Exogenous C8-Ceramide Induces Apoptosis by Overproduction of ROS and the Switch of Superoxide Dismutases SOD1 to SOD2 in Human Lung Cancer Cells
Abstract
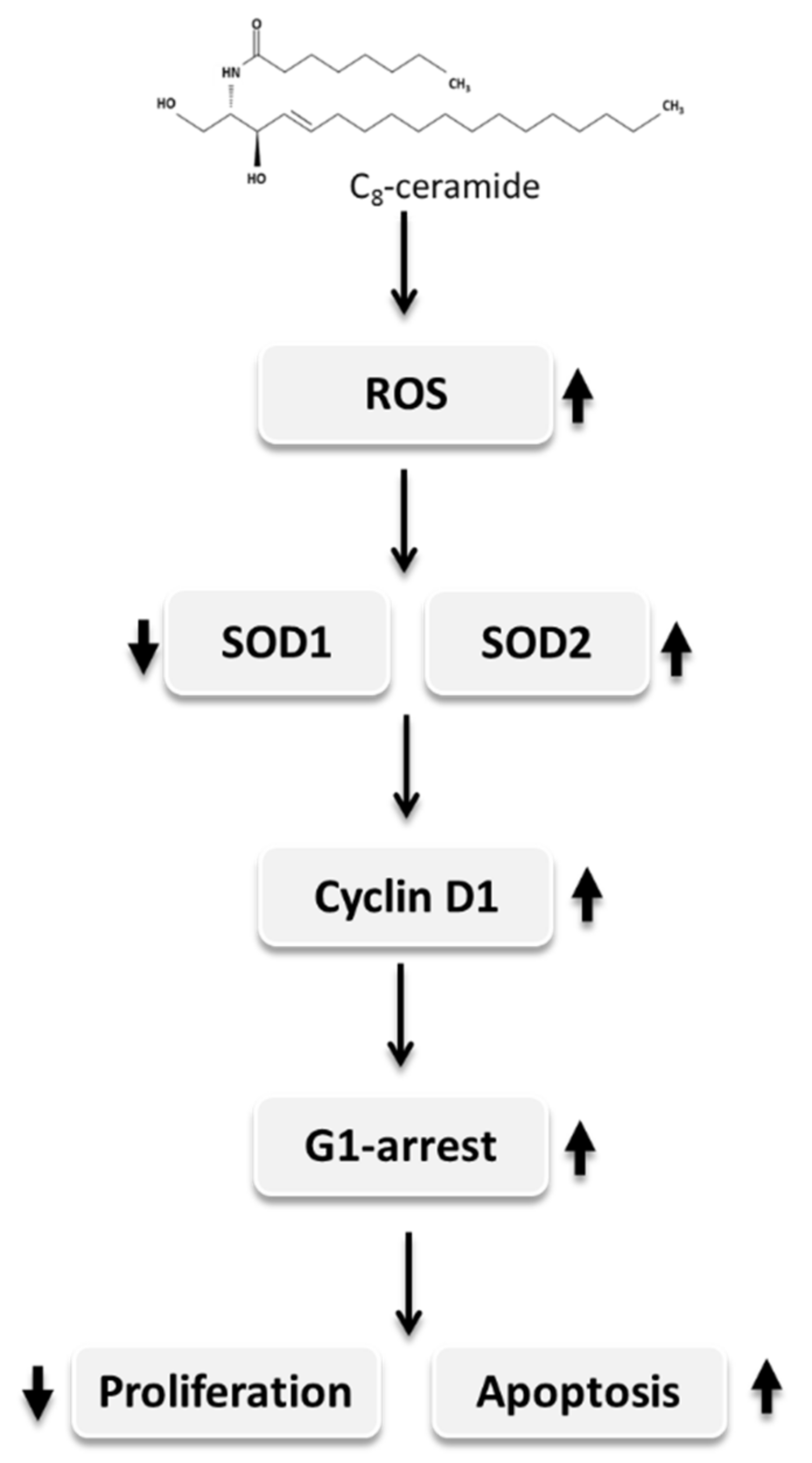
:1. Introduction
2. Results
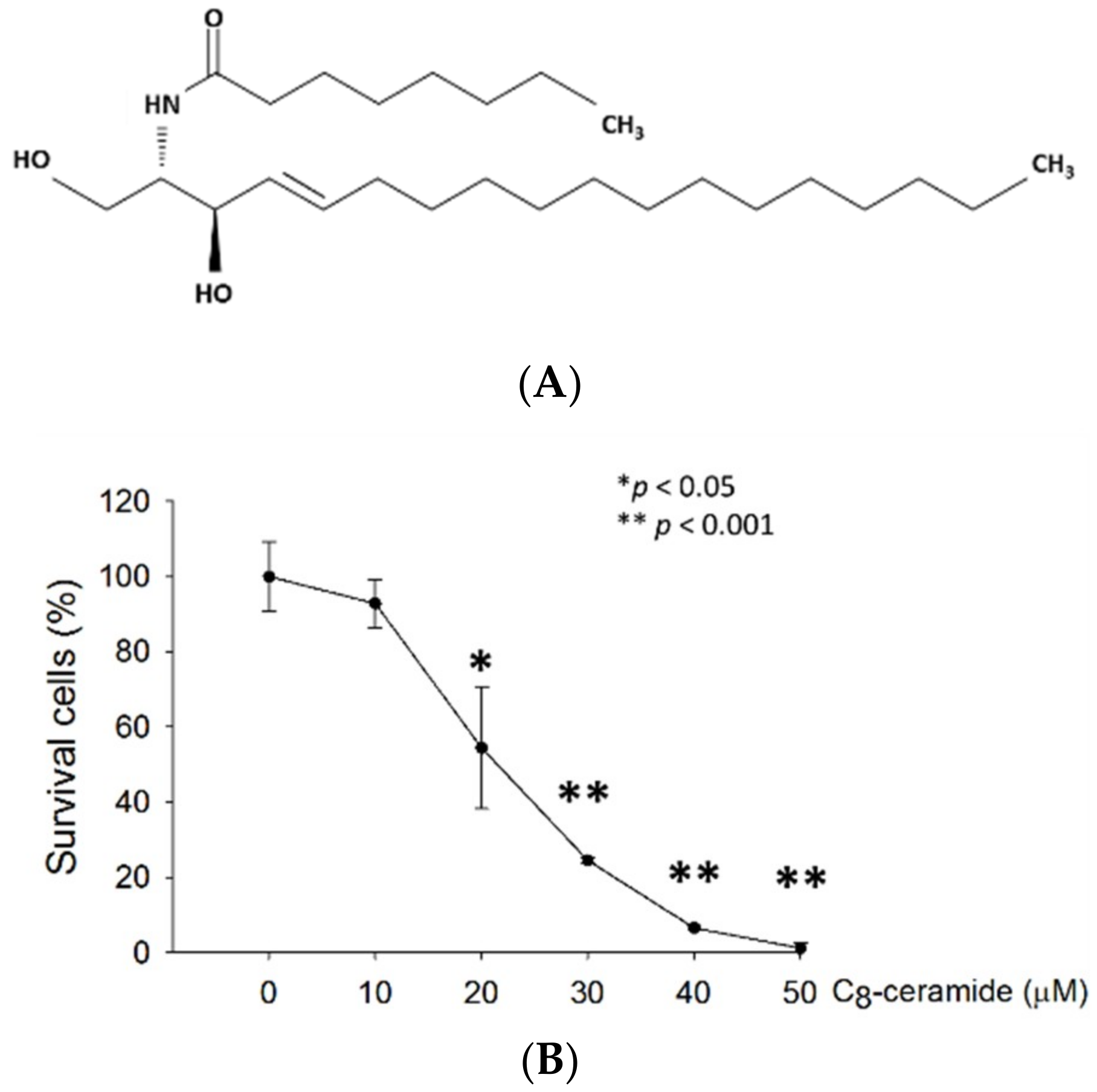
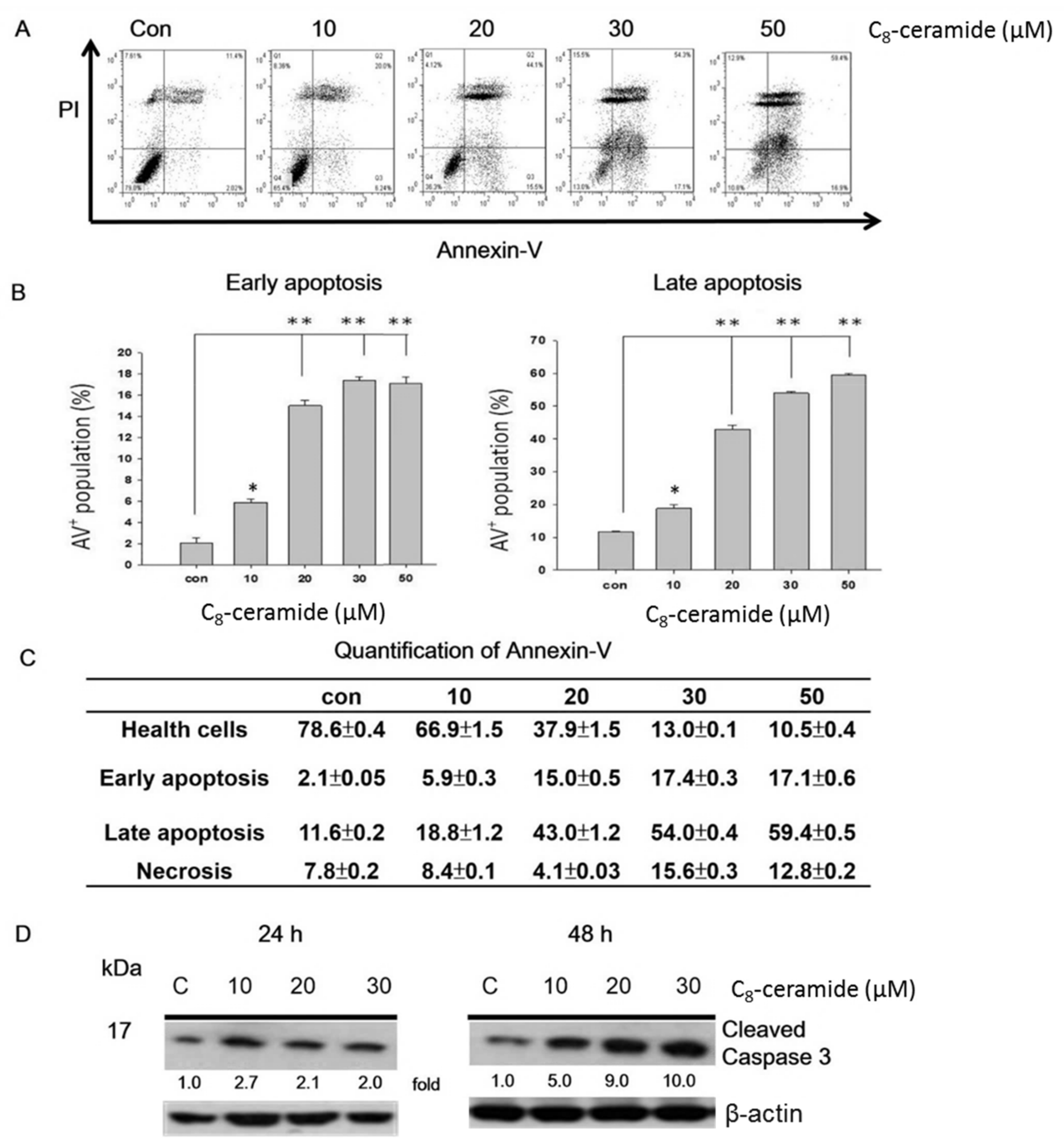
2.1. C8-ceramide Exerts the Anti-Proliferation Potential against H1299 Lung Cancer Cells.
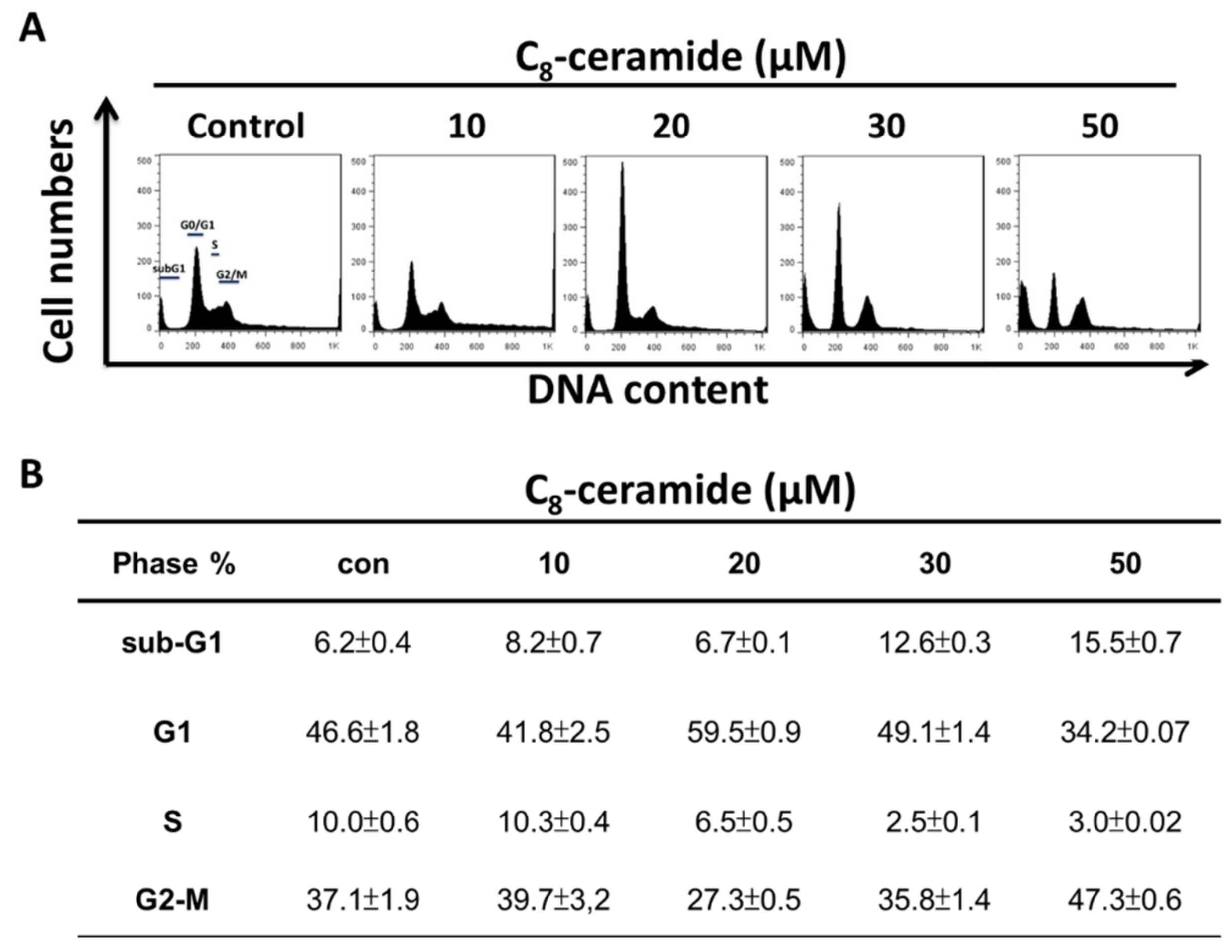
2.2. C8-Ceramide May Cause the G1 Arrest of H1299 Cells
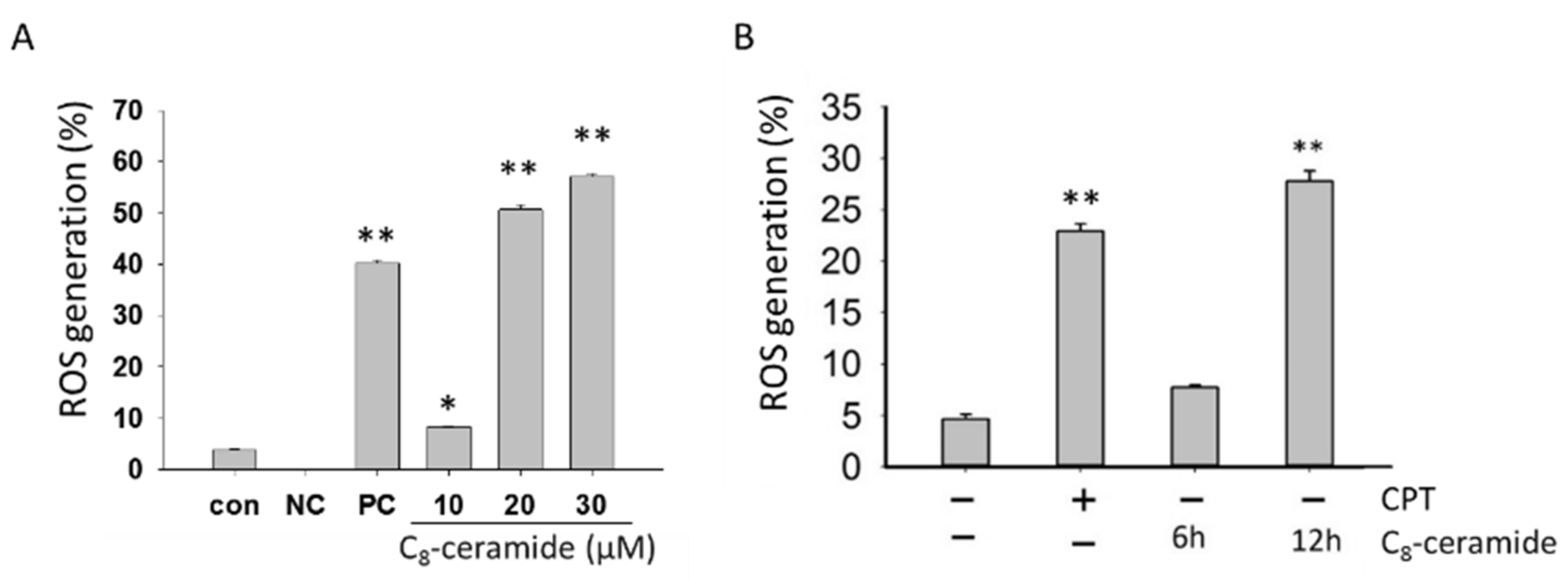
2.3. The Detection of Endogenous ROS in C8-Ceramide-Treated H1299 Cells
2.4. Assessment of Migration in C8-ceramide-treated H1299 cells
2.5. The Modulation of SOD1 and SOD2 in C8-Ceramide Treated H1299 Cells
3. Discussion
4. Materials and Methods
4.1. Preparation of C8-Ceramide
4.2. Cell Cultures
4.3. Cell Proliferation Assay
4.4. Apoptosis Assessment
4.5. Cell Cycle Distribution
4.6. Flow Cytometry-based ROS assessment
4.7. Wound Healing Assay
4.8. Boyden’s Transwell Assay
4.9. Western Blotting Assay
4.10. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Okazaki, I.; Ishikawa, S.; Sohara, Y. Genes Associated with Susceptibility to Lung Adenocarcinoma among Never Smokers Suggest the Mechanism of Disease. Anticancer Res. 2014, 34, 5229–5240. [Google Scholar] [PubMed]
- Tseng, R.C.; Lee, C.C.; Hsu, H.S.; Tzao, C.; Wang, Y.C. Distinct Hic1-Sirt1-P53 Loop Deregulation in Lung Squamous Carcinoma and Adenocarcinoma Patients. Neoplasia 2009, 11, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Pirker, R.; Minar, W. Chemotherapy of Advanced Non-Small Cell Lung Cancer. Front. Radiat. Ther. Oncol. 2010, 42, 157–163. [Google Scholar] [PubMed]
- Alghamdi, H.I.; Alshehri, A.F.; Farhat, G.N. An Overview of Mortality & Predictors of Small-Cell and Non-Small Cell Lung Cancer among Saudi Patients. J. Epidemiol. Glob. Health 2018, 7, S1–S6. [Google Scholar] [PubMed]
- Travis, W.D.; Brambilla, E.; Noguchi, M.; Nicholson, G.A.; Geisinger, K.; Yatabe, Y.; Powell, C.A.; Beer, D.; Riely, G.; Garg, K.; et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society: International Multidisciplinary Classification of Lung Adenocarcinoma: Executive Summary. Proc. Am. Thorac. Soc. 2011, 8, 381–385. [Google Scholar] [PubMed]
