1. Introduction
Several researchers have attempted to estimate the respective participation of current assimilation (resulting from photosynthesis productivity) and re-mobilization of pre-anthesis stored assimilates to winter cereal grain yield. Most studies were based on source–sink manipulation, sometimes complemented by the use of the heavy radioactive isotope
14C [
1,
2] or the stable isotope
13C [
3,
4]. Several works on grain filling have concentrated on the interaction between the source (provider of assimilates to the grain during filling) and the sink (the ability of kernel to incorporate the provided and available carbohydrates). Under favorable conditions, grain is mostly filled by current photoassimilates produced by green organs. When climatic conditions (mainly water deficit and high temperatures) are limiting, the contribution of photosynthesis to grain filling decreases and the remobilization of carbohydrates from senescent tissues rises. The wide variation observed in these estimations is likely to depend on the different protocols that have been used, but also relates to the effects of genotype and/or environment. There have been few attempts to quantify the impact of those factors on the relative contribution of different vegetative or reproductive organs to grain filling and final grain yield [
5]. Sanchez-Bragado et al. [
6] reported a higher contribution of ear to grain filling in landraces than in modern cultivars. In contrast, Maydup et al. [
7] did not find any clear participation of awns to grain filling in bread wheat. Labelling by radioactive carbon isotope can permit us to determine part of the translocation and photosynthesis and determine the source of carbon for grain filling. However, this methodology is very difficult and tiresome to achieve. Stable isotope carbon (
13C) is easier and more suitable for this approach. Carbon isotope discrimination (Δ) provides an integrated measure of photosynthetic gas exchange components and is therefore an integrated measure of transpiration efficiency during the entire period in which the sample tissue is growing. Moreover, values of Δ of different plant organs may reflect the variation of plant water status during the season, and the isotopic imprints of different organs on the final isotope composition of the grain could help advise their contribution to grain filling.
Under drought conditions, CO
2 assimilation is more affected than the re-mobilization of carbon to the grain [
8,
9,
10]. As a consequence, there is an increase in the contribution of stored dry matter to grain filling relative to current assimilation [
11]. The contribution of the re-mobilization of carbon products stored in vegetative parts may vary between 5% and 10% under near optimal conditions [
12] to 40% and 60% under water stress [
13]. Merah and Monneveux [
14] estimated this contribution to be 19.4–29.9%. There are two components involved in the contribution of stored assimilates to grain yield in wheat: i.e., the ability to store assimilates in stem and other plant parts, and the efficacy with which these stored reserves are re-mobilized and transported into the grain [
11,
15]. Both components depend on genetic factors [
16,
17].
The relative contribution of ear (spike and awns) photosynthesis to final grain weight varies between 10% and 76% according to environmental and genetic factors [
11,
18]. Ear acquires a major role in photosynthesis and yield under drought [
19] and high temperature [
20] conditions. These studies did not emphasize the environmental and genotype effects on the contribution of different organs to grain filling. Indeed, most of these works have been carried out on one genotype under controlled conditions. In the present article, these effects were estimated by analyzing the effects of source–sink manipulations on grain weight and using the stable isotope
13C as an indicator of current photosynthesis and re-mobilization [
14,
21,
22]. Associations between the contribution and carbon isotope discrimination (Δ) values of the different organs and their morphological characteristics were examined under rainfed conditions on six field grown genotypes of durum wheat. The relationships between the contribution of Δ of the different organs to grain Δ (expressed as the difference between the two values) and the morphological characteristics of these organs were also analyzed.
