Cost-Effective Biochar Produced from Agricultural Residues and Its Application for Preparation of High Performance Form-Stable Phase Change Material via Simple Method
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural Properties
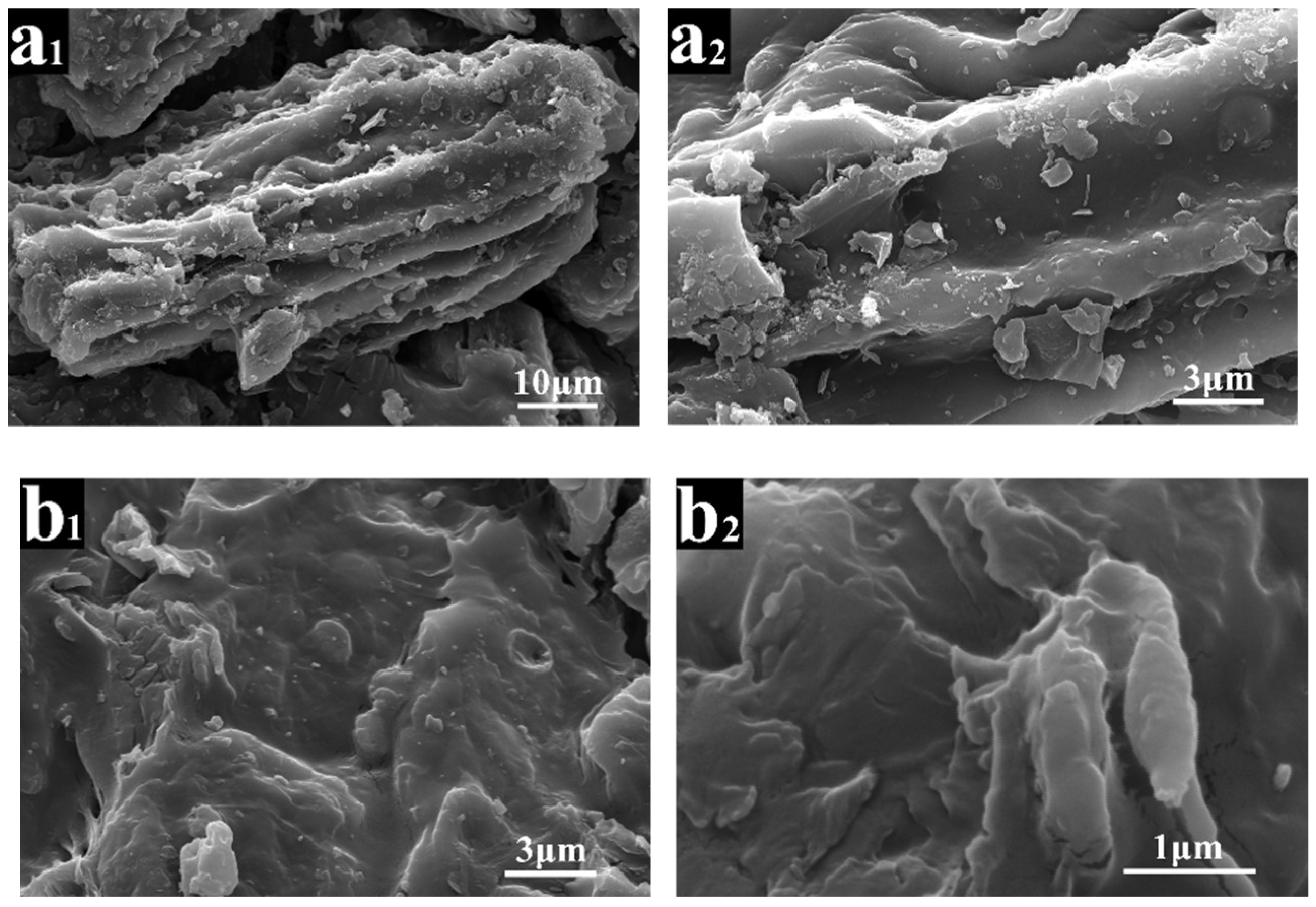
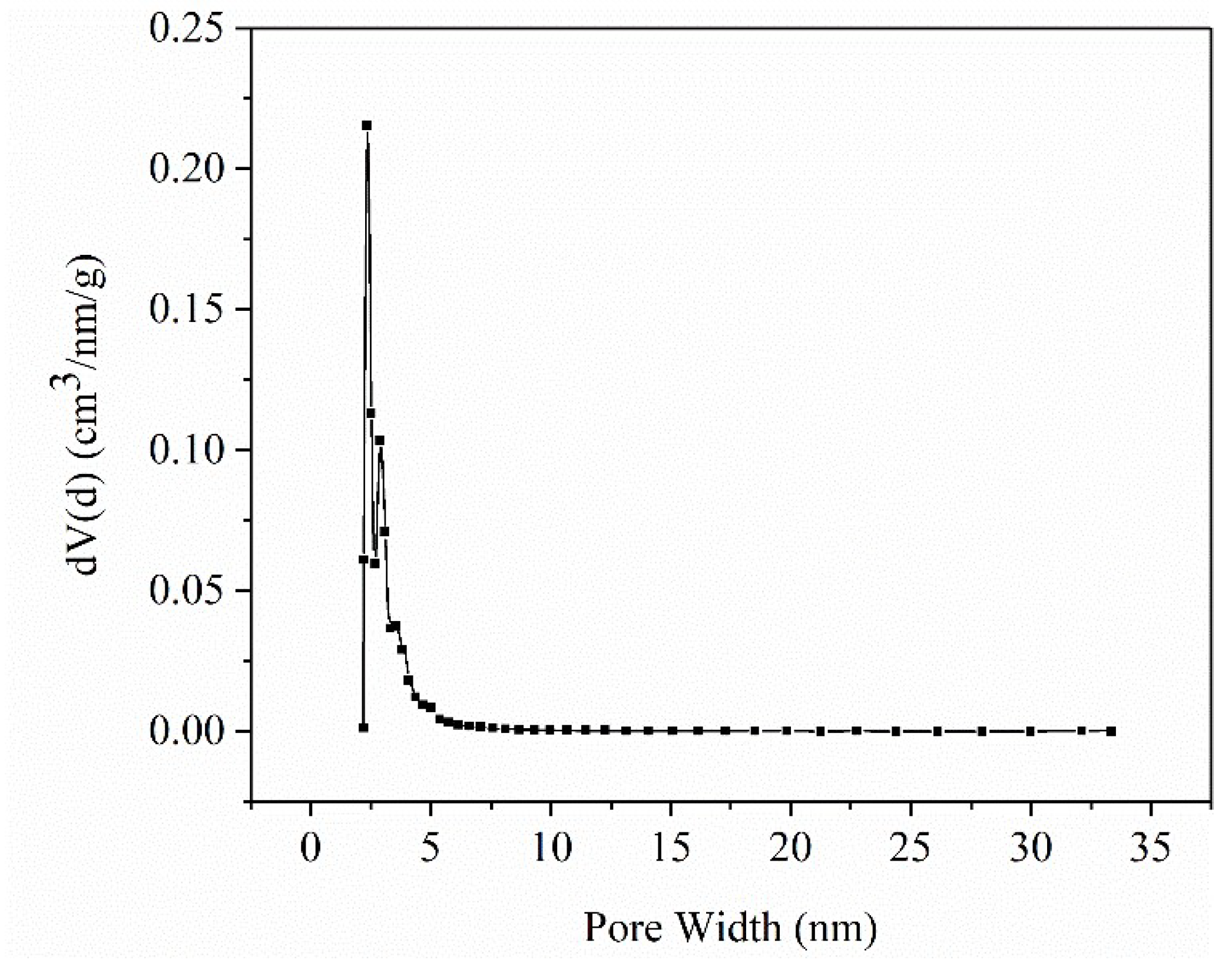
2.1.1. Microstructure of ASB
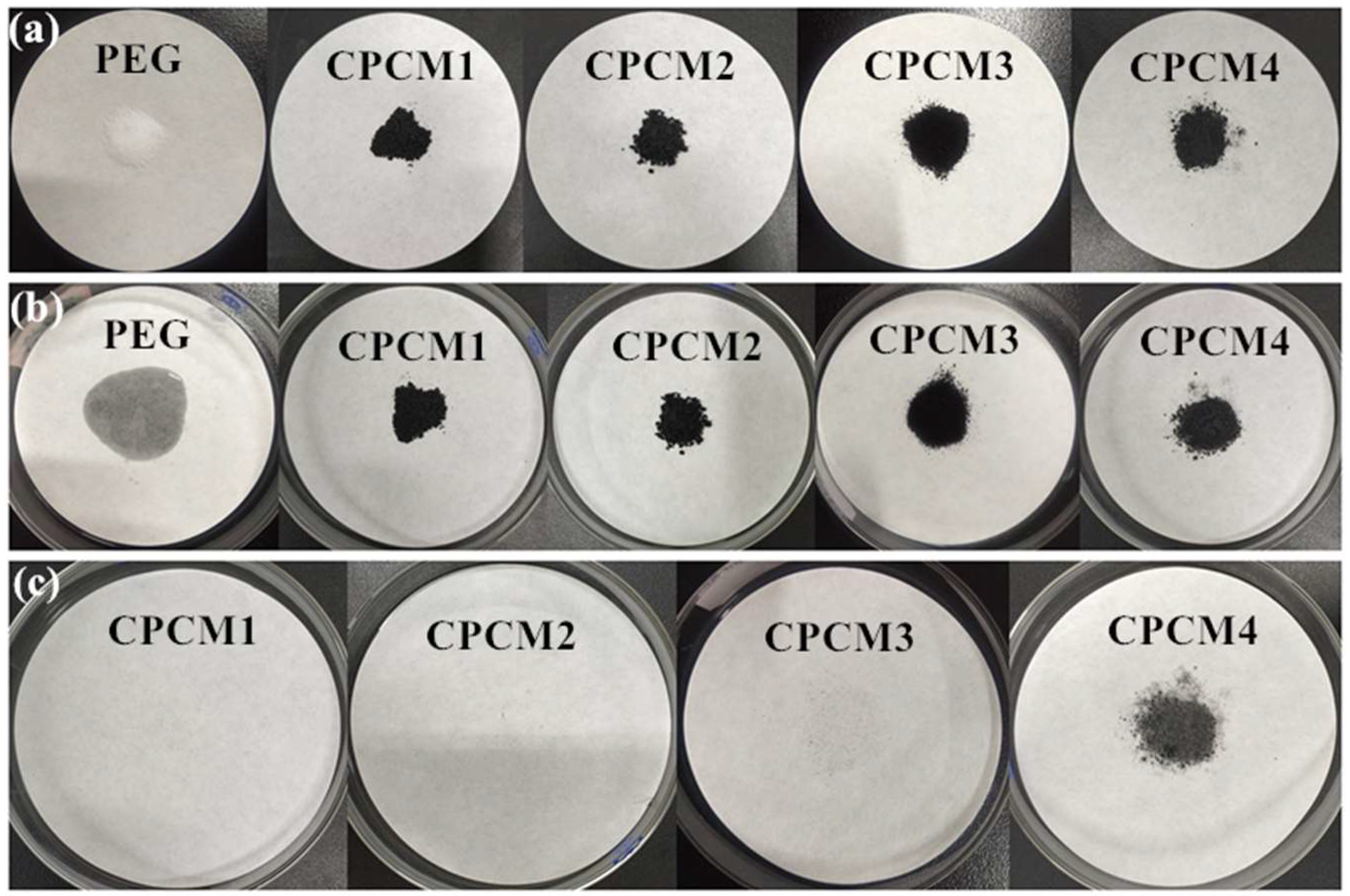
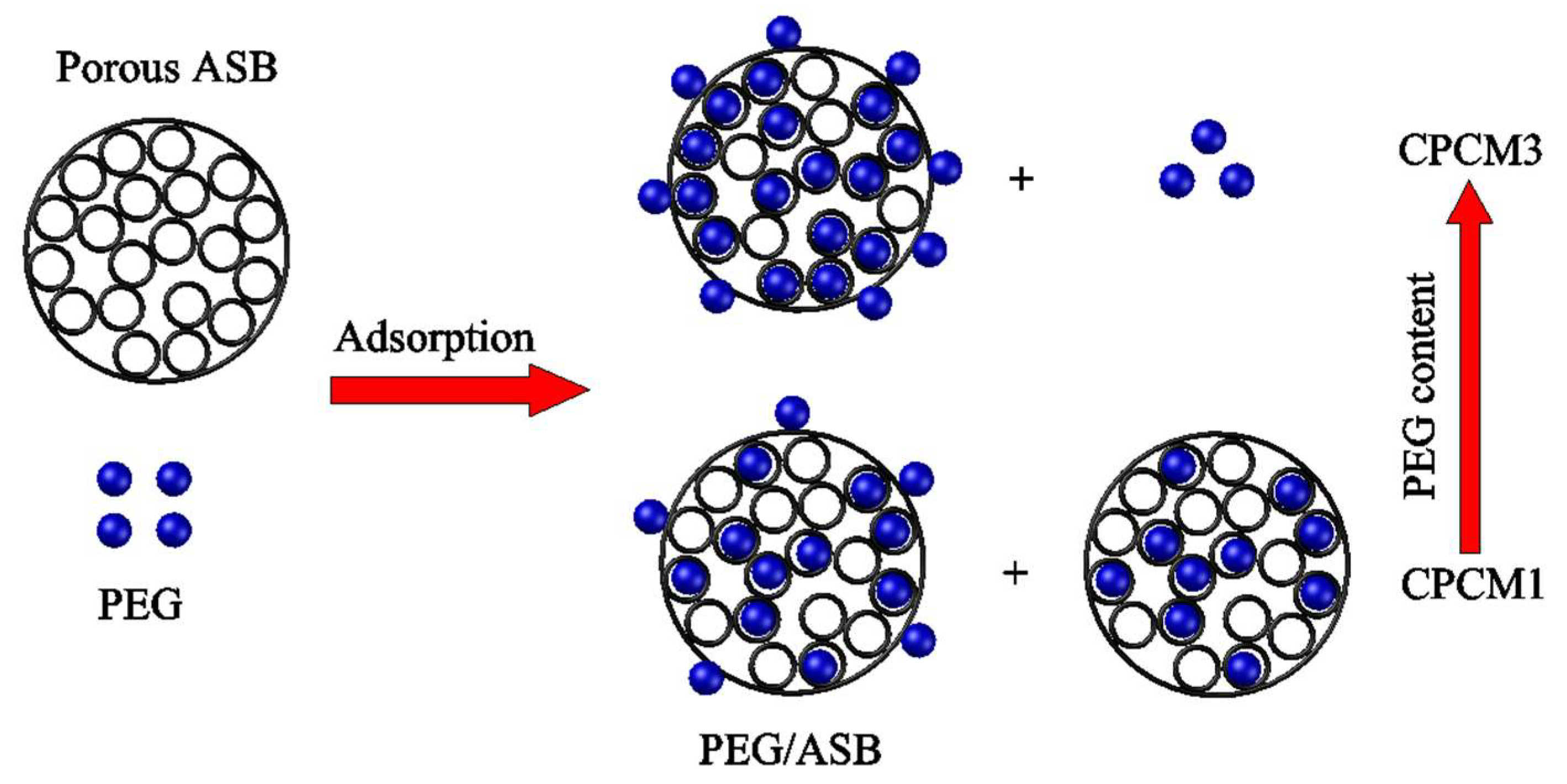
2.1.2. Leakage Test of PEG/ASB Composites
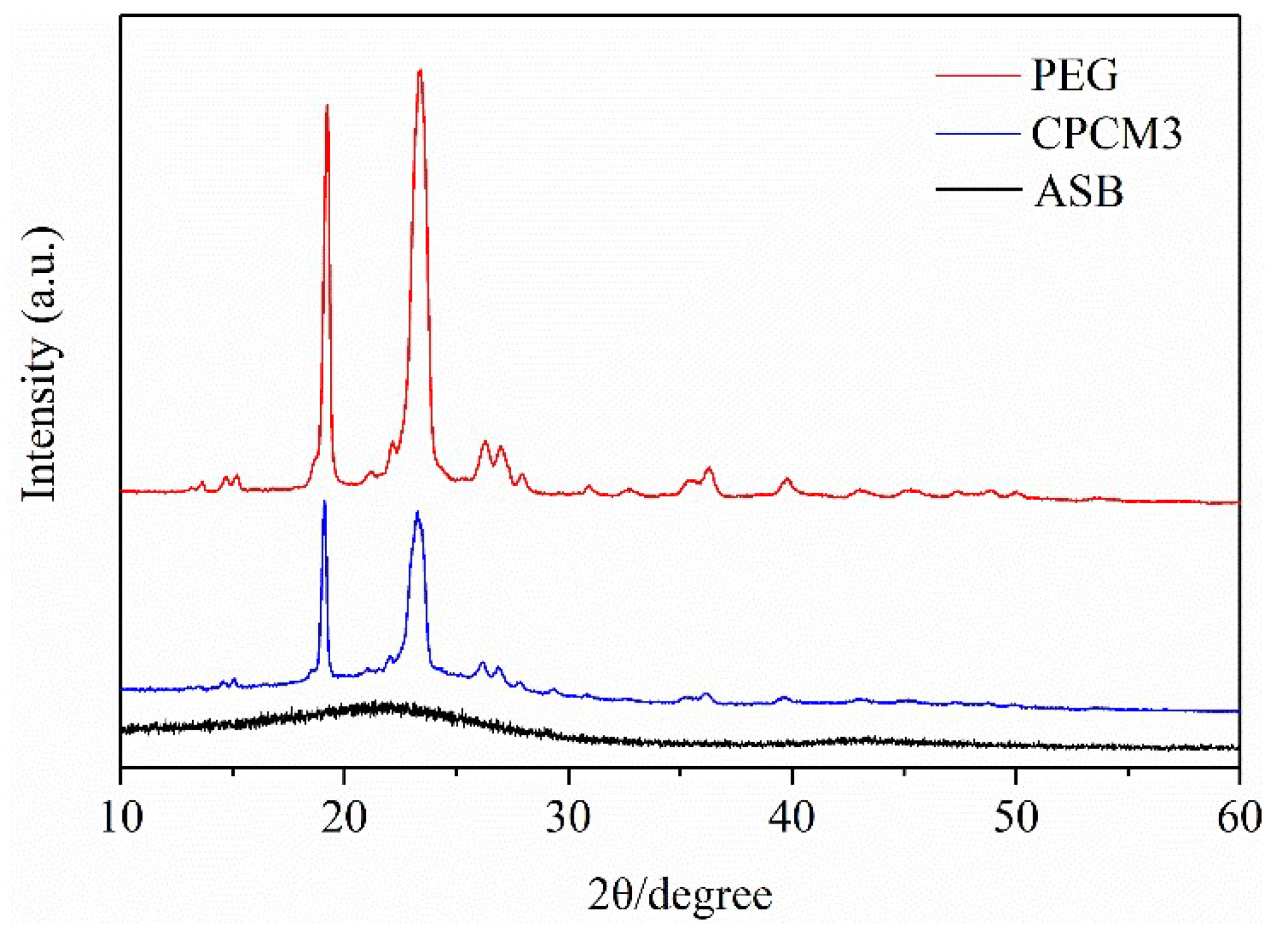
2.1.3. XRD Patterns of PEG/ASB Composites
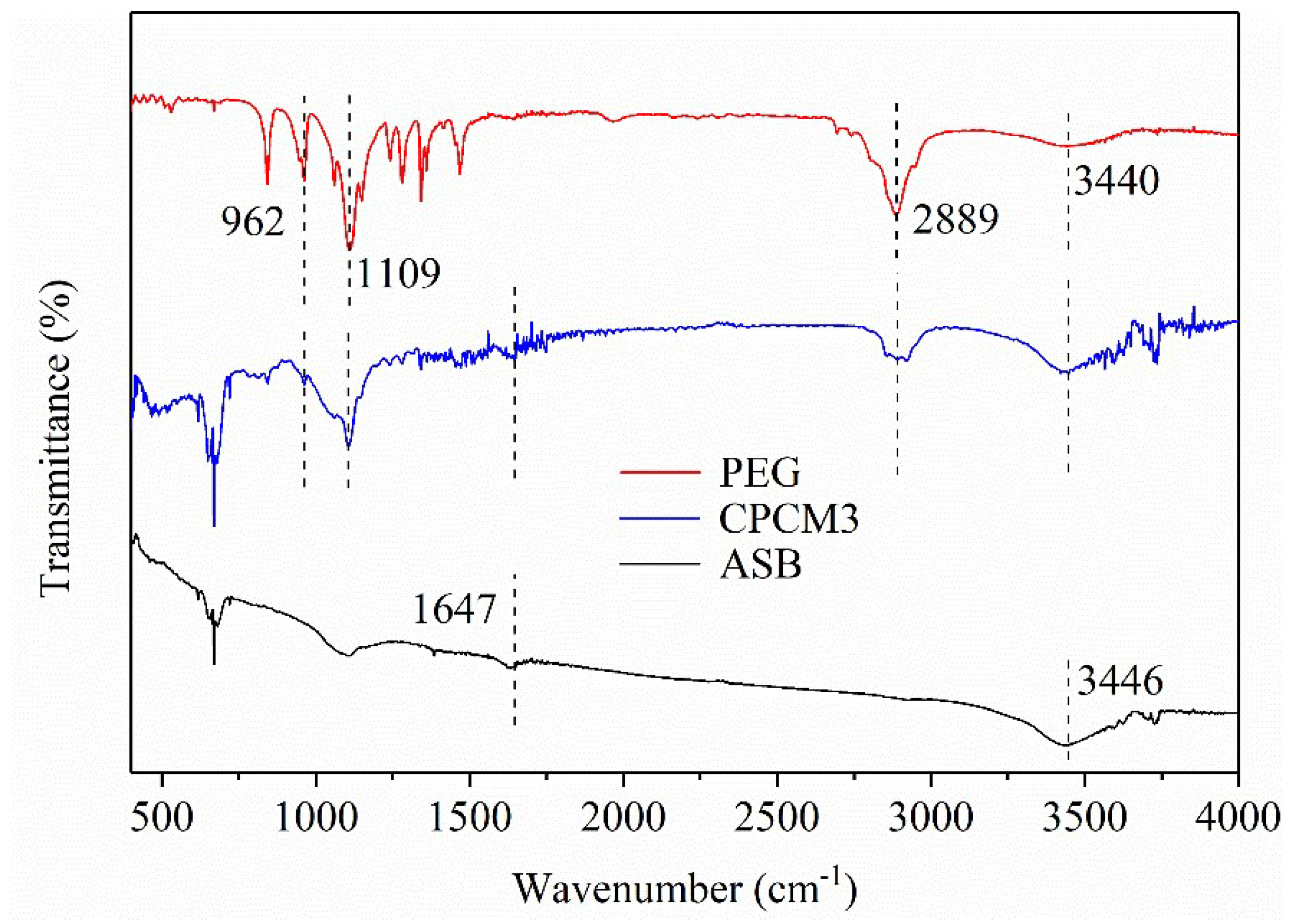
2.1.4. FT-IR Analysis of PEG/ASB Composites
2.2. Thermal Properties
2.2.1. Thermal Stability of PEG/ASB Composites
2.2.2. Interaction between PEG and ASB
2.2.3. Phase Change Behaviors of PEG/ASB Composites
2.2.4. Thermal Reliability of PEG/ASB Composites
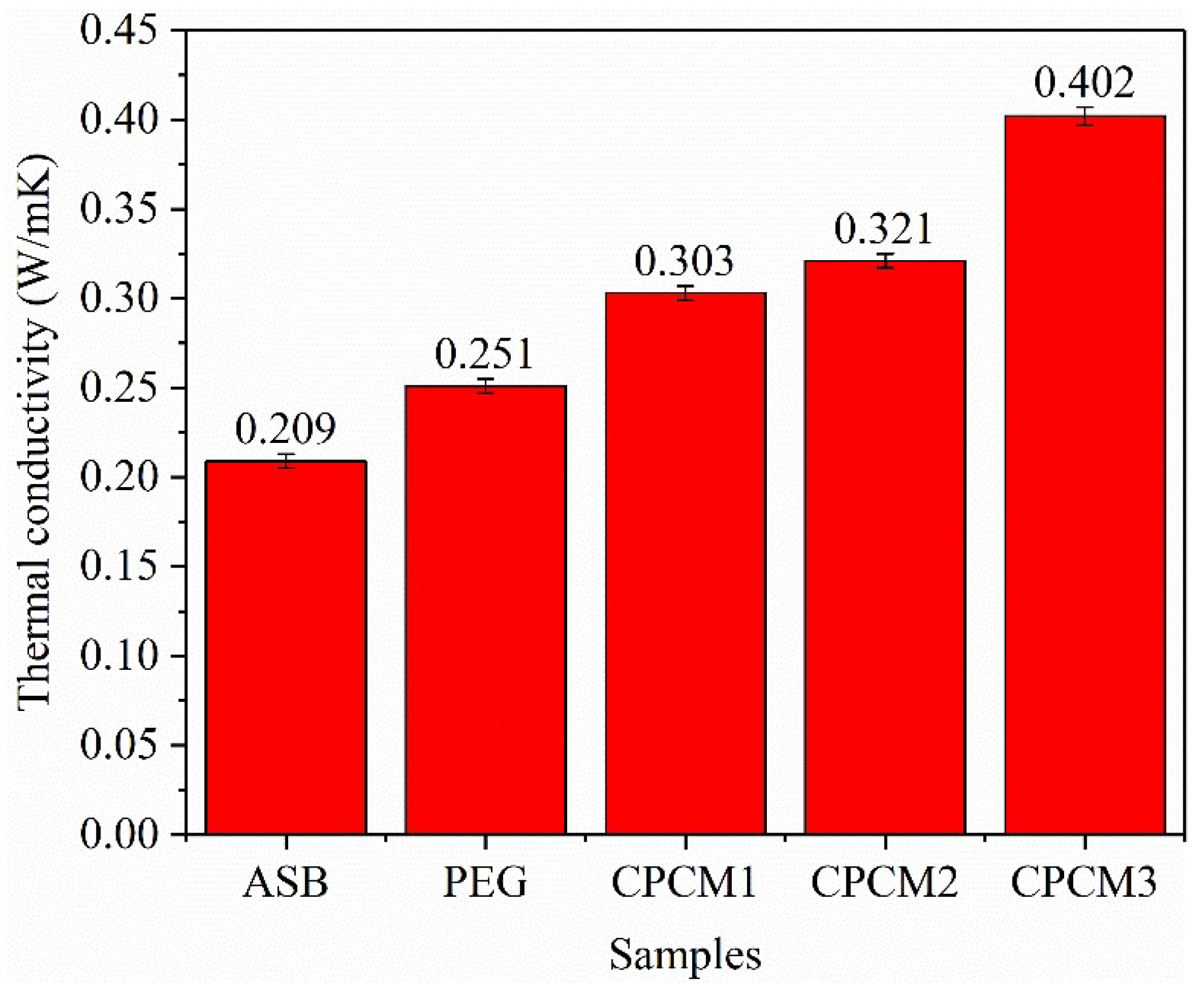
2.2.5. Thermal Conductivity of PEG/ASB Composites
3. Materials and Methods
3.1. Materials
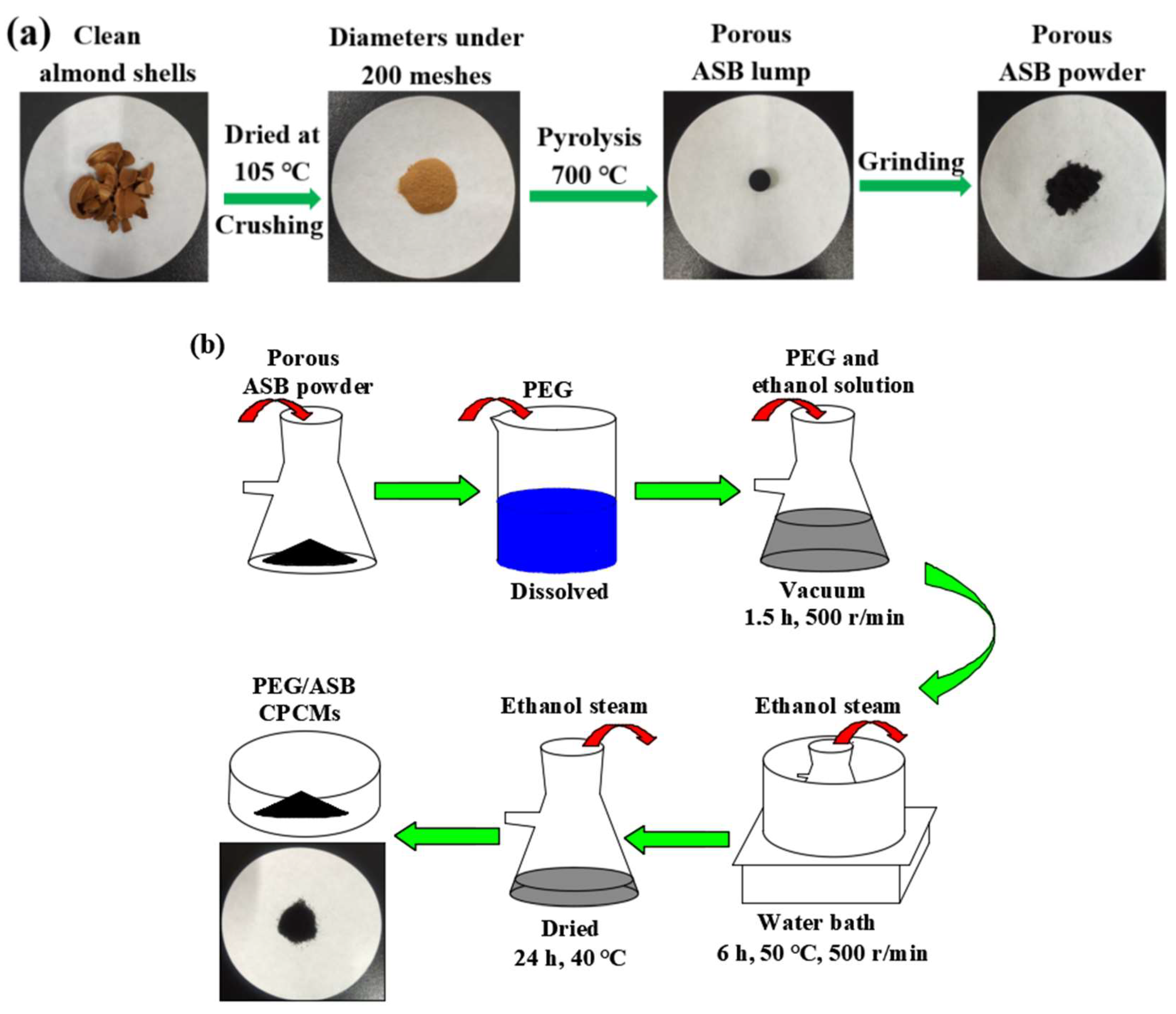
3.2. Pyrolysis Procedure
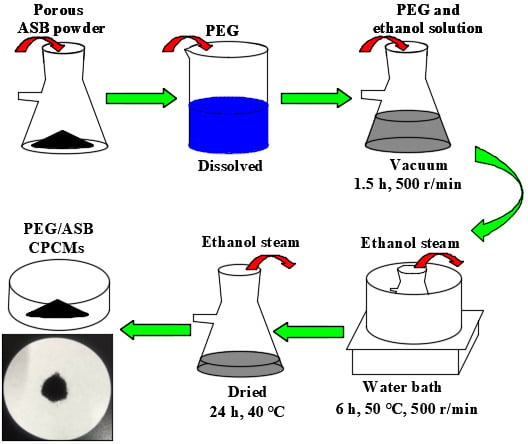
3.3. Preparation of PEG/ASB CPCMs
3.4. Analysis Methods
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, J.; He, L.; Liu, T.; Cao, X.; Zhu, H. Preparation and characterization of PEG/SiO2 composites as shape-stabilized phase change materials for thermal energy storage. Sol. Energy Mater. Sol. Cells 2013, 118, 48–53. [Google Scholar] [CrossRef]
- Wardziak, Ł.; Jaworski, M. Computer simulations of heat transfer in a building integrated heat storage unit made of PCM composite. Therm. Sci. Eng. Prog. 2017, 2, 109–118. [Google Scholar] [CrossRef]