- Dacic, S.; Nikiforova, M.N. Present and Future Molecular Testing of Lung Carcinoma. Adv. Anat. Pathol. 2014, 21, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Wagner, T.D.; Yang, G.Y. The Role of Chemotherapy and Radiation in the Treatment of Locally Advanced Non-Small Cell Lung Cancer (NSCLC). Curr. Drug Targets. 2010, 11, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.M.; Wood, D.E. Minimally Invasive Resection of Early Lung Cancers. Oncology 2015, 29. [Google Scholar]
- Skolova, B.; Hudska, K.; Pullmannova, P.; Kovacik, A.; Palat, K.; Roh, J.; Fleddermann, J.; Estrela-Lopis, I.; Vavrova, K. Different Phase Behavior and Packing of Ceramides with Long (C16) and Very Long (C24) Acyls in Model Membranes: Infrared Spectroscopy Using Deuterated Lipids. J. Phys. Chem. B 2014, 118, 10460–10470. [Google Scholar] [CrossRef] [PubMed]
- Korbelik, M.; Banath, J.; Sun, J.; Canals, D.; Hannun, Y.A.; Separovic, D. Ceramide and Sphingosine-1-Phosphate Act as Photodynamic Therapy-Elicited Damage-Associated Molecular Patterns: Cell Surface Exposure. Int. Immunopharmacol. 2014, 20, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Glasauer, A.; Sena, L.A.; Diebold, L.P.; Mazar, A.P.; Chandel, N.S. Targeting Sod1 Reduces Experimental Non-Small-Cell Lung Cancer. J. Clin. Investig. 2014, 124, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Goldkorn, T.; Chung, S.; Filosto, S. Lung Cancer and Lung Injury: The Dual Role of Ceramide. Handb. Exp. Pharmacol. 2013, 93–113. [Google Scholar]
- Morad, S.A.; Messner, M.C.; Levin, J.C.; Abdelmageed, N.; Park, H.; Merrill Jr, A.H.; Cabot, M.C. Potential Role of Acid Ceramidase in Conversion of Cytostatic to Cytotoxic End-Point in Pancreatic Cancer Cells. Cancer Chemother. Pharmacol. 2013, 71, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Flowers, M.; Fabrias, G.; Delgado, A.; Casas, J.; Abad, J.L.; Cabot, M.C. C6-Ceramide and Targeted Inhibition of Acid Ceramidase Induce Synergistic Decreases in Breast Cancer Cell Growth. Breast Cancer Res. Treat. 