3. Discussion
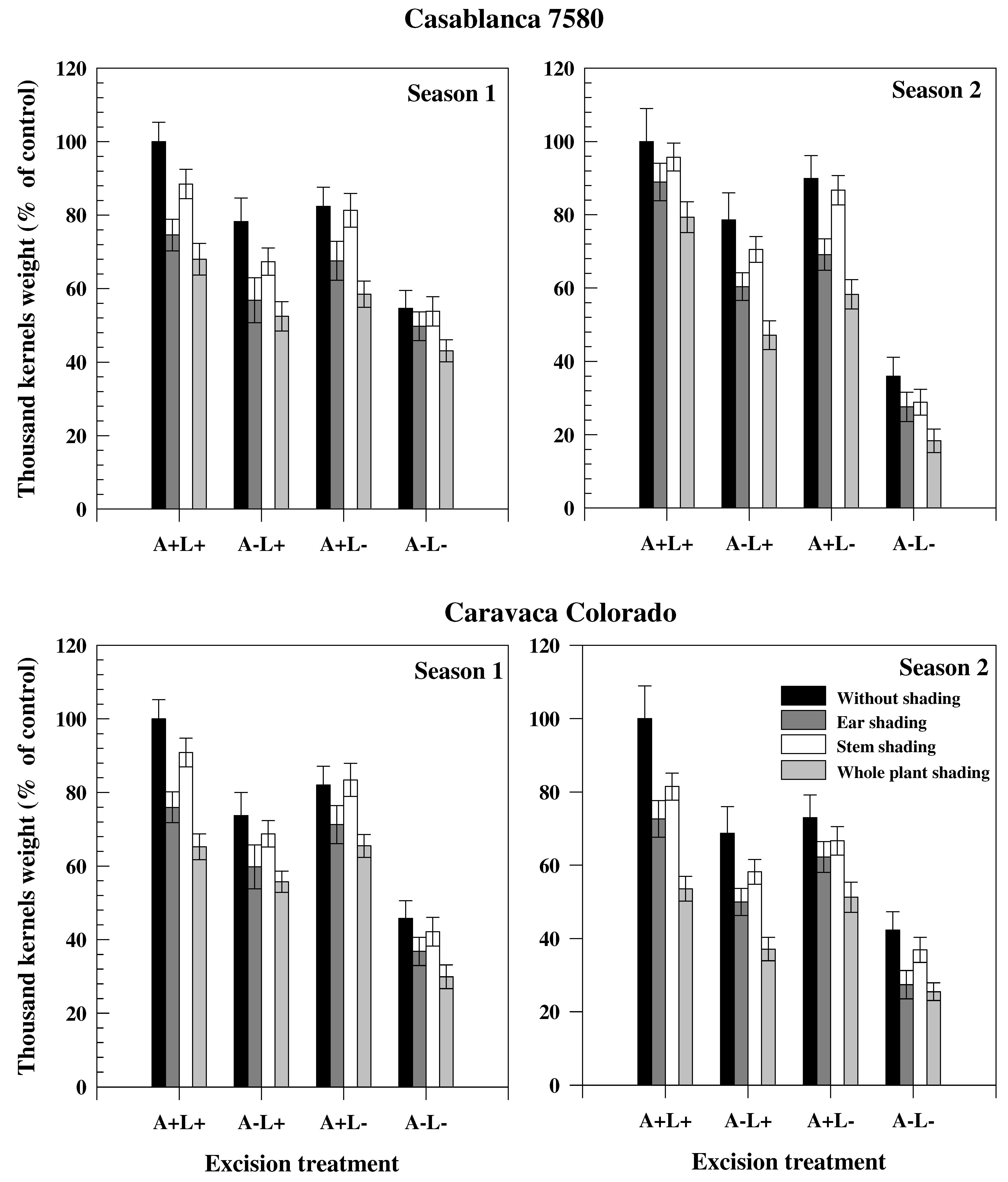
The differences in GY, TKW and ΔG
m between the two seasons were related to the amount of precipitation during the growing season and are in agreement with those reported for a wider set of genotypes tested concurrently [
15,
23]. RWC values observed in this study (
Table 1) were lower than those observed by Merah [
24] on a large collection of durum wheat genotypes under favourable conditions. This mirrored that these genotypes under these conditions have suffered a moderate water deficit at anthesis [
23,
24]. TKW and GY were lower in Season 1 as a consequence of the strong post-anthesis water stress experienced by the crop (
Figure 1). Conversely, ΔG
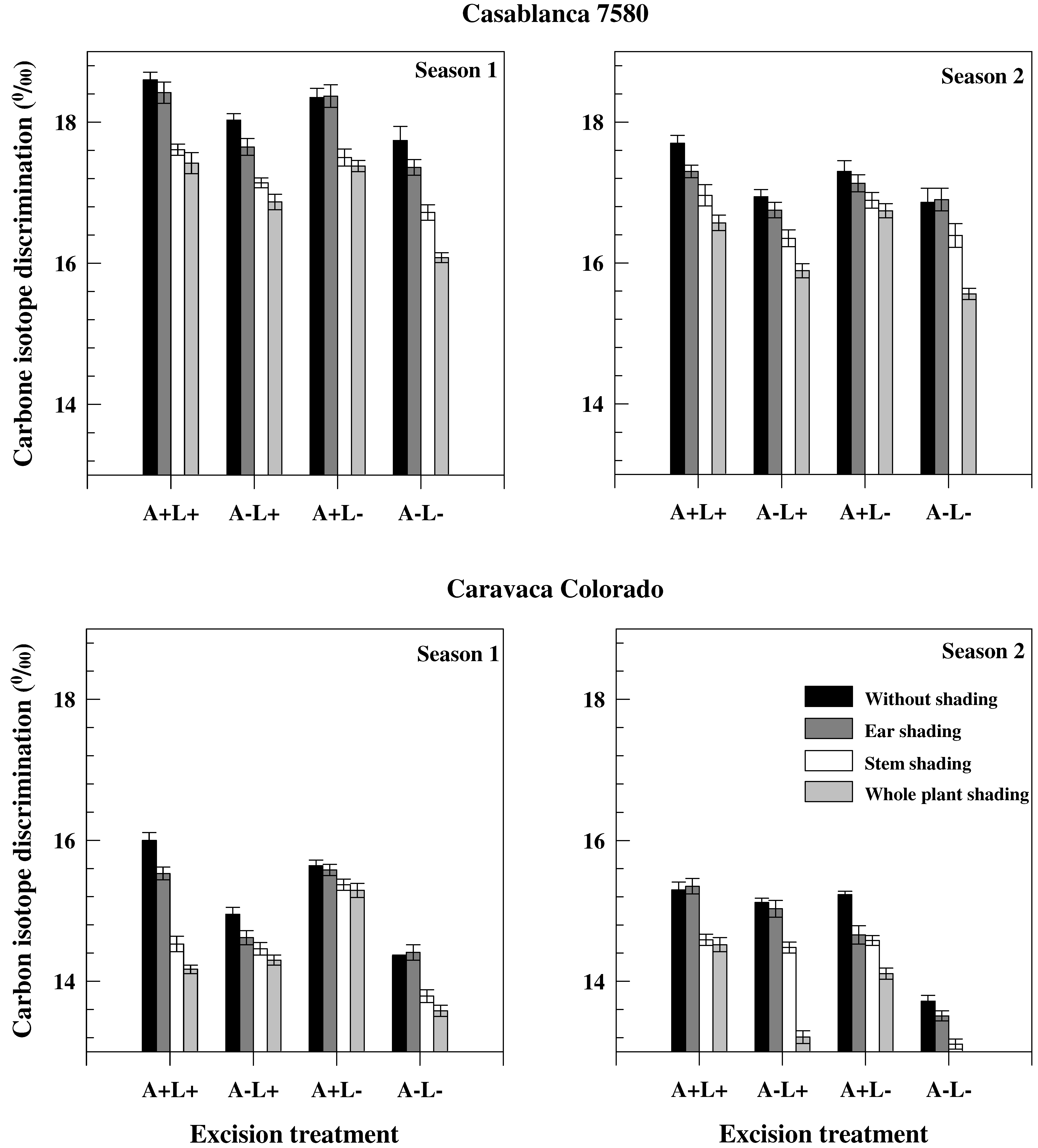
m was higher in Season 1, probably because of the enhanced re-mobilization of C products having high Δ value, contributing to an increase of Δ in the grain (
Figure 2 and
Table 4). The correlation across genotypes between ΔG
m and yield (r = 0.84,
p < 0.01) confirmed previous [
24,
25,
26] and was stronger when rainfall distribution corresponded to typical post-anthesis water stress (i.e., Season 1), as has already noted by Monneveux et al. [
25] in wheat and Tsialtas et al. [
27] in dry bean. The close association between GY and dry matter Δ across treatments confirmed the potential interest of Δ as an indicator of yield effects of source–sink manipulations [
3,
21]. Carbon isotope discrimination in sap (Δ
s) was lower in chaff and awns than in leaves in both cultivars, and significant correlations were found between Δ
s and dry matter Δ (r = 0.903,
p < 0.05 in Casablanca 7580 and r = 0.962,
p < 0.01 in Caravaca Colorado). ΔC
s was also correlated with TKW and GY. ΔA
s correlated with GY in Casablanca 7580 but not in Caravaca Colorado. All told, the data suggest that ΔC
s could also be used as an indicator of yield effects of source-sink manipulations.