- Niu, X.; Xu, Q.; Zhang, Y.; Zhang, Y.; Yan, Y.; Liu, T. Fabrication and properties of micro-nano encapsulated phase change materials for internally-cooled liquid desiccant dehumidification. Nanomaterials 2017, 7, 96. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Guo, Y.; Su, J.; Zhang, X.; Han, N.; Wang, X. Microstructure and thermal reliability of microcapsules containing phase change material with self-assembled graphene/organic nano-hybrid shells. Nanomaterials 2018, 8, 364. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yan, Z.; Fu, L.; Yang, H. Hierarchical nano activated silica nanosheets for thermal energy storage. Sol. Energy Mater. Sol. Cells 2017, 167, 140–149. [Google Scholar] [CrossRef]
- Deng, Y.; Li, J.; Qian, T.; Guan, W.; Li, Y.; Yin, X. Thermal conductivity enhancement of polyethylene glycol/expanded vermiculite shape-stabilized composite phase change materials with silver nanowire for thermal energy storage. Chem. Eng. J. 2016, 295, 427–435. [Google Scholar] [CrossRef]
- Karaman, S.; Karaipekli, A.; Sarı, A.; Bicer, A. Polyethylene glycol (PEG)/diatomite composite as a novel form-stable phase change material for thermal energy storage. Sol. Energy Mater. Sol. Cells 2011, 95, 1647–1653. [Google Scholar] [CrossRef]
- Wen, R.; Zhang, X.; Huang, Y.; Yin, Z.; Huang, Z.; Fang, M.; Liu, Y.; Wu, X. Preparation and properties of fatty acid eutectics/expanded perlite and expanded vermiculite shape-stabilized materials for thermal energy storage in buildings. Energy Build. 2017, 139, 197–204. [Google Scholar] [CrossRef]
- Zhang, X.; Wen, R.; Tang, C.; Wu, B.; Huang, Z.; Min, X.; Huang, Y.; Liu, Y.; Fang, M.; Wu, X. Thermal conductivity enhancement of polyethylene glycol/expanded perlite with carbon layer for heat storage application. Energy Build. 2016, 130, 113–121. [Google Scholar] [CrossRef]