2012, 133, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zhang, Y.; Liu, X.; Li, Z.; Xu, W.; He, S.; Huang, Y.; Zhang, H. Acid Sphingomyelinase Contributes to Evodiamine-Induced Apoptosis in Human Gastric Cancer Sgc-7901 Cells. DNA Cell Biol. 2011, 30, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Fabrias, G.; Bedia, C.; Casas, J.; Abad, J.L.; Delgado, A. Ceramidases in Hematological Malignancies: Senseless or Neglected Target? Anticancer Agents Med. Chem. 2011, 11, 830–843. [Google Scholar] [CrossRef] [PubMed]
- Modrak, D.E.; Leon, E.; Goldenberg, D.M.; Gold, D.V. Ceramide Regulates Gemcitabine-Induced Senescence and Apoptosis in Human Pancreatic Cancer Cell Lines. Mol. Cancer Res. 2009, 7, 890–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demarchi, F.; Bertoli, C.; Greer, P.A.; Schneider, C. Ceramide Triggers an Nf-Κb-Dependent Survival Pathway through Calpain. Cell Death Differ. 2005, 12, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Deng, X. Suppression of Cancer Cell Migration and Invasion by Protein Phosphatase 2a through Dephosphorylation of mu- and m-Calpains. J. Biol. Chem. 2006, 281, 35567–35575. [Google Scholar] [CrossRef] [PubMed]
- Ohno, Y. Elucidation of the Synthetic Mechanism of Acylceramide, an Essential Lipid for Skin Barrier Function. Yakugaku Zasshi 2017, 137, 1201–1208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamanaka, S.; Suzuki, A.; Hara, M.; Nishio, H.; Otsuka, F.; Uchida, Y. Human Epidermal Glucosylceramides Are Major Precursors of Stratum Corneum Ceramides. J. Investig. Dermatol. 2002, 119, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Zelko, I.N.; Mariani, T.J.; Folz, R.J. Superoxide Dismutase Multigene Family: A Comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and Ec-SOD (SOD3) Gene Structures, Evolution, and Expression. Free Radic. Biol. Med. 2002, 33, 337–349. [Google Scholar] [CrossRef]
- Kuzu, M.; Kandemir, F.M.; Yildirim, S.; Kucukler, S.; Caglayan, C.; Turk, E. Morin Attenuates Doxorubicin-Induced Heart and Brain Damage by Reducing Oxidative Stress, Inflammation and Apoptosis. Biomed. Pharmacother. 