Review of the Electrical Characterization of Metallic Nanowires on DNA Templates
Abstract
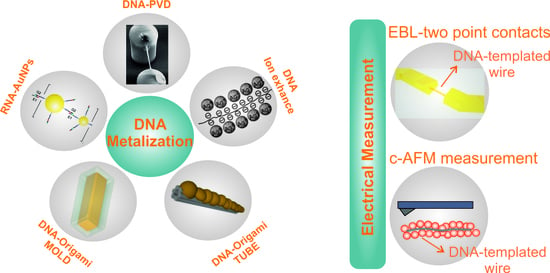
:1. Introduction
2. DNA Metalization
2.1. Activation by Metal Ions and Subsequent Metalization
2.2. Placement of Metal Nanoparticles on DNA Nanostructures
2.3. Direct Metalization
2.4. Metal Growth on and in DNA Structures
3. Electrical Characterization of DNA-Based Metallic Nanowires
3.1. Lithographically Defined Contacts and In Situ/Ex Situ I-V Measurements
3.2. Conductive AFM Measurements
3.3. DNA Origami-Based Metal Nanostructures
- Gold alignment marks (with a mutual distance of in each direction) were fabricated on a SiO2 substrate.
- SEM images were taken to register the location of the nanowires with respect to the alignment marks.
- Electrical contacts to the individual wires were defined by EBL using the precise position measured in the SEM images.
4. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rothemund, P. Folding DNA to create nanoscale shapes and patterns. Nature 2006, 440, 297–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dietz, H.; Douglas, S.M.; Shih, W.M. Folding DNA into twisted and curved nanoscale shapes. Science 2009, 325, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Seeman, N.C. Synthesis from DNA of a molecule with the connectivity of a cube. Nature 1991, 350, 631–633. [Google Scholar] [CrossRef] [PubMed]
- Kuzuya, A.; Wang, R.; Sha, R.; Seeman, N.C. Six-helix and Eight-helix DNA nanotubes assembled from half-tubes. Nano Lett. 2007, 7, 1757–1763. [Google Scholar] [CrossRef] [PubMed]
- Seeman, N.C. Nanomaterials based on DNA. Annu. Rev. Biochem. 2010, 79, 65–87. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Dai, M.; Yin, P. Complex shapes self-assembled from single-stranded DNA tiles. Nature 2012, 485, 623–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schreiber, R.; Do, J.; Roller, E.M.; Zhang, T.; Schueller, V.J.; Nickels, P.C.; Feldmann, J.; Liedl, T. Hierarchical assembly of metal nanoparticles, quantum dots and organic dyes using DNA origami scaffolds. Nat. Nanotechnol. 2014, 9, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Hemmig, E.A.; Fitzgerald, C.; Maffeo, C.; Hecker, L.; Ochmann, S.E.; Aksimentiev, A.; Tinnefeld, P.; Keyser, U.F. Optical voltage sensing using DNA origami. Nano Lett. 2018, 18, 1962–1971. [Google Scholar] [CrossRef] [PubMed]
- Cohen, H.; Nogues, C.; Ullien, D.; Daube, S.; Naaman, R.; Porath, D. Electrical characterization of self-assembled single- and double-stranded DNA monolayers using conductive AFM. Faraday Discuss. 2006, 131, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Lund, J.; Dong, J.C.; Deng, Z.X.; Mao, C.D.; Parviz, B.A. Electrical conduction in 7 nm wires constructed on lambda-DNA. Nanotechnology 2006, 17, 2752–2757. [Google Scholar] [CrossRef]
- Richter, J.; Mertig, M.; Pompe, W.; Mönch, I.; Schackert, H.K. Construction of highly conductive nanowires on a DNA template. Appl. Phys. Lett. 2001, 78, 536. [Google Scholar] [CrossRef]
