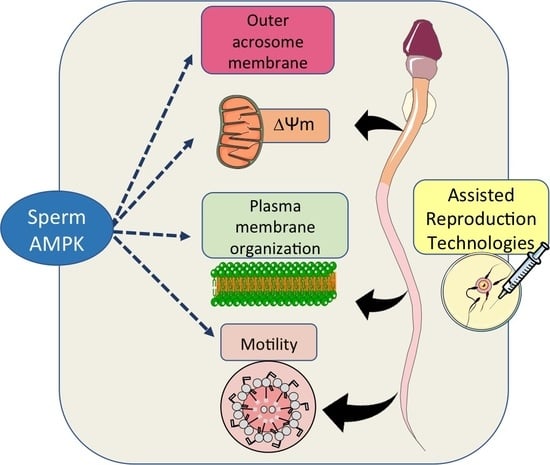
AMPK Function in Mammalian Spermatozoa
Abstract
:1. Introduction
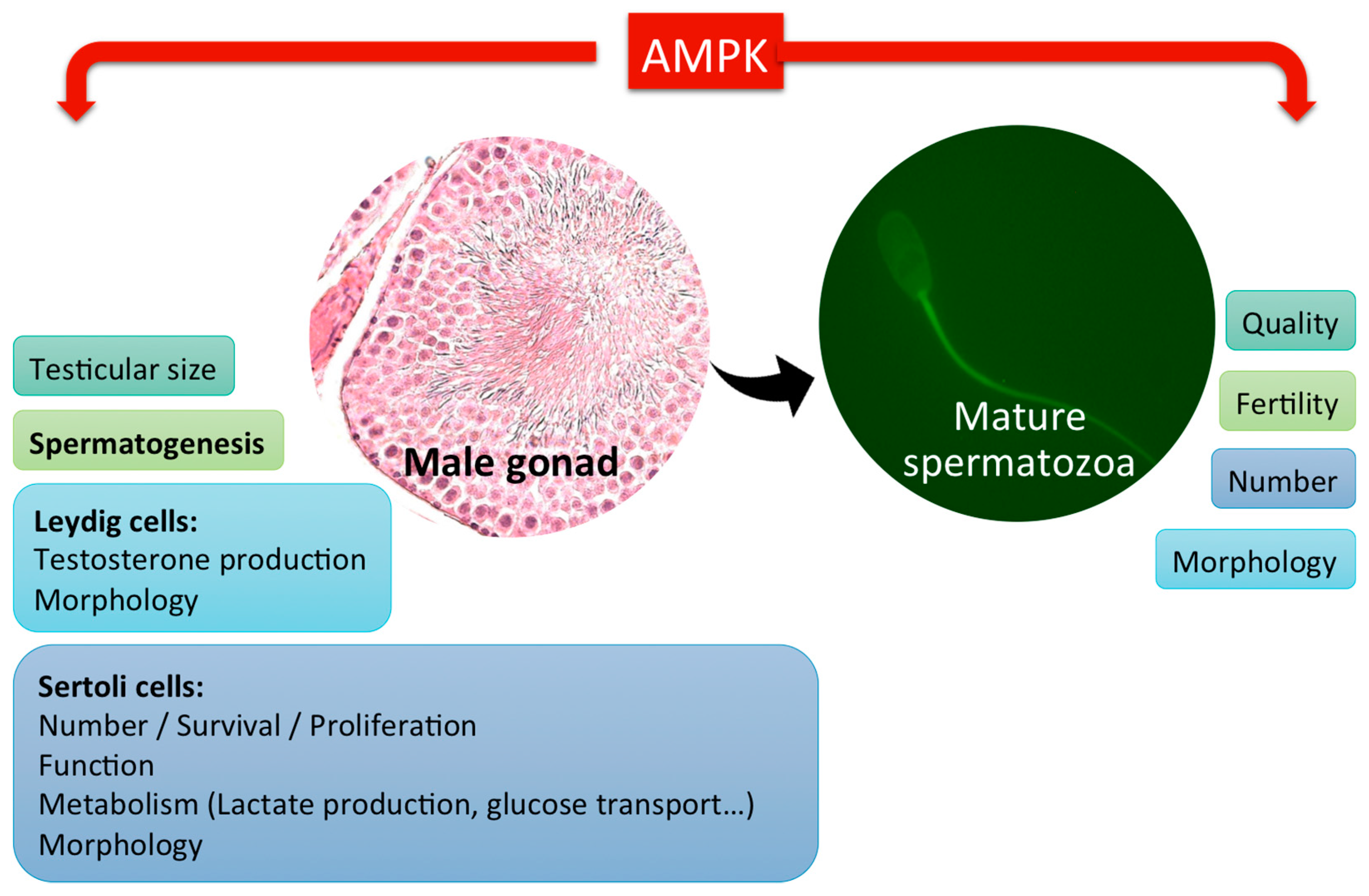
2. Role of AMPK in Male Gonads and Spermatogenesis
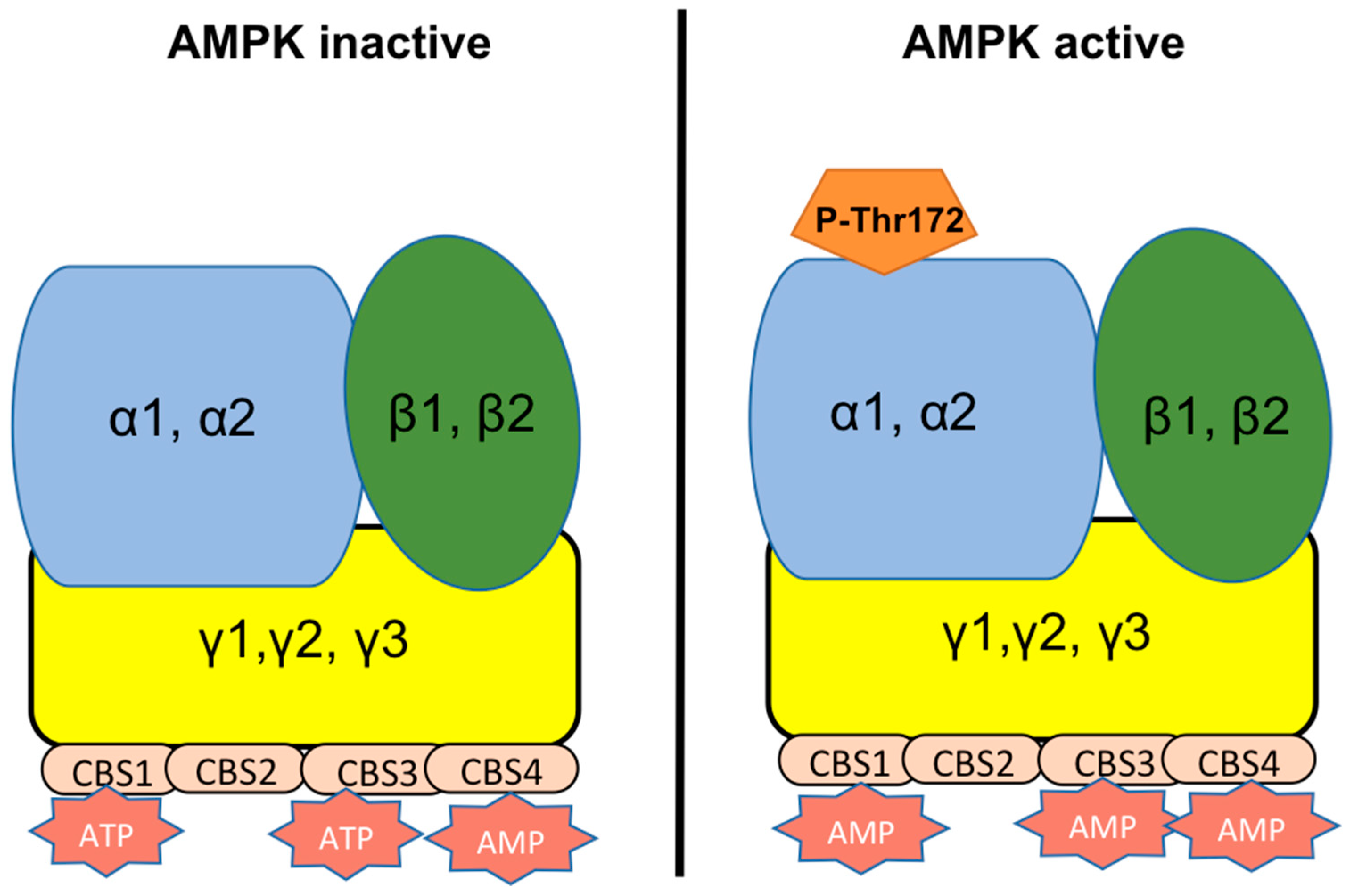
3. Localization of AMPK in Mammalian Spermatozoa
4. Functions of AMPK in Mammalian Spermatozoa
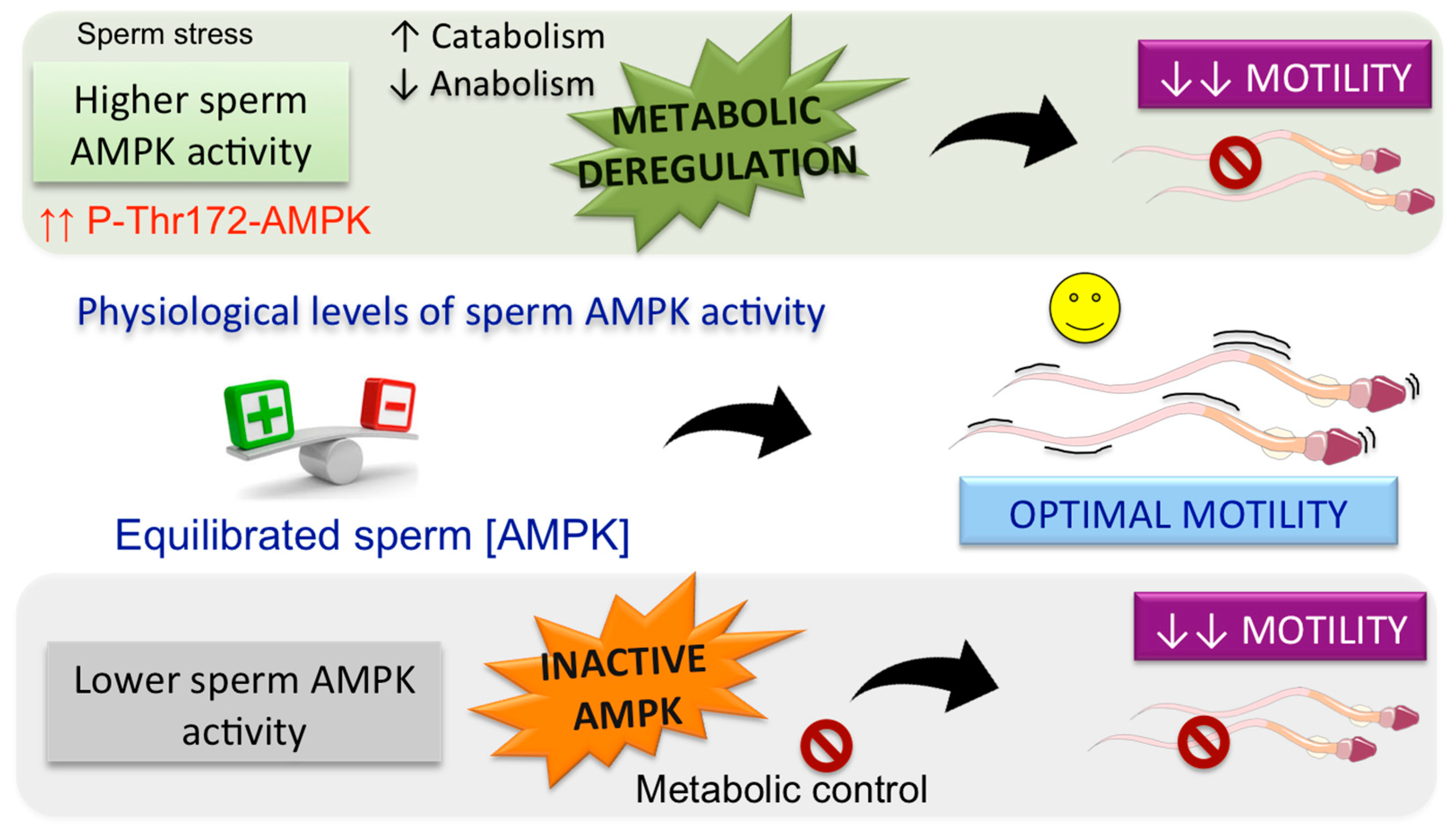
4.1. Role of AMPK in the Regulation of Spermatozoa Motility
4.2. Role of AMPK in the Regulation of Spermatozoa Mitochondrial Activity
4.3. Role of AMPK in the Regulation of Spermatozoa Membranes
4.4. Role of AMPK in the Regulation of Spermatozoa Acrosome Reaction
5. Signaling Pathways Leading to AMPK Activation in Spermatozoa
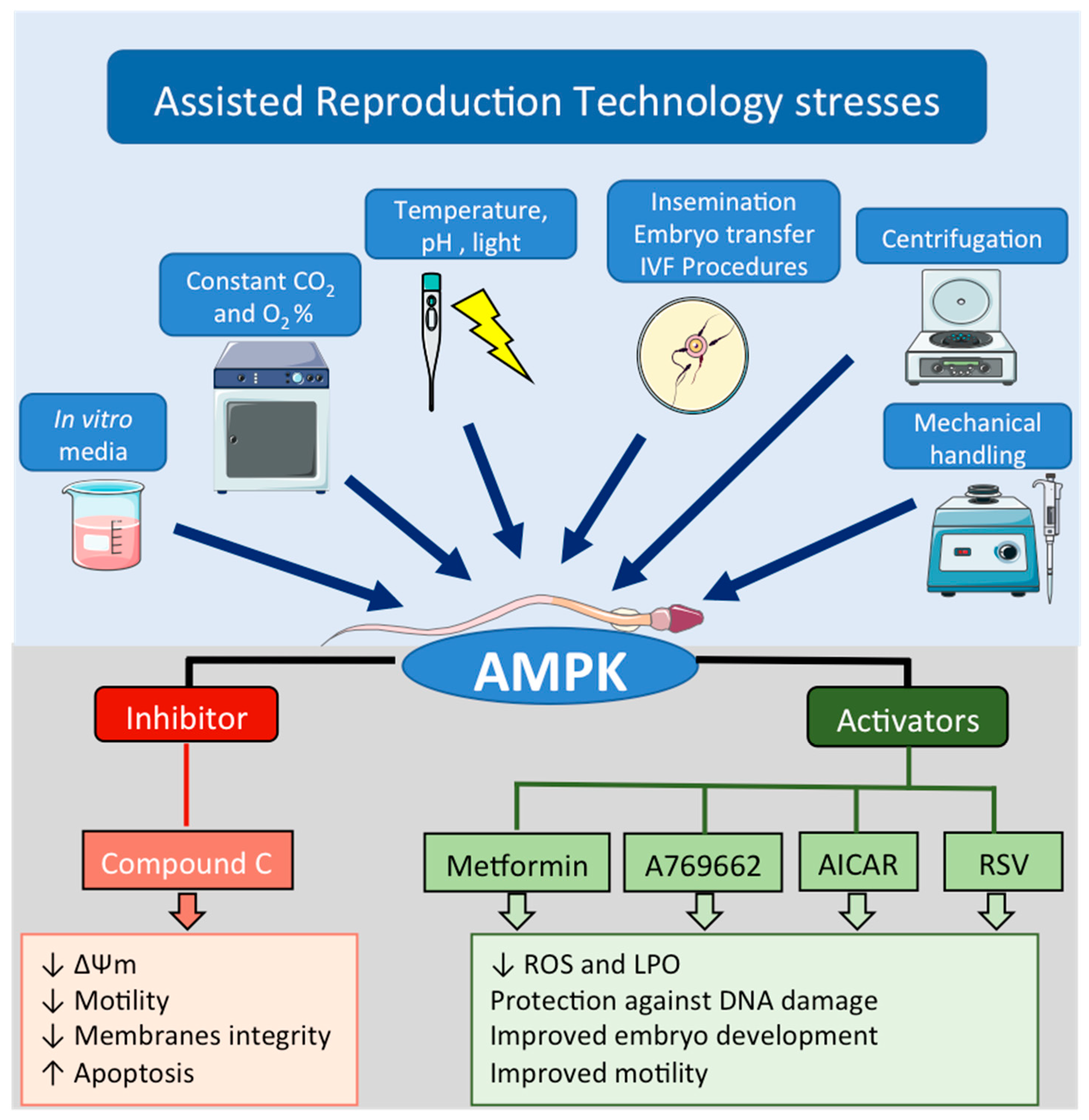
6. Role of AMPK during Assisted Reproduction Techniques: Semen Preservation
6.1. Why AMPK Protein Is Important in ART?
6.2. AMPK as a Tool to Improve ART
7. AMPK in Spermatozoa: A Physiological Link between Fertility and Energy Metabolism
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AMPK | AMP-activated protein kinase |
| ART | Assisted reproduction techniques |
| ICSI | Intracytoplasmatic sperm injection |
| IVF | In Vitro Fertilization |
| SC | Sertoli cells |
| LC | Leydig cells |
| sAC | Soluble adenylate cyclase |
| cAMP | Cyclic adenosine mono phosphate |
| PKA | cAMP-dependent protein kinase |
| PKC | Protein kinase C |
| LKB1 | Liver kinase B1 |
| CaMKKα/β | Ca2+-calmodulin dependent kinase kinases II |
| TSSK1/2 | Testis specific serine/threonine kinases 1/2 |
| AICAR | 5-aminoimidazol-4-carboxamide-1-B-D-ribofuranoside |
| CC | Compound C or dorsomorphin |
| ∆Ψm | Mitochondrial membrane potential |
| PMA | Phorbol 12-myristate 13-acetate |
| ROS | Reactive oxygen species |
| RSV | Resveratrol |
References
- Carling, D. AMPK signalling in health and disease. Curr. Opin. Cell Biol. 2017, 45, 31–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardie, D.G.; Schaffer, B.E.; Brunet, A. AMPK: An Energy-Sensing Pathway with Multiple Inputs and Outputs. Trends Cell Biol. 2016, 26, 190–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tartarin, P.; Guibert, E.; Toure, A.; Ouiste, C.; Leclerc, J.; Sanz, N.; Brière, S.; Dacheux, J.L.; Delaleu, B.; McNeilly, J.R.; et al. Inactivation of AMPKalpha1 induces asthenozoospermia and alters spermatozoa morphology. Endocrinology 2012, 153, 3468–3481. [Google Scholar] [CrossRef] [PubMed]
