem. Biophys. Res. Commun. 2003, 308, 950–955. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Q.; Zhang, X.; Li, R.; Jia, Y.; Ef, A.A.; Jia, A.; Hu, L.; Hu, X. Hydrogen sulfide mediates nicotine biosynthesis in tobacco (Nicotiana tabacum) under high temperature conditions. Plant Physiol. Biochem. 2016, 104, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Liu, H.B.; Xiong, L.Z. Endogenous auxin and jasmonic acid levels are differentially modulated by abiotic stresses in rice. Front. Plant Sci. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Jiang, L.; Wang, F.; Yu, D. Jasmonate regulates the inducer of cbf expression-C-repeat binding factor/DRE binding factor1 cascade and freezing tolerance in Arabidopsis. Plant Cell 2013, 25, 2907–2924. [Google Scholar] [CrossRef] [PubMed]
- Mur, L.A.J.; Prats, E.; Pierre, S.; Hall, M.A.; Hebelstrup, K.H. Integrating nitric oxide into salicylic acid and jasmonic acid/ethylene plant defense pathways. Front. Plant Sci. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, J.; Ji, J.; Li, P.; Yu, L.; Abd Allah, E.F.; Luo, Y.; Hu, L.; Hu, X. High Temperature Induces Expression of Tobacco Transcription Factor NtMYC2a to Regulate Nicotine and JA Biosynthesis. Front. Physiol. 2016, 7, 465. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Jia, F.; Shao, S.; Zhang, H.; Li, G.; Xia, X.; Zhou, Y.; Yu, J.; Shi, K. Involvement of nitric oxide in the jasmonate-dependent basal defense against root-knot nematode in tomato plants. Front. Plant Sci. 2015, 6, 193. [Google Scholar] [CrossRef] [PubMed]
- Chun, H.J.; Park, H.C.; Koo, S.C.; Lee, J.H.; Park, C.Y.; Choi, M.S.; Kang, C.H.; Baek, D.; Cheong, Y.H.; Yun, D.J.; et al. Constitutive expression of mammalian nitric oxide synthase in tobacco plants triggers disease resistance to pathogens. Mol. Cells 2012, 34, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Song, J.B.; Zhao, W.T.; Yang, Z.M. AtHO1 is Involved in Iron Homeostasis in an NO-Dependent Manner. Plant Cell Physiol. 2013, 54, 1105–1117. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Memelink, J. Jasmonate-responsive transcription factors regulating plant secondary metabolism. Biotechnol. Adv. 2016, 34, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, M.A.; Trowbridge, A.M.; Raffa, K.F.; Lindroth, R.L. Consequences of climate warming and altered precipitation patterns for plant-insect and multitrophic interactions. Plant Physiol. 2012, 160, 1719–1727. [Google Scholar] [CrossRef] [PubMed]
- Vadassery, J.; Reichelt, M.; Hause, B.; Gershenzon, J.; Boland, W.; Mithofer, A. CML42-mediated calcium signaling coordinates responses to Spodoptera herbivory and abiotic stresses in Arabidopsis. Plant Physiol. 2012, 159, 1159–1175. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Kong, X.; Wang, C.; Ma, L.; Zhao, J.; Wei, J.; Zhang, X.; Loake, G.J.; Zhang, T.; Huang, J.; et al. Proteasome-mediated degradation of FRIGIDA modulates flowering time in Arabidopsis during vernalization. Plant Cell 2014, 26, 4763–4781. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.D.; Cho, Y.H.; Sheen, J. Arabidopsis mesophyll protoplasts: A versatile cell system for transient gene expression analysis. Nat. Protoc. 2007, 2, 1565–1572. [Google Scholar] [CrossRef] [PubMed]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, T.; Hu, L.; Wang, P.; Yang, X.; Peng, Y.; Lu, Y.; Chen, J.; Shi, J. Carbon Monoxide Potentiates High Temperature-Induced Nicotine Biosynthesis in Tobacco. Int. J. Mol. Sci. 2018, 19, 188. https://doi.org/10.3390/ijms19010188
Cheng T, Hu L, Wang P, Yang X, Peng Y, Lu Y, Chen J, Shi J. Carbon Monoxide Potentiates High Temperature-Induced Nicotine Biosynthesis in Tobacco. International Journal of Molecular Sciences. 2018; 19(1):188. https://doi.org/10.3390/ijms19010188
Chicago/Turabian StyleCheng, Tielong, Liwei Hu, Pengkai Wang, Xiuyan Yang, Ye Peng, Ye Lu, Jinhui Chen, and Jisen Shi. 2018. "Carbon Monoxide Potentiates High Temperature-Induced Nicotine Biosynthesis in Tobacco" International Journal of Molecular Sciences 19, no. 1: 188. https://doi.org/10.3390/ijms19010188
APA StyleCheng, T., Hu, L., Wang, P., Yang, X., Peng, Y., Lu, Y., Chen, J., & Shi, J. (2018). Carbon Monoxide Potentiates High Temperature-Induced Nicotine Biosynthesis in Tobacco. International Journal of Molecular Sciences, 19(1), 188. https://doi.org/10.3390/ijms19010188