- Braun, E.; Eichen, Y.; Sivan, U.; Ben-Yoseph, G. DNA-templated assembly and electrode attachment of a conducting silver wire. Nature 1998, 391, 775–778. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Barish, R.; Li, H.; Reif, J.H.; Finkelstein, G.; Yan, H.; LaBean, T.H. Three-helix bundle DNA tiles self-assemble into 2D lattice or 1D templates for silver nanowires. Nano Lett. 2005, 5, 693–696. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Prior, M.W.; LaBean, T.H.; Finkelstein, G. Optimized fabrication and electrical analysis of silver nanowires templated on DNA molecules. Appl. Phys. Lett. 2006, 89, 033901. [Google Scholar] [CrossRef]
- Yan, H. DNA-templated self-assembly of protein arrays and highly conductive nanowires. Science 2003, 301, 1882–1884. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhang, C.; Jiang, B.; Han, W.; Lin, Z. Flow-enabled self-assembly of large-scale aligned nanowires. Angew. Chem. Int. Ed. 2015, 54, 4250–4254. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Park, S.H.; Reif, J.H.; LaBean, T.H. DNA nanotubes self-assembled from triple-crossover tiles as templates for conductive nanowires. Proc. Natl. Acad. Sci. USA 2004, 101, 717–722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ongaro, A.; Griffin, F.; Beecher, P.; Nagle, L.; Iacopino, D.; Quinn, A.; Redmond, G.; Fitzmaurice, D. DNA-templated assembly of conducting gold nanowires between gold electrodes on a silicon oxide substrate. Chem. Mater. 2005, 17, 1959–1964. [Google Scholar] [CrossRef]
- Brun, C.; Elchinger, P.H.; Nonglaton, G.; Diagne, C.T.; Tiron, R.; Thuaire, A.; Gasparutto, D.; Baillin, X. Metallic conductive nanowires elaborated by PVD metal deposition on suspended DNA bundles. Small 2017, 13, 1700956. [Google Scholar] [CrossRef] [PubMed]
- Miele, E.; Accardo, A.; Falqui, A.; Marini, M.; Giugni, A.; Leoncini, M.; De Angelis, F.; Krahne, R.; Di Fabrizio, E. Writing and functionalisation of suspended DNA nanowires on superhydrophobic pillar arrays. Small 2014, 11, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Hernández-Neuta, I.; Madaboosi, N.; Nilsson, M.; van der Wijngaart, W. Efficient DNA-assisted synthesis of trans-membrane gold nanowires. Microsyst. Nanoeng. 2018, 4, 17084. [Google Scholar] [CrossRef] [Green Version]
- Tapio, K.; Leppiniemi, J.; Shen, B.; Hytönen, V.P.; Fritzsche, W.; Toppari, J.J. Toward single electron nanoelectronics using self-assembled DNA structure. Nano Lett. 2016, 16, 6780–6786. [Google Scholar] [CrossRef] [PubMed]
- Himuro, T.; Sato, S.; Takenaka, S.; Yasuda, T. Formation and electrical evaluation of a single metallized DNA nanowire in a nanochannel. Electroanalysis 2016, 28, 1448–1454. [Google Scholar] [CrossRef]
- Tian, C.; Cordeiro, M.A.L.; Lhermitte, J.; Xin, H.L.; Shani, L.; Liu, M.; Ma, C.; Yeshurun, Y.; DiMarzio, D.; Gang, O. Supra-nanoparticle functional assemblies through programmable stacking. ACS Nano 2017, 11, 7036–7048. [Google Scholar] [CrossRef] [PubMed]
- Al-Hinai, M.N.; Hassanien, R.; Wright, N.G.; Horsfall, A.B.; Houlton, A.; Horrocks, B.R. Networks of DNA-templated palladium nanowires: Structural and electrical characterisation and their use as hydrogen gas sensors. Faraday Discuss. 2013, 164, 71–91. [Google Scholar] [PubMed]