2018, 106, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Girerd, S.; Tosca, L.; Herault, O.; Vignon, C.; Biard, D.; Aggoune, D.; Dkhissi, F.; Bonnet, M.L.; Sorel, N.; Desterke, C.; et al. Superoxide Dismutase 2 (SOD2) Contributes to Genetic Stability of Native and T315i-Mutated Bcr-Abl Expressing Leukemic Cells. Biochem. Biophys. Res. Commun. 2018, 498, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Papa, L.; Manfredi, G.; Germain, D. Sod1, an Unexpected Novel Target for Cancer Therapy. Genes Cancer 2014, 5, 15–21. [Google Scholar] [PubMed]
- Papa, L.; Hahn, M.; Marsh, E.L.; Evans, B.S.; Germain, D. Sod2 to Sod1 Switch in Breast Cancer. J. Biol. Chem. 2014, 289, 5412–5416. [Google Scholar] [CrossRef] [PubMed]
- Albero, R.; Enjuanes, A.; Demajo, S.; Castellano, G.; Pinyol, M.; García, N.; Capdevila, C.; Clot, G.; Suárez-Cisneros, H.; Shimada, M.; et al. Cyclin D1 Overexpression Induces Global Transcriptional Downregulation in Lymphoid Neosplasms. J. Clin. Investig. 2018. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.D.; Boudreau, M.; Kriz, J.; Couillard-Després, S.; Kaplan, D.R.; Julien, J.P. Cell Cycle Regulators in the Neuronal Death Pathway of Amyotrophic Lateral Sclerosis Caused by Mutant Superoxide Dismutase 1. J. Neurosci. 2003, 23, 2131–2140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.H.; Zhou, C.L.; Zhu, H.; Li, J.H.; He, C.D. A Functional SNP in the Mdm2 Promoter Mediates E2f1 Affinity to Modulate Cyclin D1 Expression in Tumor Cell Proliferation. Asian Pac. J. Cancer Prev. 2014, 15, 3817–3823. [Google Scholar] [CrossRef] [PubMed]
- Bethea, C.L.; Reddy, A.P. Effect of Ovarian Hormones on Survival Genes in Laser Captured Serotonin Neurons from Macaques. J. Neurochem. 2008, 105, 1129–1143. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.H.; Luo, L.Q.; Liu, Y.; Zhan, Q.X.; Luo, C.; Luo, J.; Zhang, G.M.; Feng, Z.H. Cyclin D1b Splice Variant Promotes Alphavbeta3-Mediated Adhesion and Invasive Migration of Breast Cancer Cells. Cancer Lett. 2014, 355, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, Y.; Hirata, Y.; Nakagawa, H.; Sakamoto, K.; Hikiba, Y.; Kinoshita, H.; Nakata, W.; Takahashi, R.; Tateishi, K.; Tada, M. Apoptosis Signal-Regulating Kinase 1 and Cyclin D1 Compose a Positive Feedback Loop Contributing to Tumor Growth in Gastric