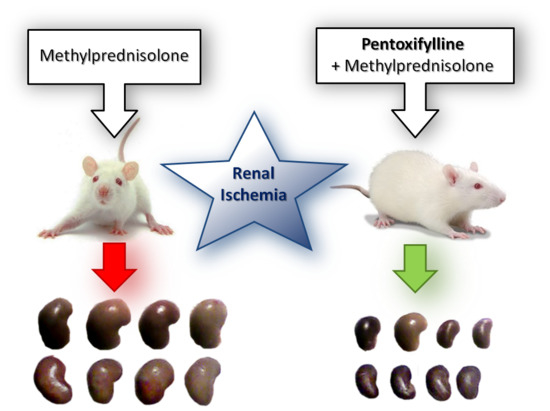
Pentoxifylline and Methylprednisolone Additively Alleviate Kidney Failure and Prolong Survival of Rats after Renal Warm Ischemia-Reperfusion
Abstract
:1. Introduction
2. Results
2.1. Acute Kidney Injury Experiment
2.2. Chronic Kidney Disease Experiment
3. Discussion
4. Materials and Methods
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| IRI | Ischemia-Reperfusion Injury |
| AKI | Acute Kidney Injury |
| ANOVA | Analysis of Variance |
| cAMP | Cyclic Adenosine Monophosphate |
| CKD | Chronic Kidney Disease |
| IL | Interleukin |
| MP | Methylprednisolone |
| NaCl | Sodium Chloride |
| NFκB | Nuclear Factor κ-light-chain-enhancer of activated B cells |
| PTX | Pentoxifylline |
| TNFα | Tumor Necrosis Factor-α |
References
- Kinsey, G.R.; Li, L.; Okusa, M.D. Inflammation in acute kidney injury. Nephron Exp. Nephrol. 2008, 109, e102–e107. [Google Scholar] [CrossRef] [PubMed]
- Gueler, F.; Gwinner, W.; Schwarz, A.; Haller, H. Long-term effects of acute ischemia and reperfusion injury. Kidney Int. 2004, 66, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Khalkhali, H.R.; Ghafari, A.; Hajizadeh, E.; Kazemnejad, A. Risk factors of long-term graft loss in renal transplant recipients with chronic allograft dysfunction. Exp. Clin. Transplant. 2010, 8, 277–282. [Google Scholar] [PubMed]
- Siedlecki, A.; Irish, W.; Brennan, D.C. Delayed graft function in the kidney transplant. Am. J. Transplant. 2011, 11, 2279–2296. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Allen, D.A.; Kieswich, J.E.; Patel, N.S.A.; Harwood, S.; Mazzon, E.; Cuzzocrea, S.; Raftery, M.J.; Thiemermann, C.; Yaqoob, M.M. Dexamethasone ameliorates renal ischemia-reperfusion injury. J. Am. Soc. Nephrol. 2009, 20, 2412–2425. [Google Scholar] [CrossRef] [PubMed]
- Fontana, J.; Vogt, A.; Hohenstein, A.; Vettermann, U.; Doroshenko, E.; Lammer, E.; Yard, B.A.; Hoeger, S. Impact of Steroids on the Inflammatory Response after Ischemic Acute Kidney Injury in Rats. Indian J. Nephrol. 2017, 27, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Whitlock, R.P.; Devereaux, P.J.; Teoh, K.H.; Lamy, A.; Vincent, J.; Pogue, J.; Paparella, D.; Sessler, D.I.; Karthikeyan, G.; Villar, J.C.; et al. Methylprednisolone in patients undergoing cardiopulmonary bypass (SIRS): A randomised, double-blind, placebo-controlled trial. Lancet 2015, 386, 1243–1253. [Google Scholar] [CrossRef]
- Lloris Carsi, J.M.; Cejalvo Lapeña, D.; Toledo, A.H.; Zaragoza Fernandez, C.; Toledo Pereyra, L.H. Pentoxifylline protects the small intestine after severe ischemia and reperfusion. Exp. Clin. Transplant. 2013, 11, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Seifi, B.; Kadkhodaee, M.; Delavari, F.; Mikaeili, S.; Shams, S.; Ostad, S.N. Pretreatment with pentoxifylline and N-acetylcysteine in liver ischemia reperfusion-induced renal injury. Ren. Fail. 2012, 34, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Wystrychowski, W.; Wystrychowski, G.; Zukowska-Szczechowska, E.; Obuchowicz, E.; Grzeszczak, W.; Więcek, A.; Wystrychowski, A. Nephroprotective effect of pentoxifylline in renal ischemia-reperfusion in rat depends on the timing of its administration. Transplant. Proc. 2014, 46, 2555–2557. [Google Scholar] [CrossRef] [PubMed]