- Qi, G.; Yang, J.; Bao, R.; Liu, Z.; Yang, W.; Xie, B.; Yang, M. Enhanced comprehensive performance of polyethylene glycol based phase change material with hybrid graphene nanomaterials for thermal energy storage. Carbon 2015, 88, 196–205. [Google Scholar] [CrossRef]
- Feng, L.; Zhao, W.; Zheng, J.; Frisco, S.; Song, P.; Li, X. The shape-stabilized phase change materials composed of polyethylene glycol and various mesoporous matrices (AC, SBA-15 and MCM-41). Sol. Energy Mater. Sol. Cells 2011, 95, 3550–3556. [Google Scholar] [CrossRef]
- Qian, T.; Li, J.; Feng, W.; Nian, H. Single-walled carbon nanotube for shape stabilization and enhanced phase change heat transfer of polyethylene glycol phase change material. Energy Convers. Manag. 2017, 143, 96–108. [Google Scholar] [CrossRef]
- Wang, C.; Wang, W.; Xin, G.; Li, G.; Zheng, J.; Tian, W.; Li, X. Phase change behaviors of PEG on modified graphene oxide mediated by surface functional groups. Eur. Polym. J. 2016, 74, 43–50. [Google Scholar] [CrossRef]
- Deng, Y.; Li, J.; Qian, T.; Guan, W.; Wang, X. Preparation and characterization of KNO3/diatomite shape-stabilized composite phase change material for high temperature thermal energy storage. J. Mater. Sci. Technol. 2017, 33, 198–203. [Google Scholar] [CrossRef]
- Chen, Z.; Shan, F.; Cao, L.; Fang, G. Synthesis and thermal properties of shape-stabilized lauric acid/activated carbon composites as phase change materials for thermal energy storage. Sol. Energy Mater. Sol. Cells 2012, 102, 131–136. [Google Scholar] [CrossRef]