- Pate, J.; Zamora, F.; Watson, S.M.D.; Wright, N.G.; Horrocks, B.R.; Houlton, A. Solution-based DNA-templating of sub-10 nm conductive copper nanowires. J. Mater. Chem. C 2014, 2, 9265–9273. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, H.D.A.; Watson, S.M.D.; Horrocks, B.R.; Houlton, A. Chemical and electrochemical routes to DNA-templated rhodium nanowires. J. Mater. Chem. C 2014, 3, 438–446. [Google Scholar]
- Harnack, O.; Ford, W.E.; Yasuda, A.; Wessels, J.M. Tris(hydroxymethyl)phosphine-capped gold particles templated by DNA as nanowire precursors. Nano Lett. 2002, 2, 919–923. [Google Scholar] [CrossRef]
- Watson, J.D.; Crick, F.H.C. Molecular structure of nucleic acids: A structure for deoxyribose nucleic acid. Nature 1953, 171, 737–738. [Google Scholar] [CrossRef] [PubMed]
- Endres, R.; Cox, D.; Singh, R. Colloquium: The quest for high-conductance DNA. Rev. Mod. Phys. 2004, 76, 195. [Google Scholar] [CrossRef]
- Liu, S.P.; Artois, J.; Schmid, D.; Wieser, M.; Bornemann, B.; Weisbrod, S.; Marx, A.; Scheer, E.; Erbe, A. Electronic transport through short dsDNA measured with mechanically controlled break junctions: New thiol-gold binding protocol improves conductance. Phys. Status Solidi B 2013, 250, 2342–2348. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, P.; Li, X.; Tao, N. Direct conductance measurement of single DNA molecules in aqueous solution. Nano Lett. 2004, 4, 1105–1108. [Google Scholar] [CrossRef]
- Stern, A.; Eidelshtein, G.; Zhuravel, R.; Livshits, G.I.; Rotem, D.; Kotlyar, A.; Porath, D. Highly conductive thin uniform gold-coated DNA nanowires. Adv. Mater. 2018, 30, 1800433. [Google Scholar] [CrossRef] [PubMed]
- Pearson, A.C.; Liu, J.; Pound, E.; Uprety, B.; Woolley, A.T.; Davis, R.C.; Harb, J.N. DNA origami metallized site specifically to form electrically conductive nanowires. J. Phys. Chem. B 2012, 116, 10551–10560. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Pearson, A.C.; Gates, E.P.; Uprety, B.; Davis, R.C.; Harb, J.N.; Woolley, A.T. Electrically conductive gold- and copper-metallized DNA origami nanostructures. Langmuir 2013, 29, 3482–3490. [Google Scholar] [CrossRef] [PubMed]
- Teschome, B.; Facsko, S.; Schoenherr, T.; Kerbusch, J.; Keller, A.; Erbe, A. Temperature-dependent charge transport through individually contacted DNA origami-based Au nanowires. Langmuir 2016, 32, 10159–10165. [Google Scholar] [CrossRef] [PubMed]
- Uprety, B.; Westover, T.; Stoddard, M.; Brinkerhoff, K.; Jensen, J.; Davis, R.C.; Woolley, A.T.; Harb, J.N. Anisotropic electroless deposition on DNA origami templates to form small diameter conductive nanowires. Langmuir 2017, 33, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Bayrak, T.; Helmi, S.; Ye, J.; Kauert, D.; Nano, J.K. DNA-mold templated assembly of conductive gold nanowires. ACS Publ. 2018, 18, 2116–2123. [Google Scholar] [CrossRef] [PubMed]
- Aryal, B.R.; Westover, T.R.; Ranasinghe, D.R.; Calvopiña, D.G.; Uprety, B.; Harb, J.N.; Davis, R.C.; Woolley, A.T. Four-point probe electrical measurements on templated gold nanowires formed on single DNA origami tiles. Langmuir 2018. [Google Scholar] [CrossRef] [PubMed]