- Hurtado de Llera, A.; Martin-Hidalgo, D.; Gil, M.C.; Garcia-Marin, L.J.; Bragado, M.J. AMP-Activated Kinase AMPK Is Expressed in Boar Spermatozoa and Regulates Motility. PLoS ONE 2012, 7, e38840. [Google Scholar] [CrossRef] [PubMed]
- Handa, N.; Takagi, T.; Saijo, S.; Kishishita, S.; Takaya, D.; Toyama, M.; Terada, T.; Shirouzu, M.; Suzuki, A.; Lee, S.; et al. Structural basis for compound C inhibition of the human AMP-activated protein kinase alpha2 subunit kinase domain. Acta Crystallogr. D Biol. Crystallogr. 2011, 67, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Dite, T.A.; Langendorf, C.G.; Hoque, A.; Galic, S.; Rebello, R.J.; Ovens, A.J.; Lindqvist, L.M.; Ngoei, K.R.W.; Ling, N.X.Y.; Furic, L.; et al. AMP-activated protein kinase selectively inhibited by the type II inhibitor SBI-0206965. J. Biol. Chem. 2018, 293, 8874–8885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olivier, S.; Foretz, M.; Viollet, B. Promise and challenges for direct small molecule AMPK activators. Biochem. Pharmacol. 2018, 153, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Myers, R.; Li, Y.; Chen, Y.; Shen, X.; Fenyk-Melody, J.; Wu, M.; Ventre, J.; Doebber, T.; Fujii, N.; et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Investig. 2001, 108, 1167–1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouyang, J.; Parakhia, R.A.; Ochs, R.S. Metformin activates AMP kinase through inhibition of AMP deaminase. J. Biol. Chem. 2011, 286, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ben-Sahra, I.; Regazzetti, C.; Robert, G.; Laurent, K.; Le Marchand-Brustel, Y.; Auberger, P.; Tanti, J.F.; Giorgetti-Peraldi, S.; Bost, F. Metformin, independent of AMPK, induces mTOR inhibition and cell-cycle arrest through REDD1. Cancer Res. 2011, 71, 4366–4372. [Google Scholar] [CrossRef] [PubMed]
- Corominas-Faja, B.; Quirantes-Pine, R.; Oliveras-Ferraros, C.; Vazquez-Martin, A.; Cufí, S.; Martin-Castillo, B.; Micol, V.; Joven, J.; Segura-Carretero, A.; Menendez, J.A. Metabolomic fingerprint reveals that metformin impairs one-carbon metabolism in a manner similar to the antifolate class of chemotherapy drugs. Aging 2012, 4, 480–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, B.; Tannahill, G.M.; Murphy, M.P.; O’Neill, L.A. Metformin Inhibits the Production of Reactive Oxygen Species from NADH: Ubiquinone Oxidoreductase to Limit Induction of Interleukin-1beta (IL-1beta) and Boosts Interleukin-10 (IL-10) in Lipopolysaccharide (LPS)-activated Macrophages. J. Biol. Chem. 2015, 290, 20348–20359. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.A.; Birnbaum, M.J. An energetic tale of AMPK-independent effects of metformin. J. Clin. Investig. 2010, 120, 2267–2270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosilio, C.; Ben-Sahra, I.; Bost, F.; Peyron, J.F. Metformin: A metabolic disruptor and anti-diabetic drug to target human leukemia. Cancer Lett. 2014, 346, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, R.; Parsons, H.L.; Wambolt, R.B.; Paulson, K.; Sharma, V.; Dyck, J.R.; Brownsey, R.W.; Allard, M.F. Metabolic actions of metformin in the heart can occur by AMPK-independent mechanisms. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H2497–H2506. [Google Scholar] [CrossRef] [PubMed]
- Scotland, S.; Saland, E.; Skuli, N.; de Toni, F.; Boutzen, H.; Micklow, E.; Sénégas, I.; Peyraud, R.; Peyriga, L.; Théodoro, F.; et al. Mitochondrial energetic and AKT status mediate metabolic effects and apoptosis of metformin in human leukemic cells. Leukemia 2013, 27, 2129–2138. [Google Scholar] [CrossRef] [PubMed]
- Owen, M.R.; Doran, E.; Halestrap, A.P. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem. J. 2000, 348, 607–614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Mir, M.Y.; Nogueira, V.; Fontaine, E.; Averet, N.; Rigoulet, M.; Leverve, X. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J. Biol. Chem. 2000, 275, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Bertoldo, M.J.; Faure, M.; Dupont, J.; Froment, P. AMPK: A master energy regulator for gonadal function. Front. Neurosci. 2015, 9, 235. [Google Scholar] [CrossRef] [PubMed]
- Towler, M.C.; Fogarty, S.; Hawley, S.A.; Pan, D.A.; Martin, D.M.; Morrice, N.A.; McCarthy, A.; Galardo, M.N.; Meroni, S.B.; Cigorraga, S.B.; et al. A novel short splice variant of the tumour suppressor LKB1 is required for spermiogenesis. Biochem. J. 2008, 416, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, F.; Wang, M.; Huang, X.; Yue, Q.; Wei, X.; Dou, X.; Peng, X.; Jia, Y.; Zheng, K.; Wu, T.; et al. Differential regulation of spermatogenic process by Lkb1 isoforms in mouse testis. Cell Death Dis. 2017, 8, e3121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, B.; Hao, Z.; Jha, K.N.; Zhang, Z.; Urekar, C.; Digilio, L.; Pulido, S.; Strauss, J.F., 3rd; Flickinger, C.J.; Herr, J.C. Targeted deletion of Tssk1 and 2 causes male infertility due to haploinsufficiency. Dev. Biol. 2008, 319, 211–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russell, L.D.; Tallon-Doran, M.; Weber, J.E.; Wong, V.; Peterson, R.N. Three-dimensional