- Feng, L.; Zheng, J.; Yang, H.; Guo, Y.; Li, W.; Li, X. Preparation and characterization of polyethylene glycol/active carbon composites as shape-stabilized phase change materials. Sol. Energy Mater. Sol. Cells 2011, 95, 644–650. [Google Scholar] [CrossRef]
- Khadiran, T.; Hussein, M.Z.; Zainal, Z.; Rusli, R. Shape-stabilized n-octadecane/activated carbon nanocomposite phase change material for thermal energy storage. J. Taiwan Inst. Chem. Eng. 2015, 55, 189–197. [Google Scholar] [CrossRef]
- Tian, B.; Yang, W.; Luo, L.; Wang, J.; Zhang, K.; Fan, J.; Wu, J.; Xing, T. Synergistic enhancement of thermal conductivity for expanded graphite and carbon fiber in paraffin/EVA form-stable phase change materials. Sol. Energy 2016, 127, 48–55. [Google Scholar] [CrossRef]
- Qian, T.; Li, J.; Min, X.; Deng, Y.; Guan, W.; Ning, L. Radial-like mesoporous silica sphere: A promising new candidate of supporting material for storage of low-, middle-, and high temperature heat. Energy 2016, 112, 1074–1083. [Google Scholar] [CrossRef]
- Wang, C.L.; Yeh, K.L.; Chen, C.W.; Lee, Y.; Lee, H.L.; Lee, T. A quick-fix design of phase change material by particle blending and spherical agglomeration. Appl. Energy 2017, 191, 239–250. [Google Scholar] [CrossRef]
- Wang, Y.; Xia, T.; Zheng, H.; Feng, H. Stearic acid/silica fume composite as form-stable phase change material for thermal energy storage. Energy Build. 2011, 43, 2365–2370. [Google Scholar] [CrossRef]
- Dawood, S.; Sen, T.K.; Phan, C. Synthesis and characterization of slow pyrolysis pine cone bio-char in the removal of organic and inorganic pollutants from aqueous solution by adsorption: Kinetic, equilibrium, mechanism and thermodynamic. Bioresour. Technol. 