Cancer. Proc. Natl. Acad. Sci. USA 2011, 108, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Aaltomaa, S.; Kärjä, V.; Lipponen, P. Isotalo, T. Kankkunen, J.P. Talja, M.; Mokka, R. Expression of Ki-67, Cyclin D1 and Apoptosis Markers Correlated with Survival in Prostate Cancer Patients Treated by Radical Prostatectomy. Anticancer Res. 2006, 26, 4873–4878. [Google Scholar] [PubMed]
- Martin, J.M.C.; Balkenende, A.; Verschoor, T.; Lallemand, F.; Michalides, R. Cyclin D1 Overexpression Enhances Radiation-Induced Apoptosis and Radiosensitivity in a Breast Tumor Cell Line. Cancer Res. 1999, 59, 1134–1140. [Google Scholar]
- Moonen, L.; Ong, F.; Gallee, M.; Verheij, M.; Horenblas, S.; Hart, A.A.; Bartelink, H. Apoptosis, Proliferation and P53, Cyclin D1, and Retinoblastoma Gene Expression in Relation to Radiation Response in Transitional Cell Carcinoma of the Bladder. Int. J. Radiat. Oncol. Biol. Phys. 2001, 49, 1305–1310. [Google Scholar] [CrossRef]
- Xie, X.; Xiao, H.; Ding, F.; Zhong, H.; Zhu, J.; Ma, N.; Mei, J. Over-Expression of Prolyl Hydroxylase-1 Blocks NF-KappaB-Mediated Cyclin D1 Expression and Proliferation in Lung Carcinoma Cells. Cancer Genet. 2014, 207, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Jiang, G.; Fan, C.; Zhang, X.; Zhang, Y.; Miao, Y.; Lin, X.; Wu, J.; Wang, L.; Liu, Y.; et al. ARMC8α Promotes Proliferation and Invasion of Non-Small Cell Lung Cancer Cells by Activating the Canonical Wnt Signaling Pathway. Tumour. Biol. 2014, 35, 8903–8911. [Google Scholar] [CrossRef] [PubMed]
- Weng, X.; Zhu, S.Q.; Cui, H.J. Artesunate Inhibits Proliferation of Glioblastoma Cells by Arresting Cell Cycle. Zhongguo Zhong Yao Za Zhi 2018, 43, 772–778. [Google Scholar] [PubMed]
- Kranenburg, O.; van der Eb, A.J.; Zantema, A. Cyclin D1 Is an Essential Mediator of Apoptotic Neuronal Cell Death. EMBO J. 1996, 15, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Katayama, K.; Dobashi, Y.; Kitagawa, M.; Kamekura, S.; Kawai, M.; Kadoya, Y.; Kameya, T. Overexpression of Cdk4/Cyclin D1 Induces Apoptosis in Pc12 Cells in the Presence of Trophic Support. FEBS Lett. 2001, 509, 382–388. [Google Scholar] [CrossRef]