The high decline in TKW induced by ear shading confirmed the high contribution of ear photosynthesis to grain filling (
Figure 1). The contribution of spikes was slightly increased in Season 2, possibly because of the capacity of spike organs to better tolerate water stress than leaves. Spikelets can maintain higher water potential than leaves under water stress conditions and are more effective at osmotic adjustment [
26]. An analysis of the role of ear parts in grain filling was attempted by Araus et al. [
28] in triticale (×
Triticosecale Wittmack) by comparing Δ values in grain and of water extracts of ear bracts, awns and flag leaves. Results indicated that a substantial portion of the photosynthetates in the grain came from ear parts. The lower Δ values in ear parts (
Table 4) also suggested higher transpiration efficiency, probably related to some distinct xeromorphic features, such as thick epidermis and cuticule in the dorsal part of ear bracts [
29,
30].
Contribution of ear and awns to grain filling was greater in Casablanca 7580 than in Caravaca Colorado, particularly in the driest season (
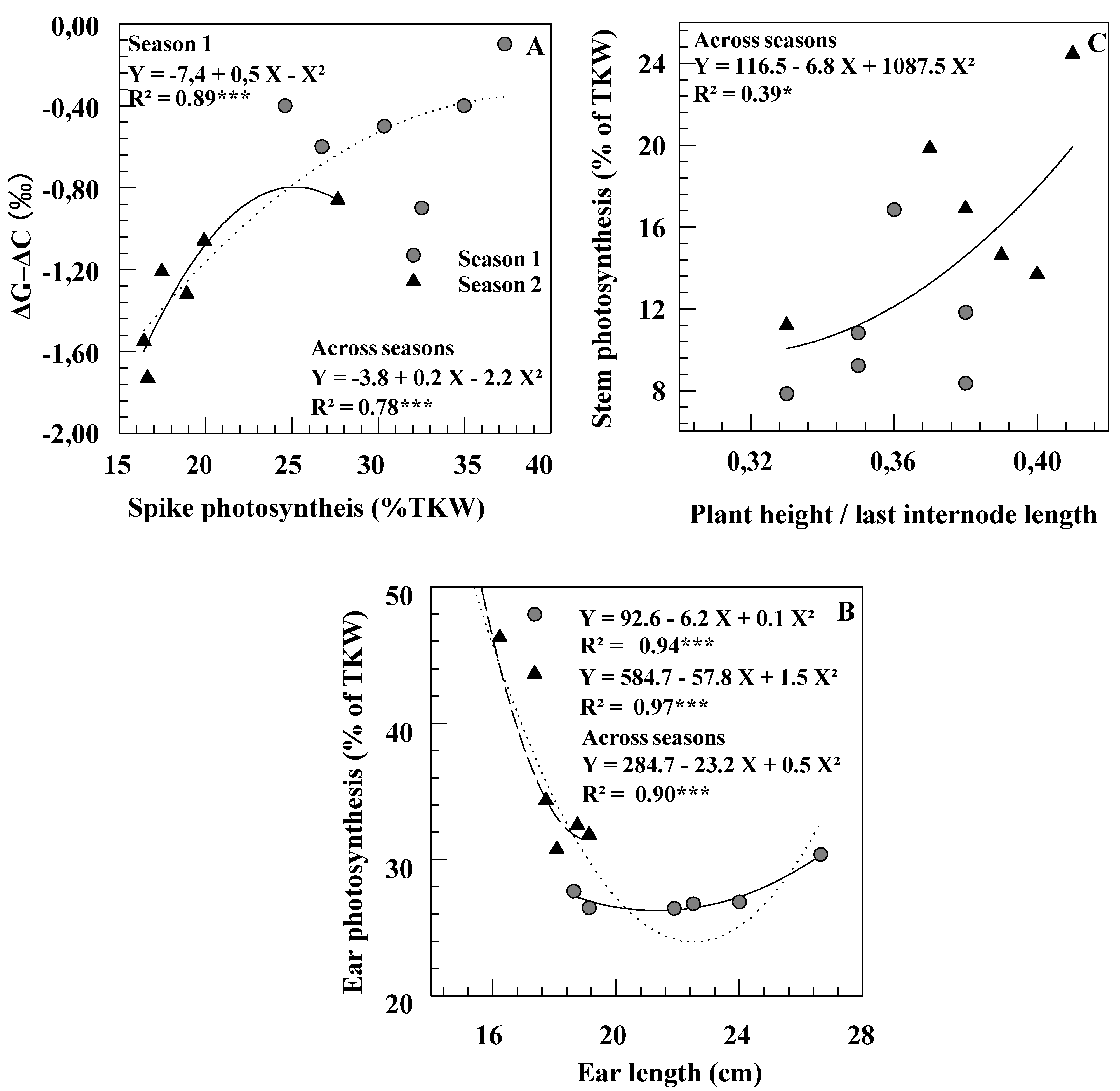
Table 3). The relationships observed here between ear photosynthesis and ear length within and across seasons (
Figure 3B) indicate that, under some conditions (Season 2, short ears), the photosynthetic capacity of the ear is a function of its total area, including the awns [
5,
31]. Chhabra and Sethi [
32] found a significant positive correlation between awn length and contribution to yield across eleven durum wheat genotypes. In the present study, however, Casablanca 7580 had shorter spikes and awns than Caravaca Colorado (
Table 1) and the relative Δ values of ear organs did not clearly relate to spike morphology (
Table 4). In contrast, Casablanca 7580 was found to have higher Δ (lower TE) in chaff, but lower Δ (higher TE) in awns than Caravaca Colorado (