2017, 246, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Kalinke, C.; Mangrich, A.S.; Marcolino-Juniora, L.H.; Bergamini, M.F. Biochar prepared from castor oil cake at different temperatures: A voltammetric study applied for Pb2+, Cd2+ and Cu2+ ions preconcentration. J. Hazard. Mater. 2016, 318, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, X.; Liu, L.; Ju, M.; Zheng, K. Adsorption Behavior of Selective Recognition Functionalized Biochar to Cd(II) in Wastewater. Materials 2018, 11, 299. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Wang, F.; Zhang, P.; Guo, J.; Sun, H. Aging effect of minerals on biochar properties and sorption capacities for atrazine and phenanthrene. Chemosphere 2018, 206, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Kalinke, C.; Oliveira, P.R.; Oliveira, G.A.; Mangrich, A.S.; Marcolino-Junior, L.H.; Bergamini, M.F. Activated biochar: Preparation, characterization and electroanalytical application in an alternative strategy of nickel determination. Anal. Chim. Acta 2017, 983, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhu, X.; Shi, L.; Li, J.; Li, S.; Lü, J.; Li, Y. Efficient removal of lead from solution by celery-derived biochars rich in alkaline minerals. Bioresour. Technol. 2017, 235, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, Q.; Guo, L.; Zhang, Y.; Lou, Z.; Wang, Y.; Qian, G. Cu(II) removal from aqueous solution by Spartina alterniflora derived Biochar. Bioresour. Technol. 2013, 141, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Wang, B. Yield, Composition, and property of biochar obtained from the two-step pyrolysis of rice husk impregnated with boric acid. Energies 2017, 10, 1814. [Google Scholar] [CrossRef]