- Renke, M.; Tylicki, L.; Rutkowski, P.; Knap, N.; Zietkiewicz, M.; Neuwelt, A.; Aleksandrowicz, E.; Łysiak-Szydłowska, W.; Woźniak, M.; Rutkowski, B. Effect of pentoxifylline on proteinuria, markers of tubular injury and oxidative stress in non-diabetic patients with chronic kidney disease—Placebo controlled, randomized, cross-over study. Acta Biochim. Pol. 2010, 57, 119–123. [Google Scholar] [PubMed]
- Navarro-González, J.F.; Mora-Fernández, C.; Muros de Fuentes, M.; Chahin, J.; Méndez, M.L.; Gallego, E.; Macía, M.; del Castillo, N.; Rivero, A.; Getino, M.A.; et al. Effect of pentoxifylline on renal function and urinary albumin excretion in patients with diabetic kidney disease: The PREDIAN trial. J. Am. Soc. Nephrol. 2015, 26, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Pollice, P.F.; Rosier, R.N.; Looney, R.J.; Puzas, J.E.; Schwarz, E.M.; O’Keefe, R.J. Oral pentoxifylline inhibits release of tumor necrosis factor-α from human peripheral blood monocytes: A potential treatment for aseptic loosening of total joint components. J. Bone Jt. Surg. 2001, 83A, 1057–1061. [Google Scholar] [CrossRef]
- Marcinkiewicz, J.; Grabowska, A.; Lauterbach, R.; Bobek, M. Differential effects of pentoxifylline, a non-specific phosphodiesterase inhibitor, on the production of IL-10, IL-12 p40 and p35 subunits by murine peritoneal macrophages. Immunopharmacology 2000, 49, 335–343. [Google Scholar] [CrossRef]
- Beshay, E.; Croze, F.; Prud’homme, G. J. The phosphodiesterase inhibitors pentoxifylline and rolipram suppress macrophage activation and nitric oxide production in vitro and in vivo. Clin. Immunol. 2001, 98, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Vukanić, Z.S.; Colić, M.; Dimitrijević, M. Effect of pentoxifylline on differentiation and maturation of human monocyte-derived dendritic cells in vitro. Int. Immunopharmacol. 2007, 7, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.P.; Umezawa, Y.; Ikushima, H.; Munakata, Y.; Schlossman, S.F.; Morimoto, C. Different regulatory effects of pentoxifylline on human T cell activation pathways. J. Clin. Immunol. 1997, 17, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Deree, J.; Lall, R.; Melbostad, H.; Grant, M.; Hoyt, D.B.; Coimbra, R. Neutrophil degranulation and the effects of phosphodiesterase inhibition. J. Surg. Res. 2006, 133, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Peterson, T.C. Inhibition of fibroproliferation by pentoxifylline. Activity of metabolite-1 and lack of role of adenosine receptors. Biochem. Pharmacol. 1996, 52, 597–602. [Google Scholar] [CrossRef]
- Chen, Y.M.; Wu, K.D.; Tsai, T.J.; Hsieh, B.S. Pentoxifylline inhibits PDGF-induced proliferation of and TGF-β-stimulated collagen synthesis by vascular smooth muscle cells. J. Mol. Cell. Cardiol. 1999, 31, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zheng, F.; Hou, F. Inhibition of tubulointerstitial fibrosis by pentoxifylline is associated with improvement of vascular endothelial growth factor expression. Acta Pharmacol. Sin. 2009, 30, 98–106. [Google Scholar] [CrossRef] [PubMed]
