Tuning Nanopore Diameter of Titanium Surfaces to Improve Human Gingival Fibroblast Response
Abstract
:1. Introduction
2. Results and Discussion
2.1. Surface Characterization
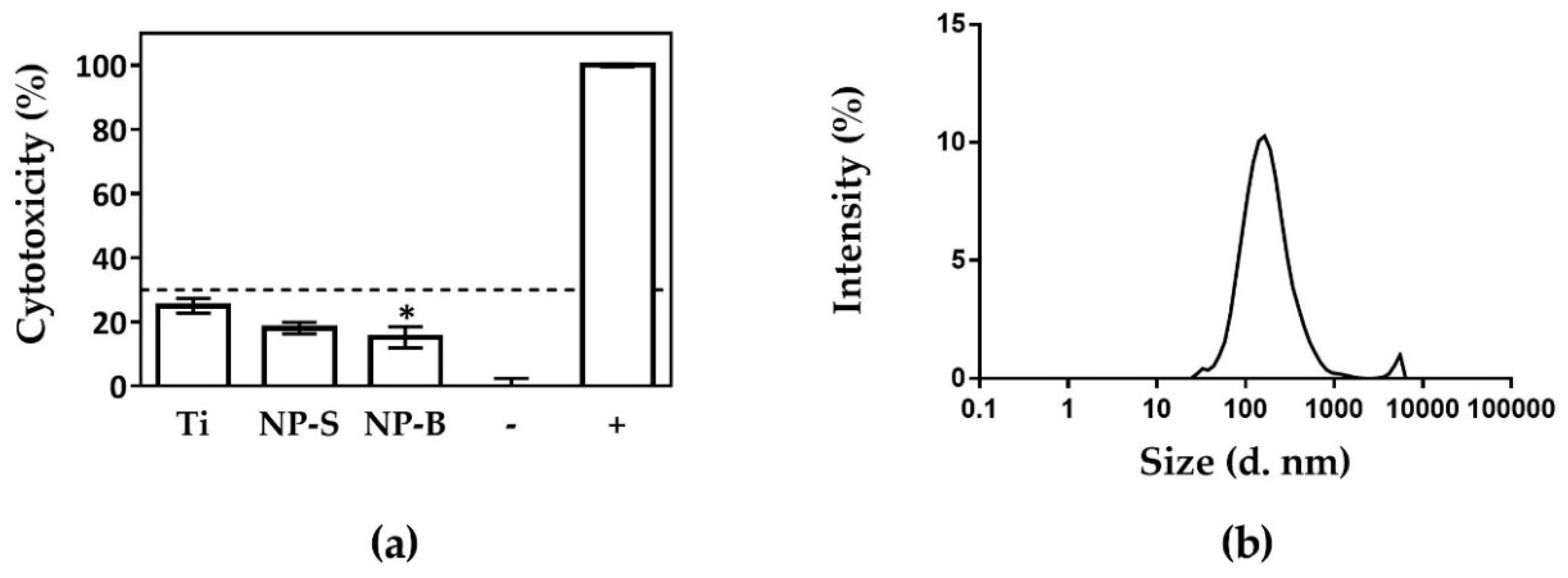
2.2. Cell Response to Nanoporous Surfaces
3. Materials and Methods
3.1. Preparation of Nanoporous Layers on Ti Foil
3.2. Characterization of Ti Nanopore Arrays
3.2.1. SEM
3.2.2. AFM
3.2.3. Contact Angle or Surface Wettability
3.2.4. Nanoparticle Release
3.3. Cell Culture
3.4. Cell Cytotoxicity
3.5. Cell Adhesion and Metabolic Activity
3.6. Gene Expression by Real-Time Polymerase Chain Reaction (RT-PCR)
3.7. Collagen Quantification
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| hGF | Human gingival fibroblasts |
| MSCs | Mesenchymal stem cells |
| Ti | Titanium |
| TiO2 | Titanium dioxide |
| NP | Nanopore |
| SEM | Scanning electronic microscope |
| Ra | Average surface roughness |
| Rq | Root Mean Square (RMS) roughness |
| Rmax | Maximum surface roughness |
| Rskw | Skewness |
| Rkur | Kurtosis |
| Rpc | Peak counts |
| Rsa | Surface Area difference |
| AFM | Atomic force microscope |
| LDH | Lactic dehydrogenase |
| NADH | Nicotinamide adenine dinucleotide |
| DLS | Dynamic light scattering |
| S.E.M. | Standard Error of the Mean |
| ANOVA | Analysis of variance |
| LSD | Least significant difference |
| ECM | Extracellular matrix |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| COL1A1 | α-1 type I collagen |
| COL3A1 | α-1 type III collagen |
| DCN | Decorin |
| TCP | Tissue culture plastic |
References
- Demetrescu, I.; Pirvu, C.; Mitran, V. Effect of nano-topographical features of Ti/TiO2 electrode surface on cell response and electrochemical stability in artificial saliva. Bioelectrochemistry 2010, 79, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, M.; Mazare, A.; Gongadze, E.; Perutkova, Š.; Kralj-Iglič, V.; Milošev, I.; Schmuki, P.; Iglič, A.; Mozetič, M. Titanium nanostructures for biomedical applications. Nanotechnology 2015, 26, 062002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Florit, M.; Ramis, J.M.; Xing, R.; Taxt-Lamolle, S.; Haugen, H.J.; Lyngstadaas, S.P.; Monjo, M. Differential response of human gingival fibroblasts to titanium- and titanium-zirconium-modified surfaces. J. Periodontal Res. 2014, 49, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Yeung, S. Biological basis for soft tissue management in implant dentistry. Aust. Dent. J. 2008, 53, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, Q.; Chu, P.K.; Mei, S.; Ji, K.; Jin, L. Concentration- and time-dependent response of human gingival fibroblasts to fibroblast growth factor 2 immobilized on titanium dental implants. Int. J. Nanomed. 2012, 7, 1965. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Florit, M. Testing of Biomolecules and Novel Surfaces for Periodontal and Peri-Implant Regeneration. Ph.D. Thesis, Balearic Islands University, Palma de Mallorca, Spain, 2015. [Google Scholar]