- Gao, M.Q.; Gao, H.; Han, M.; Liu, K.L.; Peng, J.J.; Han, Y.T. Hispidulin Suppresses Tumor Growth and Metastasis in Renal Cell Carcinoma by Modulating Ceramide-Sphingosine 1-Phosphate Rheostat. Am. J. Cancer Res. 2017, 7, 1501–1514. [Google Scholar] [PubMed]
- Hage-Sleiman, R.; Esmerian, M.O.; Kobeissy, H.; Dbaibo, G. P53 and Ceramide as Collaborators in the Stress Response. Int. J. Mol. Sci. 2013, 14, 4982–5012. [Google Scholar] [CrossRef] [PubMed]
- Kurinna, S.M.; Tsao, C.C.; Nica, A.F.; Jiffar, T.; Ruvolo, P.P. Ceramide Promotes Apoptosis in Lung Cancer-Derived A549 Cells by a Mechanism Involving C-Jun Nh2-Terminal Kinase. Cancer Res. 2004, 64, 7852–7856. [Google Scholar] [CrossRef] [PubMed]
- Dinkins, M.B.; Dasgupta, S.; Wang, G.; Zhu, G.; He, Q.; Kong, J.N.; Bieberich, E. The 5xfad Mouse Model of Alzheimer’s Disease Exhibits an Age-Dependent Increase in Anti-Ceramide Igg and Exogenous Administration of Ceramide Further Increases Anti-Ceramide Titers and Amyloid Plaque Burden. J. Alzheimers Dis. 2015. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Kollmeyer, J.; Symolon, H.; Momin, A.; Munter, E.; Wang, E.; Kelly, S.; Allegood, J.C.; Liu, Y.; Peng, Q.; Ramaraju, H. Ceramides and Other Bioactive Sphingolipid Backbones in Health and Disease: Lipidomic Analysis, Metabolism and Roles in Membrane Structure, Dynamics, Signaling and Autophagy. Biochim. Biophys. Acta. 2006, 1758, 1864–1884. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.; Nam, H.J.; Kim, S.Y.; Juhnn, Y.S. Inhibitory Heterotrimeric Gtp-Binding Proteins Inhibit Hydrogen Peroxide-Induced Apoptosis by up-Regulation of Bcl-2 Via Nf-Kappab in H1299 Human Lung Cancer Cells. Biochem. Biophys. Res. Commun. 2009, 381, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Shatz, M.; Lustig, G.; Reich, R.; Liscovitch, M. Caveolin-1 Mutants P132l and Y14f Are Dominant Negative Regulators of Invasion, Migration and Aggregation in H1299 Lung Cancer Cells. Exp. Cell Res. 2010, 316, 1748–1762. [Google Scholar] [CrossRef] [PubMed]