- De Bosscher, K.; Haegeman, G. Minireview: Latest perspectives on antiinflammatory actions of glucocorticoids. Mol. Endocrinol. 2009, 23, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Funk, J.O.; Ernst, M.; Schönharting, M.M.; Zabel, P. Pentoxifylline exerts synergistic immunomodulatory effects in combination with dexamethasone or cyclosporin A. Int. J. Immunopharmacol. 1995, 17, 1007–1016. [Google Scholar] [CrossRef]
- Han, J.; Thompson, P.; Beutler, B. Dexamethasone and pentoxifylline inhibit endotoxin-induced cachectin/tumor necrosis factor synthesis at separate points in the signaling pathway. J. Exp. Med. 1990, 172, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.-Y.; Zhang, H.-S.; Wu, L.-Y.; Zhang, X.-S.; Zhou, M.-L.; Hang, C.-H. Pentoxifylline Alleviates Early Brain Injury after Experimental Subarachnoid Hemorrhage in Rats: Possibly via Inhibiting TLR 4/NF-κB Signaling Pathway. Neurochem. Res. 2017, 42, 963–974. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Meng, F.; Song, J.; Zhang, L.; Wang, J.; Li, D.; Li, L.; Dong, P.; Yang, B.; Chen, Y. Pentoxifylline Ameliorates Cardiac Fibrosis, Pathological Hypertrophy, and Cardiac Dysfunction in Angiotensin II-induced Hypertensive Rats. J. Cardiovasc. Pharmacol. 2016, 67, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Salam, O.M.E.; Baiuomy, A.R.; El-Shenawy, S.M.; Arbid, M.S. The anti-inflammatory effects of the phosphodiesterase inhibitor pentoxifylline in the rat. Pharmacol. Res. 2003, 47, 331–340. [Google Scholar] [CrossRef]
- Andrade Wde, C.; Silva, L.F.; Coelho, M.C.; Tannuri, A.C.A.; Alves, V.A.F.; Tannuri, U. Effects of the administration of pentoxifylline and prednisolone on the evolution of portal fibrogenesis secondary to biliary obstruction in growing animals: Immunohistochemical analysis of the expression of TGF-β and VEGF. Clinics 2012, 67, 1455–1461. [Google Scholar] [CrossRef]
- De, B.K.; Gangopadhyay, S.; Dutta, D.; Baksi, S.D.; Pani, A.; Ghosh, P. Pentoxifylline versus prednisolone for severe alcoholic hepatitis: A randomized controlled trial. World J. Gastroenterol. 2009, 15, 1613–1619. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Kim, D.J.; Kim, Y.S.; Yim, H.J.; Tak, W.Y.; Lee, H.J.; Sohn, J.H.; Yoon, K.T.; Kim, I.H.; Kim, H.S.; et al. Pentoxifylline vs. corticosteroid to treat severe alcoholic hepatitis: A randomised, non-inferiority, open trial. J. Hepatol. 2014, 61, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Mathurin, P.; Louvet, A.; Duhamel, A.; Nahon, P.; Carbonell, N.; Boursier, J.; Anty, R.; Diaz, E.; Thabut, D.; Moirand, R.; et al. Prednisolone with vs without pentoxifylline and survival of patients with severe alcoholic hepatitis: A randomized clinical trial. JAMA 2013, 310, 1033–1041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, T.-L.; Wen, Y.-T.; Chang, C.-H.; Chang, S.-W.; Lin, K.-H.; Tsai, R.-K. Early Methylprednisolone Treatment Can Stabilize the Blood-Optic Nerve Barrier in a Rat Model of Anterior Ischemic Optic Neuropathy (rAION). Investig. Ophthalmol. Vis. Sci. 2017, 58, 1628–1636. [Google Scholar] [CrossRef] [PubMed]