- Tomsia, A.P.; Lee, J.S.; Wegst, U.G.K.; Saiz, E. Nanotechnology for dental implants. Int. J. Oral Maxillofac. Implants 2013, 28, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Minagar, S.; Wang, J.; Berndt, C.C.; Ivanova, E.P.; Wen, C. Cell response of anodized nanotubes on titanium and titanium alloys. J. Biomed. Mater. Res. Part A 2013, 101, 2726–2739. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chu, P.K.; Ding, C. Surface nano-functionalization of biomaterials. Mater. Sci. Eng. R Rep. 2010, 70, 275–302. [Google Scholar] [CrossRef]
- Roy, P.; Berger, S.; Schmuki, P. TiO2 nanotubes: synthesis and applications. Angew. Chem. Int. Ed. 2011, 50, 2904–2939. [Google Scholar] [CrossRef] [PubMed]
- Ponsonnet, L.; Reybier, K.; Jaffrezic, N.; Comte, V.; Lagneau, C.; Lissac, M.; Martelet, C. Relationship between surface properties (roughness, wettability) of titanium and titanium alloys and cell behaviour. Mater. Sci. Eng. C 2003, 23, 551–560. [Google Scholar] [CrossRef]
- Jemat, A.; Ghazali, M.J.; Razali, M.; Otsuka, Y. Surface modifications and their effects on titanium dental implants. BioMed Res. Int. 2015, 2015, 791725. [Google Scholar] [CrossRef] [PubMed]
- Dobbenga, S.; Fratila-Apachitei, L.E.; Zadpoor, A.A. Nanopattern-induced osteogenic differentiation of stem cells—A systematic review. Acta Biomater. 2016, 46, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Metavarayuth, K.; Sitasuwan, P.; Zhao, X.; Lin, Y.; Wang, Q. Influence of surface topographical cues on the differentiation of mesenchymal stem cells in vitro. ACS Biomater. Sci. Eng. 2016, 2, 142–151. [Google Scholar] [CrossRef]
- Macak, J.M.; Tsuchiya, H.; Ghicov, A.; Yasuda, K.; Hahn, R.; Bauer, S.; Schmuki, P. TiO2 nanotubes: Self-organized electrochemical formation, properties and applications. Curr. Opin. Solid State Mater. Sci. 2007, 11, 3–18. [Google Scholar] [CrossRef]
- Guo, Z.; Li, X.; Zhang, X.; Guan, Z.; He, T. Controlled morphology modulation of anodic TiO2 nanotubes via changing the composition of organic electrolytes. Phys. Chem. Chem. Phys. 2014, 16, 11502. [Google Scholar] [CrossRef] [PubMed]
- Gong, D.; Grimes, C.A.; Varghese, O.K.; Hu, W.; Singh, R.S.; Chen, Z.; Dickey, E.C. Titanium oxide nanotube arrays prepared by anodic oxidation. J. Mater. Res. 2001, 16, 3331–3334. [Google Scholar] [CrossRef]
- Macak, J.M.; Albu, S.P.; Schmuki, P. Towards ideal hexagonal self-ordering of TiO2 nanotubes. Phys. Status Solidi Rapid Res. Lett. 2007, 1, 181–183. [Google Scholar] [CrossRef]
- Huang, J.-Y.; Zhang, K.-Q.; Lai, Y.-K. Fabrication, modification, and emerging applications of TiO2 nanotube arrays by electrochemical synthesis: A review. Int. J. Photoenergy 2013, 2013, 761971. [Google Scholar] [CrossRef]