reconstruction of a rat stage V Sertoli cell: III. A study of specific cellular relationships. Am. J. Anat. 1983, 167, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Crisostomo, L.; Alves, M.G.; Gorga, A.; Sousa, M.; Riera, M.F.; Galardo, M.N.; Meroni, S.B.; Oliveira, P.F. Molecular Mechanisms and Signaling Pathways Involved in the Nutritional Support of Spermatogenesis by Sertoli Cells. Methods Mol. Biol. 2018, 1748, 129–155. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Chen, Z.; Wang, C.; Xiong, Z.; Zhao, W.; Jia, C.; Lin, J.; Lin, Y.; Yuan, W.; Zhao, A.Z.; et al. Rictor Regulates Spermatogenesis by Controlling Sertoli Cell Cytoskeletal Organization and Cell Polarity in the Mouse Testis. Endocrinology 2015, 156, 4244–4256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanwar, P.S.; Zhang, L.; Teixeira, J.M. Adenomatous polyposis coli (APC) is essential for maintaining the integrity of the seminiferous epithelium. Mol. Endocrinol. 2011, 25, 1725–1739. [Google Scholar] [CrossRef] [PubMed]
- Tanwar, P.S.; Kaneko-Tarui, T.; Zhang, L.; Teixeira, J.M. Altered LKB1/AMPK/TSC1/TSC2/mTOR signaling causes disruption of Sertoli cell polarity and spermatogenesis. Hum. Mol. Genet. 2012, 21, 4394–4405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riera, M.F.; Regueira, M.; Galardo, M.N.; Pellizzari, E.H.; Meroni, S.B.; Cigorraga, S.B. Signal transduction pathways in FSH regulation of rat Sertoli cell proliferation. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E914–E923. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.R.; Liao, T.T.; Bao, Z.Q.; Zhou, C.Q.; Luo, H.Y.; Lu, C.; Pan, M.H.; Wang, X.Z. Role of AMPK in the expression of tight junction proteins in heat-treated porcine Sertoli cells. Theriogenology 2018, 121, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Galardo, M.N.; Riera, M.F.; Pellizzari, E.H.; Sobarzo, C.; Scarcelli, R.; Denduchis, B.; Lustig, L.; Cigorraga, S.B.; Meroni, S.B. Adenosine regulates Sertoli cell function by activating AMPK. Mol. Cell. Endocrinol. 2010, 330, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Galardo, M.N.; Riera, M.F.; Pellizzari, E.H.; Cigorraga, S.B.; Meroni, S.B. The AMP-activated protein kinase activator, 5-aminoimidazole-4-carboxamide-1-b-D-ribonucleoside, regulates lactate production in rat Sertoli cells. J. Mol. Endocrinol. 