AMP-Activated Protein (AMPK) in Pathophysiology of Pregnancy Complications
Abstract
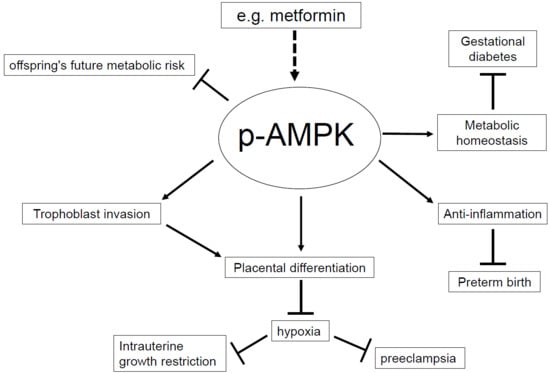
:1. Introduction
2. Intrauterine Growth Restriction (IUGR)
3. Gestational Diabetes Mellitus (GDM)
4. Preeclampsia
5. Preterm Birth (PTB)
6. Reprogramming
7. Perspective: Catechol-O-Methyltransferase and Pregnancy
8. Conclusions
Funding
Conflicts of Interest
References
- Alkema, L.; Chou, D.; Hogan, D.; Zhang, S.; Moller, A.B.; Gemmill, A.; Fat, D.M.; Boerma, T.; Temmerman, M.; Mathers, C.; et al. Global, regional, and national levels and trends in maternal mortality between 1990 and 2015, with scenario-based projections to 2030: A systematic analysis by the UN maternal mortality estimation inter-agency group. Lancet 2016, 387, 462–474. [Google Scholar] [CrossRef]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawley, S.A.; Davison, M.; Woods, A.; Davies, S.P.; Beri, R.K.; Carling, D.; Hardie, D.G. Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J. Biol. Chem. 1996, 271, 27879–27887. [Google Scholar] [CrossRef] [PubMed]
- Fogarty, S.; Hawley, S.A.; Green, K.A.; Saner, N.; Mustard, K.J.; Hardie, D.G. Calmodulin-dependent protein kinase kinase-beta activates AMPK without forming a stable complex: Synergistic effects of Ca2+ and AMP. Biochem. J. 2010, 426, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, G.R.; Kemp, B.E. AMPK in health and disease. Physiol. Rev. 2009, 89, 1025–1078. [Google Scholar] [CrossRef] [PubMed]
- Trewin, A.J.; Berry, B.J.; Wojtovich, A.P. Exercise and mitochondrial dynamics: Keeping in Shape with ROS and AMPK. Antioxidants 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Moreira, D.; Silvestre, R.; Cordeiro-da-Silva, A.; Estaquier, J.; Foretz, M.; Viollet, B. AMP-activated Protein Kinase as a target for pathogens: Friends or foes? Curr. Drug Targets 2016, 17, 942–953. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Hsu, C.N. AMP-activated protein kinase as a reprogramming strategy for hypertension and kidney disease of developmental origin. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Saud, S.M.; Young, M.R.; Chen, G.; Hua, B. Targeting AMPK for cancer prevention and treatment. Oncotarget 2015, 6, 7365–7378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carey, E.A.; Albers, R.E.; Doliboa, S.R.; Hughes, M.; Wyatt, C.N.; Natale, D.R.; Brown, T.L. AMPK knockdown in placental trophoblast cells results in altered morphology and function. Stem Cells Dev. 2014, 23, 2921–2930. [Google Scholar] [CrossRef] [PubMed]
- Martinez de Morentin, P.B.; Lage, R.; Gonzalez-Garcia, I.; Ruiz-Pino, F.; Martins, L.; Fernandez-Mallo, D.; Gallego, R.; Ferno, J.; Senaris, R.; Saha, A.K.; et al. Pregnancy induces resistance to the anorectic effect of hypothalamic malonyl-CoA and the thermogenic effect of hypothalamic AMPK inhibition in female rats. Endocrinology 2015, 156, 947–960. [Google Scholar] [CrossRef] [PubMed]
- Skeffington, K.L.; Higgins, J.S.; Mahmoud, A.D.; Evans, A.M.; Sferruzzi-Perri, A.N.; Fowden, A.L.; Yung, H.W.; Burton, G.J.; Giussani, D.A.; Moore, L.G. Hypoxia, AMPK activation and uterine artery vasoreactivity. J. Physiol. 2016, 594, 1357–1369. [Google Scholar] [CrossRef] [PubMed]