Table 4). Combined results indicate that genetic variation in yield contribution of the ear might depend not only on morphological features, but also on differences in ear transpiration efficiency [
4,
5].
Contribution from stem re-mobilization was higher in the driest season [
1,
2,
8]. Thus, a higher amount of reserves stored in vegetative organs before anthesis and available for later translocation to the grain could buffer grain yield against environmental stresses [
1,
17,
33]. The increase in the contribution of re-mobilization from the stem with drought conditions was more noticeable in the short statured cultivar Casablanca 7580 than in Caravaca Colorado. However, no relationship was found in the set of six genotypes between contribution of stem re-mobilization and plant height (
Figure 3C). These results confirmed that tall cultivars were not more efficient than dwarf ones at utilizing their reserves to grain filling [
22,
34]. Moreover, some short genotypes have shown even better re-mobilization efficiency than tall ones [
17,
33]. This suggests, as postulated by Zhu et al. [
11], that the relative importance of the contribution of stem reserves to final yield is related to more efficient re-mobilization of C-products (possibly related to sink strength) more than to morphological characteristics of the plant (such as stem height).
The estimated contribution of leaves to photosynthesis and re-mobilization showed variation with season, despite the strong reduction of leaf area in Season 2 (−15.2%) (
Table 3). Chhabra and Sethi [
32] reported that removal of flag leaf affected more adversely GY in dwarf genotypes than in taller ones. In the present work, leaf area was 37% and 41% greater in Caravaca Colorado than in Casablanca 7580 and the contribution of leaves to grain filling did not differ between the two cultivars (
Table 1). Martinez et al. [
35] reported that, under drought, leaves showed a great decrease of photosynthetic activity compared to ears allowing to a low participation of the leaves to grain filling.