2007, 39, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Bertoldo, M.J.; Guibert, E.; Faure, M.; Guillou, F.; Ramé, C.; Nadal-Desbarats, L.; Foretz, M.; Viollet, B.; Dupont, J.; Froment, P. Specific deletion of AMP-activated protein kinase (alpha1AMPK) in mouse Sertoli cells modifies germ cell quality. Mol. Cell. Endocrinol. 2016, 423, 96–112. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Z.J.; Yi, W.; Rong, Y.W.; Kee, J.D.; Zhong, W.X. MicroRNA-1285 Regulates 17beta-Estradiol-Inhibited Immature Boar Sertoli Cell Proliferation via Adenosine Monophosphate-Activated Protein Kinase Activation. Endocrinology 2015, 156, 4059–4070. [Google Scholar] [CrossRef] [PubMed]
- Faure, M.; Guibert, E.; Alves, S.; Pain, B.; Ramé, C.; Dupont, J.; Brillard, J.P.; Froment, P. The insulin sensitiser metformin regulates chicken Sertoli and germ cell populations. Reproduction 2016, 151, 527–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paillamanque, J.; Sanchez-Tusie, A.; Carmona, E.M.; Trevino, C.L.; Sandoval, C.; Nualart, F.; Osses, N.; Reyes, J.G. Arachidonic acid triggers [Ca2+]i increases in rat round spermatids by a likely GPR activation, ERK signalling and ER/acidic compartments Ca2+ release. PLoS ONE 2017, 12, e0172128. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, P.F.; Sousa, M.; Barros, A.; Moura, T.; Rebelo da Costa, A. Intracellular pH regulation in human Sertoli cells: Role of membrane transporters. Reproduction 2009, 137, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Rato, L.; Alves, M.G.; Socorro, S.; Duarte, A.I.; Cavaco, J.E.; Oliveira, P.F. Metabolic regulation is important for spermatogenesis. Nat. Rev. Urol. 2012, 9, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.L.; Guan, J.Y.; Ding, J.; Huang, S.; Lian, Y.; Luo, H.Y.; Wang, X.Z. AMP-activated protein kinase negatively regulates heat treatment-induced lactate secretion in cultured boar sertoli cells. Theriogenology 2018, 121, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Teerds, K.J.; Huhtaniemi, I.T. Morphological and functional maturation of Leydig cells: From rodent models to primates. Hum. Reprod. Update 2015, 21, 310–328. [Google Scholar] [CrossRef] [PubMed]
- Abdou, H.S.; Bergeron, F.; Tremblay, J.J. A cell-autonomous molecular cascade initiated by AMP-activated protein kinase represses steroidogenesis. Mol. Cell. Biol. 2014, 34, 4257–4271. [Google Scholar] [CrossRef] [PubMed]