- Guven, M.; Sehitoglu, M.H.; Yuksel, Y.; Tokmak, M.; Aras, A.B.; Akman, T.; Golge, U.H.; Karavelioglu, E.; Bal, E.; Cosar, M. The Neuroprotective Effect of Coumaric Acid on Spinal Cord Ischemia/Reperfusion Injury in Rats. Inflammation 2015, 38, 1986–1995. [Google Scholar] [CrossRef] [PubMed]
- Wystrychowski, A.; Wystrychowski, W.; Małecki, A.; Cierpka, L.; Więcek, A. Dose-dependent nephroprotective effect of methylprednisolone in the rat model of ischemic acute renal failure. Nephrol. Dial. Transplant. 2006, 21, iv325. [Google Scholar]




| Clinical Parameters | Group 1 NaCl + NaCl | Group 2 PTX + NaCl | Group 3 NaCl + MP | Group 4 PTX + MP |
|---|---|---|---|---|
| 48 h | n = 12 | n = 12 | n = 12 | n = 12 |
| Body weight (g) | 349 ± 32 | 341 ± 19 | 338 ± 23 | 330 ± 28 |
| Diuresis (mL) | 17.5 ± 10.5 | 21.7 ± 7.0 | 31.2 ± 9.7 *,^ | 20.9 ± 13.1 # |
| Serum creatinine (µmol/L) | 451.5 ± 114.4 | 131.1 ± 94.4 * | 233.4 ± 137.0 *,^ | 60.9 ± 19.1 *,^,# |
| Creatinine clearance (mL/min/kg body weight) | 0.11 ± 0.16 | 1.47 ± 0.93 * | 0.84 ± 0.71 * | 2.21 ± 0.86 *,# |
| Fractional excretion of sodium (%) | 23.1 ± 20.7 | 0.84 ± 0.78 * | 2.59 ± 2.40 *,^ | 0.38 ± 0.22 *,# |
| Fractional excretion of potassium (%) | 717 ± 323 | 80.2 ± 60.1 * | 237 ± 223 * | 36.8 ± 14.5 *,^,# |
| Urine protein/urine creatinine (g/g) | 13.1 ± 7.6 | 3.0 ± 4.8 | 9.0 ± 7.1 | 7.1 ± 4.4 |
| 120 h | n = 8 | n = 11 | n = 11 | n = 12 |
| Body weight (g) | 319 ± 21 | 335 ± 19 | 299 ± 26 ^ | 319 ± 29 |
| Diuresis (mL) | 32.2 ± 7.6 | 16.1 ± 5.4 * | 21.9 ± 15.1 * | 11.7 ± 3.9 *,^,# |
| Serum creatinine (µmol/L) | 98.9 ± 44.2 | 47.6 ± 6.7 * | 82.1 ± 55.2 | 39.5 ± 9.8 *,^,# |
| Creatinine clearance (mL/min/kg body weight) | 2.00 ± 1.23 | 3.21 ± 1.17 $ | 2.46 ± 1.17 | 3.70 ± 1.96 $ |
| Fractional excretion of sodium (%) | 0.31 ± 0.22 | 0.17 ± 0.06 | 0.24 ± 0.25 | 0.14 ± 0.12 |
| Fractional excretion of potassium (%) | 90.7 ± 48.8 | 34.2 ± 14.8 * | 75.8 ± 82.7 | 36.4 ± 31.8 * |
| Urine protein/urine creatinine (g/g) | 1.30 ± 0.56 | 1.17 ± 0.66 | 0.98 ± 0.35 | 2.50 ± 4.58 *,^,# |
| Left kidney weight (g) | 3.26 ± 0.71 | 1.79 ± 0.34 * | 2.80 ± 0.52 ^ | 1.53 ± 0.45 *,^,# |
| Clinical Parameters | Group 0 n = 9 | Group UNX n = 16 |
|---|---|---|
| Body weight (g) | 298 ± 12 | 347 ± 33 * |
| Diuresis (mL) | 12.5 ± 3.9 | 17.1 ± 4.2 * |
| Serum creatinine (µmol/L) | 44.2 ± 4.2 | 51.9 ± 6.1 * |
| Creatinine clearance (mL/min/kg body weight) | 3.62 ± 0.72 | 3.14 ± 0.64 ^ |
| Fractional excretion of sodium (%) | 0.15 ± 0.04 | 0.37 ± 0.13 * |
| Fractional excretion of potassium (%) | 22.0 ± 3.65 | 32.7 ± 8.32 * |
| Urine protein/urine creatinine (g/g) | 0.80 ± 0.26 | 1.74 ± 0.69 * |
| Left kidney weight (g) | 1.40 ± 0.08 | 1.62 ± 0.21 * |
| Clinical Parameters | Group I NaCl + NaCl | Group II PTX + NaCl | Group III NaCl + MP | Group IV PTX + MP |
|---|---|---|---|---|
| 2 weeks | ||||
| Right kidney weight (g) | 1.84 ± 0.19 | 1.75 ± 0.26 | 1.68 ± 0.11 *,^ | 1.47 ± 0.23 *,^,# |
| 4 weeks | n = 10 | n = 9 | n = 10 | n = 9 |