- Sgarbi, G.; Gorini, G.; Liuzzi, F.; Solaini, G.; Baracca, A. Hypoxia and IF 1 Expression Promote Ros Decrease in Cancer Cells. Cells 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zheng, L.Y.; Tian, Y.; Zhang, Z.Q.; Dong, W.L.; Wang, X.F.; Zhang, X.Y.; Cao, C. C6 Ceramide Dramatically Enhances Docetaxel-Induced Growth Inhibition and Apoptosis in Cultured Breast Cancer Cells: A. Mechanism Study. Exp. Cell Res. 2015, 332, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Martín, D.; Salinas, M.; Fujita, N.; Tsuruo, T.; Cuadrado, A. Ceramide and Reactive Oxygen Species Generated by H2o2 Induce Caspase-3-Independent Degradation of Akt/Protein Kinase, B. J. Biol. Chem. 2002, 277, 42943–42952. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Long, X.; Liao, Y.; Zheng, J. Lycorine Inhibits the Proliferation and Promotes Apoptosis of Sgc-7901 Gastric Cancer Cells by Downregulating Expression of Cyclin D1. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2018, 34, 354–358. [Google Scholar] [PubMed]
- Guo, X.; Li, W.; Wang, Q.; Yang, H.S. Akt Activation by Pdcd4 Knockdown up-Regulates Cyclin D1 Expression and Promotes Cell Proliferation. Genes Cancer 2011, 2, 818–828. [Google Scholar] [CrossRef] [PubMed]
- Yodkeeree, S.; Pompimon, W.; Limtrakul, P. Crebanine, an Aporphine Alkaloid, Sensitizes TNF-alpha-Induced Apoptosis and Suppressed Invasion of Human Lung Adenocarcinoma Cells A549 by Blocking NF-KappaB-Regulated Gene Products. Tumour Biol. 2014, 35, 8615–8624. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Tang, S.C.; Xu, C.; Wang, C.; Qin, S.; Du, N.; Liu, J.; Zhang, Y.; Li, X.; Luo, G.; et al. Dicer1 Regulated Let-7 Expression Levels in P53-Induced Cancer Repression Requires Cyclin D1. J. Cell Mol. Med. 2015, 19, 1357–1365. [Google Scholar] [CrossRef] [PubMed]
- Menon, S.G.; Sarsour, E.H.; Kalen, A.L.; Venkataraman, S.; Hitchler, M.J.; Domann, F.E.; Oberley, L.W.; Goswami, P.C. Superoxide Signaling Mediates N-Acetyl-l-Cysteine-Induced G1 Arrest: Regulatory Role of Cyclin D1 and Manganese Superoxide Dismutase. Cancer Res. 2007, 67, 6392–6399. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.Y.; Wang, H.M.; Chang, K.F.; Hu, H.T.; Hwang, L.J.; Fu, T.F.; Lin, Y.C.; Chang, W.C.; Chiu, T.P.; Wen, Z.H.; et al. Feruloyl-L-Arabinose Attenuates Migration, Invasion and Production of Reactive Oxygen Species in H1299 Lung Cancer Cells. Food Chem. Toxicol. 