- Taibi, N.; Dupont, J.; Bouguermouh, Z.; Froment, P.; Ramé, C.; Anane, A.; Amirat, Z.; Khammar, F. Expression of adenosine 5′-monophosphate-Activated protein kinase (AMPK) in ovine testis (Ovis aries): In vivo regulation by nutritional state. Anim. Reprod. Sci. 2017, 178, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Sun, Y.; Li, Z. Resveratrol protects Leydig cells from nicotine-induced oxidative damage through enhanced autophagy. Clin. Exp. Pharmacol. Physiol. 2018, 45, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Dong, Y.; Tian, E.; Xie, L.; Wang, G.; Li, X.; Chen, X.; Chen, Y.; Lv, Y.; Ni, C.; et al. 4-Bromodiphenyl ether delays pubertal Leydig cell development in rats. Chemosphere 2018, 211, 986–997. [Google Scholar] [CrossRef] [PubMed]
- Cheung, P.C.; Salt, I.P.; Davies, S.P.; Hardie, D.G.; Carling, D. Characterization of AMP-activated protein kinase gamma-subunit isoforms and their role in AMP binding. Biochem. J. 2000, 346, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Duan, P.; Hu, C.; Quan, C.; Yu, T.; Zhou, W.; Yuan, M.; Shi, Y.; Yang, K. 4-Nonylphenol induces apoptosis, autophagy and necrosis in Sertoli cells: Involvement of ROS-mediated AMPK/AKT-mTOR and JNK pathways. Toxicology 2016, 341–343, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Duan, P.; Hu, C.; Quan, C.; Yu, T.; Huang, W.; Chen, W.; Tang, S.; Shi, Y.; Martin, F.L.; Yang, K. 4-Nonylphenol induces autophagy and attenuates mTOR-p70S6K/4EBP1 signaling by modulating AMPK activation in Sertoli cells. Toxicol. Lett. 2017, 267, 21–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Z.; Yan, X.; Wang, L.; Wu, J.; Jing, X.; Liu, J. Effect of Telmisartan or Insulin on the Expression of Adiponectin and its Receptors in the Testis of Streptozotocin-Induced Diabetic Rats. Horm. Metab. Res. 2016, 48, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Hallows, K.R.; Alzamora, R.; Li, H.; Gong, F.; Smolak, C.; Neumann, D.; Pastor-Soler, N.M. AMP-activated protein kinase inhibits alkaline pH- and PKA-induced apical vacuolar H+-ATPase accumulation in epididymal clear cells. Am. J. Physiol. Cell Physiol. 2009, 296, C672–C681. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.W.; Nedumaran, B.; Xie, Y.; Kim, D.K.; Kim, Y.D.; Choi, H.S. Bisphenol A bis(2,3-dihydroxypropyl) ether (BADGE.2H2O) induces orphan nuclear receptor Nur77 gene expression and increases steroidogenesis in mouse testicular Leydig cells. Mol. Cells 2008, 26, 74–80. [Google Scholar] [PubMed]