- Poudel, R.; Stanley, J.L.; Rueda-Clausen, C.F.; Andersson, I.J.; Sibley, C.P.; Davidge, S.T.; Baker, P.N. Effects of resveratrol in pregnancy using murine models with reduced blood supply to the uterus. PLoS ONE 2013, 8, e64401. [Google Scholar] [CrossRef] [PubMed]
- Perez-Perez, A.; Maymo, J.L.; Gambino, Y.P.; Guadix, P.; Duenas, J.L.; Varone, C.L.; Sanchez-Margalet, V. Activated translation signaling in placenta from pregnant women with gestational diabetes mellitus: Possible role of leptin. Horm. Metab. Res. 2013, 45, 436–442. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Gestational diabetes mellitus. Diabetes Care 2004, 27, S88–S90. [Google Scholar]
- Yao, L.; Wan, J.; Li, H.; Ding, J.; Wang, Y.; Wang, X.; Li, M. Resveratrol relieves gestational diabetes mellitus in mice through activating AMPK. Reprod. Biol. Endocrinol. 2015, 13, 118. [Google Scholar] [CrossRef] [PubMed]
- Brownfoot, F.C.; Hastie, R.; Hannan, N.J.; Cannon, P.; Tuohey, L.; Parry, L.J.; Senadheera, S.; Illanes, S.E.; Kaitu’u-Lino, T.J.; Tong, S. Metformin as a prevention and treatment for preeclampsia: Effects on soluble fms-like tyrosine kinase 1 and soluble endoglin secretion and endothelial dysfunction. Am. J. Obstet. Gynecol. 2016, 214, 356.e1–356.e15. [Google Scholar] [CrossRef] [PubMed]
- Koroglu, N.; Tola, E.; Temel Yuksel, I.; Aslan Cetin, B.; Turhan, U.; Topcu, G.; Dag, I. Maternal serum AMP-activated protein kinase levels in mild and severe preeclampsia. J. Matern. Fetal. Neonatal. Med. 2018, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Banek, C.T.; Bauer, A.J.; Needham, K.M.; Dreyer, H.C.; Gilbert, J.S. AICAR administration ameliorates hypertension and angiogenic imbalance in a model of preeclampsia in the rat. Am. J. Physiol. Heart. Circ. Physiol. 2013, 304, H1159–H1165. [Google Scholar] [CrossRef] [PubMed]
- Lim, R.; Barker, G.; Lappas, M. Activation of AMPK in human fetal membranes alleviates infection-induced expression of pro-inflammatory and pro-labour mediators. Placenta 2015, 36, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Cha, J.; Yuan, J.; Haraguchi, H.; Bartos, A.; Leishman, E.; Viollet, B.; Bradshaw, H.B.; Hirota, Y.; Dey, S.K. p53 coordinates decidual sestrin 2/AMPK/mTORC1 signaling to govern parturition timing. J. Clin. Investig. 2016, 126, 2941–2954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukami, T.; Sun, X.; Li, T.; Desai, M.; Ross, M.G. Mechanism of programmed obesity in intrauterine fetal growth restricted offspring: Paradoxically enhanced appetite stimulation in fed and fasting states. Reprod. Sci. 2012, 19, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.F.; Tang, S.J.; Shen, Z.; Wang, Y.M.; Liang, L. Growth hormone reverses dyslipidemia in adult offspring after maternal undernutrition. Sci. Rep. 2017, 7, 6038. [Google Scholar] [CrossRef] [PubMed]
- Crescenti, A.; del Bas, J.M.; Arola-Arnal, A.; Oms-Oliu, G.; Arola, L.; Caimari, A. Grape seed procyanidins administered at physiological doses to rats during pregnancy and lactation promote lipid oxidation and up-regulate AMPK in the muscle of male offspring in adulthood. J. Nutr. Biochem. 2015, 26, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Mukai, Y.; Sun, Y.; Sato, S. Azuki bean polyphenols intake during lactation upregulate AMPK in male rat offspring exposed to fetal malnutrition. Nutrition 2013, 29, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Unterscheider, J.; O’Donoghue, K.; Daly, S.; Geary, M.P.; Kennelly, M.M.; McAuliffe, F.M.; Hunter, A.; Morrison, J.J.; Burke, G.; Dicker, P.; et al. Fetal growth restriction and the risk of perinatal mortality-case studies from the multicentre PORTO study. BMC Pregnancy Childbirth 2014, 14, 63. [Google Scholar] [CrossRef] [PubMed]