- Li, S.; Zhang, G.; Guo, D.; Yu, L.; Zhang, W. Anodization fabrication of highly ordered TiO2 nanotubes. J. Phys. Chem. C 2009, 113, 12759–12765. [Google Scholar] [CrossRef]
- Yoriya, S. Effect of inter-electrode Spacing on electrolyte properties and morphologies of anodic TiO2 nanotube array films. Int. J. Electrochem. Sci. 2012, 7, 9454–9464. [Google Scholar]
- Albu, S.P.; Ghicov, A.; Aldabergenova, S.; Drechsel, P.; LeClere, D.; Thompson, G.E.; Macak, J.M.; Schmuki, P. Formation of double-walled TiO2 nanotubes and robust anatase membranes. Adv. Mater. 2008, 20, 4135–4139. [Google Scholar] [CrossRef]
- Webb, H.K.; Truong, V.K.; Hasan, J.; Fluke, C.; Crawford, R.J.; Ivanova, E.P. Roughness parameters for standard description of surface nanoarchitecture. Scanning 2012, 34, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, C.; Taylor, J.; Hong, S. Effect of surface roughness on fouling of RO and NF membranes during filtration of a high organic surficial groundwater. J. Water Supply Res. Technol. 2006, 55, 559–570. [Google Scholar] [CrossRef]
- Smith, B.S. Titania Nanotube Arrays: Interfaces for Implantable Devices. Ph.D. Thesis, Colorado State University, Fort Collins, CO, USA, 2012. [Google Scholar]
- Bartold, P.M.; Walsh, L.J.; Narayanan, A.S. Molecular and cell biology of the gingiva. Periodontol. 2000 2000, 24, 28–55. [Google Scholar] [CrossRef] [PubMed]
- Gongadze, K.; Kabaso, D.; Bauer, S.; Schmuki, P.; Slivnik, T.; Van Rienen, U.; Iglic, A. Adhesion of osteoblasts to a nanorough titanium implant surface. Int. J. Nanomed. 2011, 6, 1801. [Google Scholar] [CrossRef] [Green Version]
- Birkedal-Hansen, H.; Moore, W.G.I.; Bodden, M.K.; Windsor, L.J.; Birkedal-Hansen, B.; DeCarlo, A.; Engler, J.A. Matrix metalloproteinases: A review. Crit. Rev. Oral Biol. Med. 1993, 4, 197–250. [Google Scholar] [CrossRef] [PubMed]
- ISO/EN10993-5 B. Biological Evaluation of Medical Devices--Part 5: Tests for In Vitro Cytotoxicity, 3rd ed.; ISO 10993-5:2009; International Organization for Standardization, 2009; p. 42. [Google Scholar]
- Steinemann, S.G. Titanium—The material of choice? Periodontology 2000 1998, 17, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Bauer, S.; von der Mark, K.; Schmuki, P. Nanosize and vitality: TiO2 nanotube diameter Directs cell fate. Nano Lett. 2007, 7, 1686–1691. [Google Scholar] [CrossRef] [PubMed]
- Llopis-Grimalt, M.A.; Amengual-Tugoresa, A.M.; Monjo, M.; Ramis, J.M. Oriented cell alignment induced by a nanostructured titanium surface enhances expression of cell differentation markers. 2018. in preparation. [Google Scholar]