- Mertig, M.; Colombi Ciacchi, L.; Seidel, R.; Pompe, W.; De Vita, A. DNA as a selective metallization template. Nano Lett. 2002, 2, 841–844. [Google Scholar] [CrossRef]
- Andrew, D.B.; Benjamin, P.C.; Jonathan, M.C.; Rick, C.; Cody, G.; Andrew, G.; Luc, J.; John, L.P.; María, P.-P.; Xu, C.; et al. Construction and characterization of a gold nanoparticle wire assembled using Mg2+-dependent RNA–RNA interactions. Nano Lett. 2006, 6, 445–448. [Google Scholar]
- Helmi, S.; Ziegler, C.; Kauert, D.J.; Seidel, R. Shape-controlled synthesis of gold nanostructures using DNA origami molds. Nano Lett. 2014, 14, 6693–6698. [Google Scholar] [CrossRef] [PubMed]
- Lukatsky, D.B.; Frenkel, D. Surface and bulk dissolution properties, and selectivity of DNA-linked nanoparticle assemblies. J. Chem. Phys. 2005, 122, 214904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Veracoechea, F.J.; Mladek, B.M.; Tkachenko, A.V.; Frenkel, D. Design rule for colloidal crystals of DNA-functionalized particles. Phys. Rev. Lett. 2011, 107, 045902. [Google Scholar] [CrossRef] [PubMed]
- Takabayashi, S.; Klein, W.P.; Onodera, C.; Rapp, B.; Flores-Estrada, J.; Lindau, E.; Snowball, L.; Sam, J.T.; Padilla, J.E.; Lee, J.; et al. High precision and high yield fabrication of dense nanoparticle arrays onto DNA origami at statistically independent binding sites. Nanoscale 2014, 6, 13928–13938. [Google Scholar] [CrossRef] [PubMed]
- Teschome, B.; Facsko, S.; Gothelf, K.V.; Keller, A. Alignment of gold nanoparticle-decorated DNA origami nanotubes: Substrate prepatterning versus molecular combing. Langmuir 2015, 31, 12823–12829. [Google Scholar] [CrossRef] [PubMed]
- Gür, F.N.; Schwarz, F.W.; Ye, J.; Diez, S.; Schmidt, T.L. Toward self-assembled plasmonic devices: High-yield arrangement of gold nanoparticles on DNA origami templates. ACS Nano 2016, 10, 5374–5382. [Google Scholar] [CrossRef] [PubMed]
- Puchkova, A.; Vietz, C.; Pibiri, E.; Wünsch, B.; Sanz Paz, M.; Acuna, G.P.; Tinnefeld, P. DNA origami nanoantennas with over 5000-fold fluorescence enhancement and single-molecule detection at 25 μM. Nano Lett. 2015, 15, 8354–8359. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, R.; Luong, N.; Fan, Z.; Kuzyk, A.; Nickels, P.C.; Zhang, T.; Smith, D.M.; Yurke, B.; Kuang, W.; Govorov, A.O.; et al. Chiral plasmonic DNA nanostructures with switchable circular dichroism. Nat. Commun. 2013, 4, 2948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ketterer, P.; Willner, E.M.; Dietz, H. Nanoscale rotary apparatus formed from tight-fitting 3D DNA components. Sci. Adv. 2016, 2, e1501209. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.R.; Natan, M.J. Hydroxylamine seeding of colloidal Au nanoparticles in solution and on surfaces. Langmuir 1998, 14, 726–728. [Google Scholar] [CrossRef]
- Liu, J.; Geng, Y.; Pound, E.; Gyawali, S.; Ashton, J.R.; Hickey, J.; Woolley, A.T.; Harb, J.N. Metallization of branched DNA origami for nanoelectronic circuit fabrication. ACS Nano 2011, 5, 2240–2247. [Google Scholar] [CrossRef] [PubMed]
- Berti, L.; Alessandrini, A.; Facci, P. DNA-templated photoinduced silver deposition. J. Am. Chem. Soc. 2005, 127, 11216–11217. [Google Scholar] [CrossRef] [PubMed]