- Kumar, A.; Joseph, S.; Tsechansky, L.; Privat, K.; Schreiter, I.J.; Schüth, C.; Graber, E.R. Biochar aging in contaminated soil promotes Zn immobilization due to changes in biochar surface structural and chemical properties. Sci. Total Environ. 2018, 626, 953–961. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Shi, S.; Liu, J.; Su, S.; Liang, Q.; Zeng, X.; Li, T. Study of the effect of pyrolysis temperature on the Cd2+ adsorption characteristics of biochar. Appl. Sci. 2018, 8, 1019. [Google Scholar] [CrossRef]
- Li, B.; Yang, L.; Wang, C.; Zhang, Q.; Liu, Q.; Li, Y.; Xiao, R. Adsorption of Cd(II) from aqueous solutions by rape straw biochar derived from different modification processes. Chemosphere 2017, 175, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Fernandez, G.; Perez-Ferreras, S.; Pascual, L.; Llorente, I.; Ibanez, J.; Rojo, J.M. Electrochemical study of tetraalkylammonium tetrafluoroborate electrolytes in combination with microporous and mesoporous carbon monoliths. Electrochim. Acta 2018, 268, 121–130. [Google Scholar] [CrossRef]
- Zhao, B.; O’Connor, D.; Zhang, J.; Peng, T.; Shen, Z.; Tsang, D.C.W.; Hou, D. Effect of pyrolysis temperature, heating rate, and residence time on rapeseed stem derived biochar. J. Clean. Prod. 2018, 174, 977–987. [Google Scholar] [CrossRef]
- Liu, W.; Jiang, H.; Yu, H. Development of biochar-based functional materials: Toward a sustainable platform carbon material. Chem. Rev. 2015, 115, 12251–12285. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Zhu, L. Sorption of phenanthrene to biochar modified by base. Front. Environ. Sci. Eng. 2018, 12, 1. [Google Scholar] [CrossRef]