- Li, B.; Li, Y.; Li, J.; Fu, X.; Li, H.; Wang, H.; Xin, S.; Zhou, L.; Liang, C.; Li, C. Influence of nanostructures on the biological properties of Ti implants after anodic oxidation. J. Mater. Sci. Mater. Med. 2014, 25, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Murakami, H.; Chehroudi, B.; Textor, M.; Brunette, D.M. Effects of surface topography on the connective tissue attachment to subcutaneous implants. Int. J. Oral Maxillofac. Implants 2006, 21, 354–365. [Google Scholar] [PubMed]
- Schwarz, F.; Herten, M.; Sager, M.; Wieland, M.; Dard, M.; Becker, J. Histological and immunohistochemical analysis of initial and early subepithelial connective tissue attachment at chemically modified and conventional SLA titanium implants. A pilot study in dogs. Clin. Oral Investig. 2007, 11, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Ferrari, D.; Herten, M.; Mihatovic, I.; Wieland, M.; Sager, M.; Becker, J. Effects of surface hydrophilicity and microtopography on early stages of soft and hard tissue integration at non-submerged titanium implants: An immunohistochemical study in dogs. J. Periodontol. 2007, 78, 2171–2184. [Google Scholar] [CrossRef] [PubMed]
- Oates, C.J.; Wen, W.; Hamilton, D.W. Role of titanium surface topography and surface wettability on focal adhesion kinase mediated signaling in fibroblasts. Materials 2011, 4, 893–907. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wen, C.; Hodgson, P.; Li, Y. Biocompatibility of TiO2 nanotubes with different topographies. J. Biomed. Mater. Res. Part A 2014, 102, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Malec, K.; Goralska, J.; Hubalewska-Mazgaj, M.; Glowacz, P.; Jarosz, M.; Brzewski, P.; Sulka, G.; Jaskula, M.; Wybranska, I. Effects of nanoporous anodic titanium oxide on human adipose derived stem cells. Int. J. Nanomed. 2016, 11, 5349–5360. [Google Scholar] [CrossRef] [PubMed]
- Ponsonnet, L.; Comte, V.; Othmane, A.; Lagneau, C.; Charbonnier, M.; Lissac, M.; Jaffrezic, N. Effect of surface topography and chemistry on adhesion, orientation and growth of fibroblasts on nickel–titanium substrates. Mater. Sci. Eng. C 2002, 21, 157–165. [Google Scholar] [CrossRef]
- Zhou, W.; Zhong, X.; Wu, X.; Yuan, L.; Zhao, Z.; Wang, H.; Xia, Y.; Feng, Y.; He, J.; Chen, W. The effect of surface roughness and wettability of nanostructured TiO2 film on TCA-8113 epithelial-like cells. Surf. Coat. Technol. 2006, 200, 6155–6160. [Google Scholar] [CrossRef]
- Le Guéhennec, L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. 2007, 23, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Palaiologou, A.A.; Yukna, R.A.; Moses, R.; Lallier, T.E. Gingival, dermal, and periodontal ligament fibroblasts express different extracellular matrix receptors. J. Periodontol. 2001, 72, 798–807. [Google Scholar] [CrossRef] [PubMed]