- Watson, S.M.D.; Mohamed, H.D.A.; Horrocks, B.R.; Houlton, A. Electrically conductive magnetic nanowires using an electrochemical DNA-templating route. Nanoscale 2013, 5, 5349–5359. [Google Scholar] [CrossRef] [PubMed]
- Janke, C.; Rogowski, K.; Wloga, D.; Regnard, C.; Kajava, A.V.; Strub, J.M.; Temurak, N.; van Dijk, J.; Boucher, D.; van Dorsselaer, A.; et al. Tubulin polyglutamylase enzymes are members of the TTL domain protein family. Science 2005, 308, 1758–1762. [Google Scholar] [CrossRef] [PubMed]
- Thuaire, A.; Reynaud, P.; Brun, C.; Sordes, D.; Carmignani, C.; Rolland, E.; Baillin, X.; Cheramy, S.; Poupon, G. Innovative solutions for the nanoscale packaging of silicon-based and biological nanowires: Development of a generic characterization and integration platform. IEEE Trans. Compon. Packag. Manuf. Technol. 2016, 6, 1804–1814. [Google Scholar] [CrossRef]
- Watson, S.M.D.; Houlton, A.; Horrocks, B.R. Equilibrium and non-equilibrium thermodynamics of templating reactions for the formation of nanowires. Nanotechnology 2012, 23, 505603. [Google Scholar] [CrossRef] [PubMed]
- Richter, J.; Seidel, R.; Kirsch, R.; Mertig, M.; Pompe, W.; Plaschke, J.; Schackert, H.K. Nanoscale palladium metallization of DNA. Adv. Mater. 2000, 12, 507–510. [Google Scholar] [CrossRef]
- Russell, C.; Welch, K.; Jarvius, J.; Cai, Y.; Brucas, R.; Nikolajeff, F.; Svedlindh, P.; Nilsson, M. Gold nanowire based electrical DNA detection using rolling circle amplification. ACS Nano 2014, 8, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- Hassanien, R.; Al-Said, S.; Šiller, L.; Little, R. Smooth and conductive DNA-templated Cu2O nanowires: Growth morphology, spectroscopic and electrical characterization. Nanotechnology 2012, 23, 075601. [Google Scholar] [CrossRef] [PubMed]
- Uprety, B.; Gates, E.P.; Geng, Y.; Woolley, A.T.; Harb, J.N. Site-specific metallization of multiple metals on a single DNA origami template. Langmuir 2013, 30, 1134–1141. [Google Scholar] [CrossRef] [PubMed]



| DNA Building | Contact Method | NP | DNA Structure | Resistances | Metal Source/Metallization | Contact Metal | Substrate | Temp. | Height | Length | Width | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DNA Metallization | EBL | Pd | -DNA | 800 | Pd(Ac)2/Chemical Reduction | Cr/Au, Au, & Pd | Mica | 120–300 K | NA | 1–2 m | 7 | [10] |
| Pd | -DNA | 743 and < 5 | Pd(CH3COO)2/Chemical Reduction | Au | SiO2 | RT | NA | 6.5 m | 50 | [11] | ||
| Ag | -DNA/ds-DNA | 30 , 7 | AgNO3/Chemical Reduction | Au | Glass | RT | NA | 1.2 m | 100 | [12] | ||
| Ag | TX-DNA | 1.42– | AgNO3/Chemical Reduction | Cr/Au | Si | RT | nm | nm | 320 and 430 nm | [13] | ||
| Ag | -DNA/ds-DNA | 597 –895 (30 & 500 at 77 K) | AgNO3/Chemical Reduction | Cr/Au | Si | 77–300 K | NA | 7 m | 15–35 nm | [14] | ||
| Ag | DNA nanoribbons | 200 | Protein Array | NA | NA | RT | 25 nm | 5 m | 43 nm | [15] | ||
| Ag | ds-DNA | 500 | AgNO3/Chemical Reduction | NA | PDMS transferred to Si | RT | NA | 60 nm | NA | [16] | ||
| Ag | TX-DNA | k, k, k | AgNO3/Chemical Reduction | Cr/Au | Si | RT | 35 nm | 5 m | 40 nm | [17] | ||
| Au | ds-DNA | 103 k | Pyridine modified gold nanoparticles/Gold-enhancer solution | Au | SiO2 | RT | 20 nm | 1.25 m | 40 nm | [18] | ||