- Cheng, Y.; Chen, G.; Wang, L.; Kong, J.; Pan, J.; Xi, Y.; Shen, F.; Huang, Z. Triptolide-induced mitochondrial damage dysregulates fatty acid metabolism in mouse sertoli cells. Toxicol. Lett. 2018, 292, 136–150. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Luo, X.; Zhu, Y.; Zhao, L.; Li, L.; Peng, Q.; Ma, M.; Gao, Y. ATM signals to AMPK to promote autophagy and positively regulate DNA damage in response to cadmium-induced ROS in mouse spermatocytes. Environ. Pollut. 2017, 231, 1560–1568. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Fu, J.; Zhang, S.; Zhao, J.; Xie, N.; Cai, G. The proteasome inhibitor bortezomib induces testicular toxicity by upregulation of oxidative stress, AMP-activated protein kinase (AMPK) activation and deregulation of germ cell development in adult murine testis. Toxicol. Appl. Pharmacol. 2015, 285, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Mancilla, H.; Maldonado, R.; Cereceda, K.; Villarroel-Espindola, F.; Montes de Oca, M.; Angulo, C.; Castro, M.A.; Slebe, J.C.; Vera, J.C.; Lavandero, S.; et al. Glutathione Depletion Induces Spermatogonial Cell Autophagy. J. Cell. Biochem. 2015, 116, 2283–2292. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Xu, B.; Fan, W.; Zhu, X.; Wang, G.; Zhang, A. Adiponectin protects Leydig cells against proinflammatory cytokines by suppressing the nuclear factor-kappaB signaling pathway. FEBS J. 2013, 280, 3920–3927. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhu, Y.; Shi, Y.; Han, Y.; Liang, C.; Feng, Z.; Zheng, H.; Eng, M.; Wang, J. Fluoride-Induced Autophagy via the Regulation of Phosphorylation of Mammalian Targets of Rapamycin in Mice Leydig Cells. J. Agric. Food Chem. 2017, 65, 8966–8976. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Li, F.; Feng, X.; Yang, H.; Han, L.; Fan, Y.; Nie, H.; Wang, Z.; Wang, F.; Zhang, Y. Influences of different dietary energy level on sheep testicular development associated with AMPK/ULK1/autophagy pathway. Theriogenology 2018, 108, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Ham, S.; Brown, K.A.; Simpson, E.R.; Meachem, S.J. Immunolocalisation of aromatase regulators liver kinase B1, phosphorylated AMP-activated protein kinase and cAMP response element-binding protein-regulated transcription co-activators in the human testis. Reprod. Fertil. Dev. 2016. [Google Scholar] [CrossRef] [PubMed]