- Kehlet, S.N.; Willumsen, N.; Armbrecht, G.; Dietzel, R.; Brix, S.; Henriksen, K.; Karsdal, M.A. Age-related collagen turnover of the interstitial matrix and basement membrane: Implications of age- and sex-dependent remodeling of the extracellular matrix. PLoS ONE 2018, 13, e0194458. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Florit, M.; Pacha-Olivenza, M.A.; Fernández-Calderón, M.C.; Córdoba, A.; González-Martín, M.L.; Monjo, M.; Ramis, J.M. Quercitrin-nanocoated titanium surfaces favour gingival cells against oral bacteria. Sci. Rep. 2016, 6, 22444. [Google Scholar] [CrossRef] [PubMed]
- Arriero, M.D.M.; Ramis, J.M.; Perelló, J.; Monjo, M. Inositol hexakisphosphate inhibits osteoclastogenesis on RAW 264.7 cells and human primary osteoclasts. PLoS ONE 2012, 7, e43187. [Google Scholar] [CrossRef] [PubMed]




| Parameters | Ti Control | NP-S | NP-B |
|---|---|---|---|
| Pore diameter (nm) 1 | (-) | 48.2 ± 1.2 | 74.0 ± 3.3 † |
| Ra (nm) | 51.7 ± 5.71 | 54.7 ± 1.4 | 41.6 ± 5.5 |
| Rskw (-) | 0.123 ± 0.337 | 0.069 ± 0.162 | −0.310 ± 0.196 |
| Rkur (-) | 3.62 ± 0.39 | 2.62 ± 0.15 * | 2.99 ± 0.15 |
| Rpc (-) | 29.8 ± 5.0 | 22.0 ± 4.0 | 156 ± 34 * † |
| Srf. Area (µm2) | 26.4 ± 0.2 | 26.4 ± 0.5 | 30.4 ± 0.4 * † |
| Rsa (%) | 5.68 ± 0.86 | 5.41 ± 0.21 | 21.6 ± 1.6 * † |
| Contact angle (°) | 53.2 ± 2.5 | 78.6 ± 2.2 * | 65.5 ± 5.8 † |
| TiO2 Structure | First Anodizing Step 30 min | Second Anodizing Step 10 min | Interspace |
|---|---|---|---|
| NP-S | 35 V 1 | 1 V | 2.5 cm |
| NP-B | 60 V | 60 V | 5 cm |
| Gene | Primer Sequence (5′–3′) | Product Size (bp) | GenBank ID |
|---|---|---|---|
| Collagen I α1 (COL1A1) | S: AGAGCATGACCGATGGATTC A: TTCTTGAGGTTGCCAGTC | 122 | NM_000088.3 |
| Collagen III α1 (COL3A1) | S: GGCCTACTGGGCCTGGTGGT A: CCACGTTCACCAGGGGCACC | 190 | NM_000090.3 |
| Decorin (DCN) | S: ATCTCAGCTTTGAGGGCTCC A: GCCTCTCTGTTGAAACGGTC | 146 | NM_001920.3 |
| Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) | S: TGCACCACCAACTGCTTAGC A: AAGGGACTTCCTGTAACAA | 87 | NM_002046.3 |
| β-Actin (ACTBL2) | S: CTGGAACGGTGAAGGTGACA A: AAGGGACTTCCTGTAACAA | 140 | NM_001101.3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrà-Cañellas, M.D.M.; Llopis-Grimalt, M.A.; Monjo, M.; Ramis, J.M. Tuning Nanopore Diameter of Titanium Surfaces to Improve Human Gingival Fibroblast Response. Int. J. Mol. Sci. 2018, 19, 2881. https://doi.org/10.3390/ijms19102881
Ferrà-Cañellas MDM, Llopis-Grimalt MA, Monjo M, Ramis JM. Tuning Nanopore Diameter of Titanium Surfaces to Improve Human Gingival Fibroblast Response. International Journal of Molecular Sciences. 2018; 19(10):2881. https://doi.org/10.3390/ijms19102881
Chicago/Turabian StyleFerrà-Cañellas, Maria Del Mar, Maria Antonia Llopis-Grimalt, Marta Monjo, and Joana Maria Ramis. 2018. "Tuning Nanopore Diameter of Titanium Surfaces to Improve Human Gingival Fibroblast Response" International Journal of Molecular Sciences 19, no. 10: 2881. https://doi.org/10.3390/ijms19102881