| Au | -DNA/ds-DNA | (60 nm) and (80 nm) | E-beam Evaporation Gold | Ti/Au | Si/SiO2 | RT | NA | 800 nm | 60 nm and 80 nm | [19] | ||
| Au | -DNA | 30–140 | Thermal Evaporation Gold | Au | Pillars on Si or CF4 substrate | RT | 5–350 nm in diameter | >5 mm | 5–350 nm in diameter | [20] | ||
| Au | ss-DNA | < 20 | Gold nanoparticles/Gold-enhancer solution | Au | Polycarbonate memranes | RT | NA | 1.4 m | NA | [21] | ||
| AFM | Cu | ds-DNA | 107 M | Cu(NO3)2/Chemical Reduction | NA | TMS modified Si/SiO2 | RT | 11–20 nm | 1.5 m | 20 nm | [26] | |
| Pd | -DNA/ds-DNA | 0.4– G with DMAB and 2–8 G with NaBH4 | K2PdCl4/Chemical Reduction | Au | SiO2 | RT | NA | NA | 5–45 nm diameter | [25] | ||
| Au | DNA | k | THP-AuNPs/Chemical reduction | Au | Si | RT | NA | 2 m | 30–40 nm | [28] | ||
| Au | DNA | 3 k to 1 G | Au seeds/Chemical reduction | Au | Mica | RT | (10 ± 2, 13 ± 2 and 27 ± 3) nm | 10–700 nm | 25 nm | [33] | ||
| AFM | Rh | -DNA | 400–650 M and 250–350 M | RhCl3(H2O)/Chemical and electrochemical reduction | NA | SiO2 | RT | 3–31 nm in diameter | NA | 3–31 nm in diameter | [27] | |
| Dielectro- phoresis | Au | TX-DNA tiles | Coulomb Blockade | DNA modified gold nanoparticles | Au | Si/SiO2 | 4.2–300 K | 1.5 nm | 50–60 nm | NA | [22] | |
| Micro- channel | Ag | ds-DNA | 9 | Chemical modification of gold nanoparticles | Au | PDMS | RT | NA | 1 m | 40 nm | [23] | |
| Metalized DNA Origami | EBL | Au | T-shaped | 1.5– | DNA modified gold nanoparticles/Chemical Reduction | Au | SiO2 | RT | NA | 120 –240 | 33 | [34] |
| Pd | CC | 1–5 for Au/40 –1 Cu | (NH4)2PdCl4/Chemical Reduction and Gold-enhancer solution | Au | Si | RT | NA | 150 nm | 35/30 for Au, 40 nm for Cu | [35] | ||
| Au | Nanotube | 116 – G | DNA modified gold nanoparticles/Gold-enhancer solution | Ti/Au | SiO2 | –300 | 40 | 400 nm | 30nm | [36] | ||
| Au rod | Rectangular | 435 – M | DNA modified gold rod/Chemical Reduction | Cr/Au | SiO2 | RT | NA | < 410 | 13–29 nm | [37] | ||
| Au | Nanopillars | Highly resistive | DNA modified gold nanoparticles/Chemical Reduction | Pt | SiO2 | RT | NA | NA | NA | [24] | ||
| Au | Mold | 90 –30 | DNA modified gold nanoparticles/Chemical Reduction | Ti/Au | SiO2 | K–300 | 20–30 nm in diameter | NA | 20–30 nm in diameter | [38] | ||
| EBID | Au rod | plus, cross, c-shaped | –76 M | DNA modified gold rod/Chemical Reduction | Cr/Au-Pd | Si | RT | NA | 130 nm | 12 nm | [39] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bayrak, T.; Jagtap, N.S.; Erbe, A. Review of the Electrical Characterization of Metallic Nanowires on DNA Templates. Int. J. Mol. Sci. 2018, 19, 3019. https://doi.org/10.3390/ijms19103019
Bayrak T, Jagtap NS, Erbe A. Review of the Electrical Characterization of Metallic Nanowires on DNA Templates. International Journal of Molecular Sciences. 2018; 19(10):3019. https://doi.org/10.3390/ijms19103019
Chicago/Turabian StyleBayrak, Türkan, Nagesh S. Jagtap, and Artur Erbe. 2018. "Review of the Electrical Characterization of Metallic Nanowires on DNA Templates" International Journal of Molecular Sciences 19, no. 10: 3019. https://doi.org/10.3390/ijms19103019