- Longo, S.; Bollani, L.; Decembrino, L.; di Comite, A.; Angelini, M.; Stronati, M. Short-term and long-term sequelae in intrauterine growth retardation (IUGR). J. Matern. Fetal. Neonatal. Med. 2013, 26, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, J.V.; Rosario, F.J.; Nijland, M.J.; McDonald, T.J.; Wu, G.; Kanai, Y.; Powell, T.L.; Nathanielsz, P.W.; Jansson, T. Down-regulation of placental mTOR, insulin/IGF-I signaling, and nutrient transporters in response to maternal nutrient restriction in the baboon. FASEB J. 2014, 28, 1294–1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jansson, N.; Greenwood, S.L.; Johansson, B.R.; Powell, T.L.; Jansson, T. Leptin stimulates the activity of the system A amino acid transporter in human placental villous fragments. J. Clin. Endocrinol. Metab. 2003, 88, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Yiallourides, M.; Sebert, S.P.; Wilson, V.; Sharkey, D.; Rhind, S.M.; Symonds, M.E.; Budge, H. The differential effects of the timing of maternal nutrient restriction in the ovine placenta on glucocorticoid sensitivity, uncoupling protein 2, peroxisome proliferator-activated receptor-gamma and cell proliferation. Reproduction 2009, 138, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Jansson, N.; Pettersson, J.; Haafiz, A.; Ericsson, A.; Palmberg, I.; Tranberg, M.; Ganapathy, V.; Powell, T.L.; Jansson, T. Down-regulation of placental transport of amino acids precedes the development of intrauterine growth restriction in rats fed a low protein diet. J. Physiol. 2006, 576, 935–946. [Google Scholar] [CrossRef] [PubMed]
- Jansson, T.; Powell, T.L. Role of placental nutrient sensing in developmental programming. Clin. Obstet. Gynecol. 2013, 56, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Rosario, F.J.; Jansson, N.; Kanai, Y.; Prasad, P.D.; Powell, T.L.; Jansson, T. Maternal protein restriction in the rat inhibits placental insulin, mTOR, and STAT3 signaling and down-regulates placental amino acid transporters. Endocrinology 2011, 152, 1119–1129. [Google Scholar] [CrossRef] [PubMed]
- Dimasuay, K.G.; Boeuf, P.; Powell, T.L.; Jansson, T. Placental responses to changes in the maternal environment determine fetal growth. Front. Physiol. 2016, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Zamudio, S.; Moore, L.G. Altitude and fetal growth: Current knowledge and future directions. Ultrasound Obstet. Gynecol. 2000, 16, 6–8. [Google Scholar] [CrossRef] [PubMed]
- Giussani, D.A.; Salinas, C.E.; Villena, M.; Blanco, C.E. The role of oxygen in prenatal growth: Studies in the chick embryo. J. Physiol. 2007, 585, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.M.; Smith, S.D.; Furesz, T.C.; Sadovsky, Y.; Ganapathy, V.; Parvin, C.A.; Smith, C.H. Hypoxia reduces expression and function of system A amino acid transporters in cultured term human trophoblasts. Am. J. Physiol. Cell Physiol. 2003, 284, C310–C315. [Google Scholar] [CrossRef] [PubMed]