2013, 58, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.C.; Chang, H.W.; Chuang, D.W.; Chang, F.R.; Chang, Y.C.; Cheng, Y.S.; Tsai, M.T.; Chen, W.Y.; Lee, S.S.; Wang, C.K.; et al. Fern Plant-Derived Protoapigenone Leads to DNA Damage, Apoptosis, and G(2)/M Arrest in Lung Cancer; et al. Cell Line H1299. DNA Cell Biol. 2009, 28, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.C.; Liu, P.L.; Huang, K.J.; Wang, H.M.; Chang, K.F.; Chou, C.K.; Chang, F.R.; Chong, I.W.; Fang, K.; Chen, J.S.; et al. Goniothalamin Inhibits Growth of Human Lung Cancer Cells through DNA Damage, Apoptosis, and Reduced Migration Ability. J. Agric. Food Chem. 2011, 59, 4288–4293. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.C.; Chou, H.L.; Chen, B.H.; Chang, K.F.; Tseng, C.H.; Fong, Y.; Fu, T.F.; Chang, H.W.; Wu, C.Y.; Tsai, E.M.; et al. BPIQ, a Novel Synthetic Quinoline Derivative, Inhibits Growth and Induces Mitochondrial Apoptosis of Lung Cancer Cells in Vitro and in Zebrafish Xenograft Model. BMC Cancer 2015, 15, 962. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.C.; Fong, Y.; Tsai, E.-M.; Chang, Y.-G.; Chou, H.L.; Wu, C.-Y.; Teng, Y.-N.; Liu, T.-C.; Yuan, S.-S.; Chiu, C.-C. Exogenous C8-Ceramide Induces Apoptosis by Overproduction of ROS and the Switch of Superoxide Dismutases SOD1 to SOD2 in Human Lung Cancer Cells. Int. J. Mol. Sci. 2018, 19, 3010. https://doi.org/10.3390/ijms19103010
Chang YC, Fong Y, Tsai E-M, Chang Y-G, Chou HL, Wu C-Y, Teng Y-N, Liu T-C, Yuan S-S, Chiu C-C. Exogenous C8-Ceramide Induces Apoptosis by Overproduction of ROS and the Switch of Superoxide Dismutases SOD1 to SOD2 in Human Lung Cancer Cells. International Journal of Molecular Sciences. 2018; 19(10):3010. https://doi.org/10.3390/ijms19103010
Chicago/Turabian StyleChang, Yuli C., Yao Fong, Eing-Mei Tsai, Ya-Gin Chang, Han Lin Chou, Chang-Yi Wu, Yen-Ni Teng, Ta-Chih Liu, Shyng-Shiou Yuan, and Chien-Chih Chiu. 2018. "Exogenous C8-Ceramide Induces Apoptosis by Overproduction of ROS and the Switch of Superoxide Dismutases SOD1 to SOD2 in Human Lung Cancer Cells" International Journal of Molecular Sciences 19, no. 10: 3010. https://doi.org/10.3390/ijms19103010