- Zhao, Y.; Min, X.; Huang, Z.; Liu, Y.; Wu, X.; Fang, M. Honeycomb-like structured biological porous carbon encapsulating PEG: A shape-stable phase change material with enhanced thermal conductivity for thermal energy storage. Energy Build. 2018, 158, 1049–1062. [Google Scholar] [CrossRef]
- Qian, T.; Li, J.; Ma, H.; Yang, J. The preparation of a green shape-stabilized composite phase change material of polyethylene glycol/SiO2 with enhanced thermal performance based on oil shale ash via temperature-assisted sol-gel method. Sol. Energy Mater. Sol. Cells 2015, 132, 29–39. [Google Scholar] [CrossRef]
- Tan, B.; Huang, Z.; Yin, Z.; Min, X.; Liu, Y.; Wu, X.; Fang, M. Preparation and thermal properties of shape-stabilized composite phase change materials based on polyethylene glycol and porous carbon prepared from potato. RSC Adv. 2016, 6, 15821–15830. [Google Scholar] [CrossRef]
- Liu, X.; Rao, Z. Experimental study on the thermal performance of graphene and exfoliated graphite sheet for thermal energy storage phase change material. Thermochim. Acta 2017, 647, 15–21. [Google Scholar] [CrossRef]
- Li, C.; Yu, H.; Song, Y.; Zhao, M. Synthesis and characterization of PEG/ZSM-5 composite phase change materials for latent heat storage. Renew. Energy 2018, 121, 45–52. [Google Scholar] [CrossRef]
- Tang, B.; Cui, J.; Wang, Y.; Jia, C.; Zhang, S. Facile synthesis and performances of PEG/SiO2 composite form-stable phase change materials. Sol. Energy 2013, 97, 484–492. [Google Scholar] [CrossRef]
- Feng, L.; Song, P.; Yan, S.; Wang, H.; Wang, J. The shape-stabilized phase change materials composed of polyethylene glycol and graphitic carbon nitride matrices. Thermochim. Acta 2015, 612, 19–24. [Google Scholar] [CrossRef]