- Inoki, K.; Zhu, T.; Guan, K.L. TSC2 mediates cellular energy response to control cell growth and survival. Cell 2003, 115, 577–590. [Google Scholar] [CrossRef]
- Yung, H.W.; Calabrese, S.; Hynx, D.; Hemmings, B.A.; Cetin, I.; Charnock-Jones, D.S.; Burton, G.J. Evidence of placental translation inhibition and endoplasmic reticulum stress in the etiology of human intrauterine growth restriction. Am. J. Pathol. 2008, 173, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Ashwal, E.; Hod, M. Gestational diabetes mellitus: Where are we now? Clin. Chim. Acta 2015, 451, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Snider, F.; Cross, J.C. Prolactin receptor is required for normal glucose homeostasis and modulation of beta-cell mass during pregnancy. Endocrinology 2009, 150, 1618–1626. [Google Scholar] [CrossRef] [PubMed]
- Le, T.N.; Elsea, S.H.; Romero, R.; Chaiworapongsa, T.; Francis, G.L. Prolactin receptor gene polymorphisms are associated with gestational diabetes. Genet. Test. Mol. Biomark. 2013, 17, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Jauniaux, E.; Watson, A.L.; Hempstock, J.; Bao, Y.P.; Skepper, J.N.; Burton, G.J. Onset of maternal arterial blood flow and placental oxidative stress. A possible factor in human early pregnancy failure. Am. J. Pathol. 2000, 157, 2111–2122. [Google Scholar] [CrossRef]
- Bjork, O.; Persson, B.; Stangenberg, M.; Vaclavinkova, V. Spiral artery lesions in relation to metabolic control in diabetes mellitus. Acta Obstet. Gynecol. Scand. 1984, 63, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Desoye, G. The human placenta in diabetes and obesity: Friend or foe? The 2017 norbert freinkel award lecture. Diabetes Care 2018, 41, 1362–1369. [Google Scholar] [CrossRef] [PubMed]
- Salvesen, D.R.; Brudenell, J.M.; Snijders, R.J.; Ireland, R.M.; Nicolaides, K.H. Fetal plasma erythropoietin in pregnancies complicated by maternal diabetes mellitus. Am. J. Obstet. Gynecol. 1993, 168, 88–94. [Google Scholar] [CrossRef]
- Desoye, G.; Shafrir, E. Placental metabolism and its regulation in health and diabetes. Mol. Aspects Med. 1994, 15, 505–682. [Google Scholar] [CrossRef]
- Martino, J.; Sebert, S.; Segura, M.T.; Garcia-Valdes, L.; Florido, J.; Padilla, M.C.; Marcos, A.; Rueda, R.; McArdle, H.J.; Budge, H.; et al. Maternal body weight and gestational diabetes differentially influence placental and pregnancy outcomes. J. Clin. Endocrinol. Metab. 2016, 101, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.R.; Zhang, S.J.; Tsoi, B.; Huang, W.S.; Zhuang, X.J.; Chen, X.Y.; Yao, N.; Mao, Z.F.; Tang, L.P.; Wang, Q.; et al. A natural product, resveratrol, protects against high-glucose-induced developmental damage in chicken embryo. J. Asian Nat. Prod. Res. 2015, 17, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Liong, S.; Lappas, M. Activation of AMPK improves inflammation and insulin resistance in adipose tissue and skeletal muscle from pregnant women. J. Physiol. Biochem. 2015, 71, 703–717. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rodriguez, A.; Santamaria, B.; Mas-Gutierrez, J.A.; Rada, P.; Fernandez-Millan, E.; Pardo, V.; Alvarez, C.; Cuadrado, A.; Ros, M.; Serrano, M.; et al. Resveratrol treatment restores peripheral insulin sensitivity in diabetic mice in a sirt1-independent manner. Mol. Nutr. Food Res. 2015, 59, 1431–1442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.D.; Park, K.G.; Lee, Y.S.; Park, Y.Y.; Kim, D.K.; Nedumaran, B.; Jang, W.G.; Cho, W.J.; Ha, J.; Lee, I.K.; et al. Metformin inhibits hepatic gluconeogenesis through AMP-activated protein kinase-dependent regulation of the orphan nuclear receptor SHP. Diabetes 2008, 57, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Myers, R.; Li, Y.; Chen, Y.; Shen, X.; Fenyk-Melody, J.; Wu, M.; Ventre, J.; Doebber, T.; Fujii, N.; et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Investig. 2001, 108, 1167–1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egawa, T.; Hamada, T.; Kameda, N.; Karaike, K.; Ma, X.; Masuda, S.; Iwanaka, N.; Hayashi, T. Caffeine acutely activates 5′adenosine monophosphate-activated protein kinase and increases insulin-independent glucose transport in rat skeletal muscles. Metabolism 2009, 58, 1609–1617. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Seo, W.Y.; Song, K.H.; Chanda, D.; Kim, Y.D.; Kim, D.K.; Lee, M.W.; Ryu, D.; Kim, Y.H.; Noh, J.R.; et al. AMPK-dependent repression of hepatic gluconeogenesis via disruption of CREB.CRTC2 complex by orphan nuclear receptor small heterodimer partner. J. Biol. Chem. 2010, 285, 32182–32191. [Google Scholar] [CrossRef] [PubMed]
- Koh, H.J. Regulation of exercise-stimulated glucose uptake in skeletal muscle. Ann. Pediatr. Endocrinol. Metab. 2016, 21, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Witczak, C.A.; Sharoff, C.G.; Goodyear, L.J. AMP-activated protein kinase in skeletal muscle: From structure and localization to its role as a master regulator of cellular metabolism. Cell Mol. Life Sci. 2008, 65, 3737–3755. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Viana, M.; Thirumangalathu, S.; Loeken, M.R. AMP-activated protein kinase mediates effects of oxidative stress on embryo gene expression in a mouse model of diabetic embryopathy. Diabetologia 2012, 55, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Wei, D.; Loeken, M.R. Lack of metformin effect on mouse embryo AMPK activity: Implications for metformin treatment during pregnancy. Diabetes Metab. Res. Rev. 2014, 30, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Ahmadimoghaddam, D.; Zemankova, L.; Nachtigal, P.; Dolezelova, E.; Neumanova, Z.; Cerveny, L.; Ceckova, M.; Kacerovsky, M.; Micuda, S.; Staud, F. Organic cation transporter 3 (OCT3/SLC22A3) and multidrug and toxin extrusion 1 (MATE1/SLC47A1) transporter in the placenta and fetal tissues: Expression profile and fetus protective role at different stages of gestation. Biol. Reprod. 2013, 88, 55. [Google Scholar] [CrossRef] [PubMed]
- Ijas, H.; Vaarasmaki, M.; Morin-Papunen, L.; Keravuo, R.; Ebeling, T.; Saarela, T.; Raudaskoski, T. Metformin should be considered in the treatment of gestational diabetes: A prospective randomised study. BJOG 2011, 118, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Nanovskaya, T.N.; Nekhayeva, I.A.; Patrikeeva, S.L.; Hankins, G.D.; Ahmed, M.S. Transfer of metformin across the dually perfused human placental lobule. Am. J. Obstet. Gynecol. 2006, 195, 1081–1085. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Recommendations for Prevention and Treatment of Pre-Eclampsia and Eclampsia; WHO Press: Geneva, Switzerland, 2011; p. 38. ISBN 9789241548335. [Google Scholar]