| Body weight (g) | 311 ± 41 | 313 ± 42 | 322 ± 22 | 308 ± 29 |
| Diuresis (mL) | 33.3 ± 9.4 | 26.6 ± 10.3 | 27.5 ± 7.8 | 22.3 ± 7.9 |
| Serum creatinine (µmol/L) | 222.9 ± 91.4 | 118.1 ± 64.5 * | 156.9 ± 72.6 | 89.0 ± 31.9 *,# |
| Creatinine clearance (mL/min/kg body weight) | 0.77 ± 0.44 | 1.85 ± 0.89 * | 1.39 ± 0.87 * | 1.90 ± 0.67 *,# |
| Fractional excretion of sodium (%) | 2.17 ± 2.29 | 0.36 ± 0.56 * | 0.42 ± 0.36 *,^ | 0.24 ± 0.11 *,# |
| Fractional excretion of potassium (%) | 206.9 ± 120.6 | 73.7 ± 41.8 * | 123.8 ± 83.1 * | 61.4 ± 25.4 *,^,# |
| Urine protein/urine creatinine (g/g) | 2.89 ± 1.83 | 2.57 ± 0.99 | 2.78 ± 1.65 | 2.60 ± 1.58 |
| 16 weeks | n = 6 | n = 8 | n = 7 | n = 8 |
| Body weight (g) | 450 ± 63 | 483 ± 28 | 506 ± 32 | 513 ± 66 |
| Diuresis (mL) | 26.7 ± 4.1 | 22.5 ± 7.9 | 20.7 ± 5.9 | 18.7 ± 8.2 |
| Serum creatinine (µmol/L) | 150.0 ± 59.8 | 94.6 ± 58.6 | 88.3 ± 28.8 * | 63.6 ± 25.8 * |
| Creatinine clearance (mL/min/kg body weight) | 0.81 ± 0.55 | 2.07 ± 1.16 * | 1.49 ± 0.81 | 2.15 ± 1.26 * |
| Fractional excretion of sodium (%) | 0.29 ± 0.23 | 0.21 ± 0.26 | 0.77 ± 1.51 | 0.34 ± 0.56 |
| Fractional excretion of potassium (%) | 135.4 ± 89.6 | 47.9 ± 30.6 * | 66.1 ± 76.8 | 58.2 ± 63.4 |
| Urine protein/urine creatinine (g/g) | 13.7 ± 9.0 | 7.6 ± 5.2 | 9.5 ± 9.4 | 8.6 ± 5.9 |
| 24 weeks | n = 4 | n = 6 | n = 7 | n = 8 |
| Body weight (g) | 424 ± 120 | 515 ± 35 | 523 ± 29 | 544 ± 63 |
| Diuresis (mL) | 23.1 ± 4.6 | 22.8 ± 9.3 | 27.1 ± 12.2 | 20.4 ± 8.3 |
| Serum creatinine (µmol/L) | 216.0 ± 165.1 | 66.5 ± 46.4 | 86.3 ± 33.7 | 66.1 ± 39.3 $ |
| Creatinine clearance (mL/min/kg body weight) | 1.08 ± 1.14 | 2.48 ± 1.99 | 1.75 ± 1.04 | 2.44 ± 1.41 |
| Fractional excretion of sodium (%) | 0.54 ± 0.59 | 0.25 ± 0.22 | 0.19 ± 0.13 | 0.15 ± 0.12 |
| Fractional excretion of potassium (%) | 139.9 ± 146.9 | 43.2 ± 46.4 | 56.7 ± 31.0 | 43.4 ± 34.2 |
| Urine protein/urine creatinine (g/g) | 18.3 ± 13.3 | 14.7 ± 13.6 | 11.0 ± 6.8 | 10.1 ± 5.1 |
| Left kidney weight (g) | 2.23 ± 0.76 | 2.29 ± 0.44 | 2.42 ± 0.29 | 2.67 ± 0.71 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wystrychowski, G.; Wystrychowski, W.; Grzeszczak, W.; Więcek, A.; Król, R.; Wystrychowski, A. Pentoxifylline and Methylprednisolone Additively Alleviate Kidney Failure and Prolong Survival of Rats after Renal Warm Ischemia-Reperfusion. Int. J. Mol. Sci. 2018, 19, 221. https://doi.org/10.3390/ijms19010221
Wystrychowski G, Wystrychowski W, Grzeszczak W, Więcek A, Król R, Wystrychowski A. Pentoxifylline and Methylprednisolone Additively Alleviate Kidney Failure and Prolong Survival of Rats after Renal Warm Ischemia-Reperfusion. International Journal of Molecular Sciences. 2018; 19(1):221. https://doi.org/10.3390/ijms19010221
Chicago/Turabian StyleWystrychowski, Grzegorz, Wojciech Wystrychowski, Władysław Grzeszczak, Andrzej Więcek, Robert Król, and Antoni Wystrychowski. 2018. "Pentoxifylline and Methylprednisolone Additively Alleviate Kidney Failure and Prolong Survival of Rats after Renal Warm Ischemia-Reperfusion" International Journal of Molecular Sciences 19, no. 1: 221. https://doi.org/10.3390/ijms19010221