| Samples | Onset (°C) | Peak (°C) | End (°C) | Residual Weight (%) |
|---|---|---|---|---|
| PEG | 354 | 401 | 426 | 2.2 |
| CPCM3 | 346 | 391 | 426 | 47.4 |
| CPCM2 | 345 | 389 | 424 | 51.3 |
| CPCM1 | 340 | 388 | 420 | 61.1 |
| Samples | PEG Contents a | Melting | Solidification | ||||
|---|---|---|---|---|---|---|---|
| Tmpb (°C) | Tmc (°C) | Hmd (J/g) | Tspe (°C) | Tsf (°C) | Hsg (J/g) | ||
| PEG | 100% | 63.13 | 56.93 | 189.06 | 35.70 | 41.01 | 175.68 |
| CPCM3 | 60% | 62.76 | 52.47 | 82.73 | 31.97 | 38.87 | 78.76 |
| CPCM2 | 50% | 60.49 | 52.21 | 71.16 | 34.06 | 39.38 | 65.26 |
| CPCM1 | 40% | 57.04 | 50.37 | 59.37 | 33.03 | 39.41 | 57.21 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Cui, Z.; Ding, H.; Wan, Y.; Tang, Z.; Gao, J. Cost-Effective Biochar Produced from Agricultural Residues and Its Application for Preparation of High Performance Form-Stable Phase Change Material via Simple Method. Int. J. Mol. Sci. 2018, 19, 3055. https://doi.org/10.3390/ijms19103055
Chen Y, Cui Z, Ding H, Wan Y, Tang Z, Gao J. Cost-Effective Biochar Produced from Agricultural Residues and Its Application for Preparation of High Performance Form-Stable Phase Change Material via Simple Method. International Journal of Molecular Sciences. 2018; 19(10):3055. https://doi.org/10.3390/ijms19103055
Chicago/Turabian StyleChen, Yan, Zhixing Cui, Han Ding, Yechao Wan, Zhibo Tang, and Junkai Gao. 2018. "Cost-Effective Biochar Produced from Agricultural Residues and Its Application for Preparation of High Performance Form-Stable Phase Change Material via Simple Method" International Journal of Molecular Sciences 19, no. 10: 3055. https://doi.org/10.3390/ijms19103055