- Gilbert, J.S.; Ryan, M.J.; LaMarca, B.B.; Sedeek, M.; Murphy, S.R.; Granger, J.P. Pathophysiology of hypertension during preeclampsia: Linking placental ischemia with endothelial dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H541–H550. [Google Scholar] [CrossRef] [PubMed]
- Conrad, K.P.; Benyo, D.F. Placental cytokines and the pathogenesis of preeclampsia. Am. J. Reprod. Immunol. 1997, 37, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.S.; Babcock, S.A.; Granger, J.P. Hypertension produced by reduced uterine perfusion in pregnant rats is associated with increased soluble fms-like tyrosine kinase-1 expression. Hypertension 2007, 50, 1142–1147. [Google Scholar] [CrossRef] [PubMed]
- Santner-Nanan, B.; Peek, M.J.; Khanam, R.; Richarts, L.; Zhu, E.; Fazekas de St Groth, B.; Nanan, R. Systemic increase in the ratio between Foxp3+ and IL-17-producing CD4+ T cells in healthy pregnancy but not in preeclampsia. J. Immunol. 2009, 183, 7023–7030. [Google Scholar] [CrossRef] [PubMed]
- Norris, W.; Nevers, T.; Sharma, S.; Kalkunte, S. Review: hCG, preeclampsia and regulatory T cells. Placenta 2011, 32, S182–S185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, S.; Nakashima, A.; Shima, T.; Ito, M. Th1/Th2/Th17 and regulatory T-cell paradigm in pregnancy. Am. J. Reprod. Immunol. 2010, 63, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Gualdoni, G.A.; Mayer, K.A.; Goschl, L.; Boucheron, N.; Ellmeier, W.; Zlabinger, G.J. The AMP analog AICAR modulates the Treg/Th17 axis through enhancement of fatty acid oxidation. FASEB J. 2016, 30, 3800–3809. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Recommendations on Interventions to Improve Preterm Birth Outcomes; WHO Press: Geneva, Switzerland, 2015; ISBN 9789241508988. [Google Scholar]
- Godfrey, K.M. Maternal regulation of fetal development and health in adult life. Eur. J. Obstet. Gynecol. Reprod. Biol. 1998, 78, 141–150. [Google Scholar] [CrossRef]
- Cao, K.; Zheng, A.; Xu, J.; Li, H.; Liu, J.; Peng, Y.; Long, J.; Zou, X.; Li, Y.; Chen, C.; et al. AMPK activation prevents prenatal stress-induced cognitive impairment: Modulation of mitochondrial content and oxidative stress. Free Radic. Biol. Med. 2014, 75, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Hsu, C.N. Developmental programming of the metabolic syndrome: Can we reprogram with resveratrol? Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Lin, Y.J.; Sheen, J.M.; Lin, I.C.; Yu, H.R.; Huang, L.T.; Hsu, C.N. Resveratrol prevents the combined maternal plus postweaning high-fat-diets-induced hypertension in male offspring. J. Nutr. Biochem. 2017, 48, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Kanasaki, K.; Palmsten, K.; Sugimoto, H.; Ahmad, S.; Hamano, Y.; Xie, L.; Parry, S.; Augustin, H.G.; Gattone, V.H.; Folkman, J.; et al. Deficiency in Catechol-O-methyltransferase and 2-methoxyoestradiol is associated with pre-eclampsia. Nature 2008, 453, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Htun, N.C.; Miyaki, K.; Song, Y.; Ikeda, S.; Shimbo, T.; Muramatsu, M. Association of the Catechol-O-methyl transferase gene Val158Met polymorphism with blood pressure and prevalence of hypertension: Interaction with dietary energy intake. Am. J. Hypertens. 2011, 24, 1022–1026. [Google Scholar] [CrossRef] [PubMed]
- Xiu, L.; Lin, M.; Liu, W.; Kong, D.; Liu, Z.; Zhang, Y.; Ouyang, P.; Liang, Y.; Zhong, S.; Chen, C.; et al. Association of DRD3, COMT, and SLC6A4 gene polymorphisms with type 2 diabetes in southern chinese: A hospital-based case-control study. Diabetes Technol. Ther. 2015, 17, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Kanasaki, M.; Srivastava, S.P.; Yang, F.; Xu, L.; Kudoh, S.; Kitada, M.; Ueki, N.; Kim, H.; Li, J.; Takeda, S.; et al. Deficiency in catechol-o-methyltransferase is linked to a disruption of glucose homeostasis in mice. Sci. Rep. 2017, 7, 7927. [Google Scholar] [CrossRef] [PubMed]
- Ueki, N.; Kanasaki, K.; Kanasaki, M.; Takeda, S.; Koya, D. Catechol-O-Methyltransferase Deficiency leads to hypersensitivity of the pressor response against angiotensin II. Hypertension 2017, 69, 1156–1164. [Google Scholar] [CrossRef] [PubMed]
- Deji, N.; Kume, S.; Araki, S.; Isshiki, K.; Araki, H.; Chin-Kanasaki, M.; Tanaka, Y.; Nishiyama, A.; Koya, D.; Haneda, M.; et al. Role of angiotensin II-mediated AMPK inactivation on obesity-related salt-sensitive hypertension. Biochem. Biophys. Res. Commun. 2012, 418, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, J.S.; Barreto-Torres, G.; Kuznetsov, A.V.; Khuchua, Z.; Javadov, S. Crosstalk between AMPK activation and angiotensin II-induced hypertrophy in cardiomyocytes: The role of mitochondria. J. Cell Mol. Med. 2014, 18, 709–720. [Google Scholar] [CrossRef] [PubMed]

| p-AMPK Levels | |||||||
|---|---|---|---|---|---|---|---|
| Maternal | |||||||
| Hypothalamus | Liver | Vessel | Placenta | Serum | Fetal Membrane | ||
| No complication | human | ||||||
| animal model | ↓ Ref. [11] | ||||||
| IUGR | human | ||||||
| animal model | ↓ Refs. [12,13] | ||||||
| GDM | human | ↓ Ref. [14] | ↓ Ref. [15] | ||||
| animal model | ↓ Ref. [16] | ||||||
| Preeclampsia | human | ↓ (indirect) Ref. [17] | ↓ Ref. [17] | ↑ Ref. [18] | |||
| animal model | ↓ (indirect) Refs. [13,19] | ||||||
| PTB | human | ↓ Ref. [20] | |||||
| animal model | ↓ Ref. [21] | ||||||
| Fetal | |||||||
| Offspring of complicated pregnancy | human | ||||||
| animal model | ↑ Ref. [22] | ↓ Ref. [23,24,25] | |||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumagai, A.; Itakura, A.; Koya, D.; Kanasaki, K. AMP-Activated Protein (AMPK) in Pathophysiology of Pregnancy Complications. Int. J. Mol. Sci. 2018, 19, 3076. https://doi.org/10.3390/ijms19103076
Kumagai A, Itakura A, Koya D, Kanasaki K. AMP-Activated Protein (AMPK) in Pathophysiology of Pregnancy Complications. International Journal of Molecular Sciences. 2018; 19(10):3076. https://doi.org/10.3390/ijms19103076
Chicago/Turabian StyleKumagai, Asako, Atsuo Itakura, Daisuke Koya, and Keizo Kanasaki. 2018. "AMP-Activated Protein (AMPK) in Pathophysiology of Pregnancy Complications" International Journal of Molecular Sciences 19, no. 10: 3076. https://doi.org/10.3390/ijms19103076