- Vadnais, M.L.; Kirkwood, R.N.; Tempelman, R.J.; Sprecher, D.J.; Chou, K. Effect of cooling and seminal plasma on the capacitation status of fresh boar sperm as determined using chlortetracycline assay. Anim. Reprod. Sci. 2005, 87, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Cordova, A.; Strobel, P.; Vallejo, A.; Valenzuela, P.; Ulloa, O.; Burgos, R.A.; Menarim, B.; Rodríguez-Gil, J.E.; Ratto, M.; Ramírez-Reveco, A. Use of hypometabolic TRIS extenders and high cooling rate refrigeration for cryopreservation of stallion sperm: Presence and sensitivity of 5’ AMP-activated protein kinase (AMPK). Cryobiology 2014, 69, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Swegen, A.; Lambourne, S.R.; Aitken, R.J.; Gibb, Z. Rosiglitazone Improves Stallion Sperm Motility, ATP Content, and Mitochondrial Function. Biol. Reprod. 2016, 95, 107. [Google Scholar] [CrossRef] [PubMed]
- Kumar, L.; Yadav, S.K.; Kushwaha, B.; Pandey, A.; Sharma, V.; Verma, V.; Maikhuri, J.P.; Rajender, S.; Sharma, V.L.; Gupta, G. Energy Utilization for Survival and Fertilization-Parsimonious Quiescent Sperm Turn Extravagant on Motility Activation in Rat. Biol. Reprod. 2016, 94, 96. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Li, R.; Ma, G.; Bai, W.; Fan, X.; Lv, Y.; Luo, J.; Zeng, W. 5′-AMP-Activated Protein Kinase Regulates Goat Sperm Functions via Energy Metabolism in Vitro. Cell. Physiol. Biochem. 2018, 47, 2420–2431. [Google Scholar] [CrossRef] [PubMed]
- Calle-Guisado, V.; Hurtado de Llera, A.; Martin-Hidalgo, D.; Mijares, J.; Gil, M.C.; Alvarez, I.S.; Bragado, M.J.; Garcia-Marin, L.J. AMP-activated kinase in human spermatozoa: Identification, intracellular localization, and key function in the regulation of sperm motility. Asian J. Androl. 2016, 185, 848. [Google Scholar]
- Shabani, N.M.; Amidi, F.; Sedighi Gilani, M.A.; Aleyasin, A.; Bakhshalizadeh, S.; Naji, M.; Nekoonam, S. Protective features of resveratrol on human spermatozoa cryopreservation may be mediated through 5′ AMP-activated protein kinase activation. Andrology 2017, 5, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Hurtado de Llera, A.; Martin-Hidalgo, D.; Rodriguez-Gil, J.E.; Gil, M.C.; Garcia-Marin, L.J.; Bragado, M.J. AMP-activated kinase, AMPK, is involved in the maintenance of plasma membrane organization in boar spermatozoa. Biochim. Biophys. Acta 2013, 1828, 2143–2151. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.M.; Alves, S.; Grasseau, I.; Metayer-Coustard, S.; Praud, C.; Froment, P.; Blesbois, E. Central role of 5′-AMP-activated protein kinase in chicken sperm functions. Biol. Reprod. 2014, 91, 121. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Hao, Z.; Jha, K.N.; Digilio, L.; Urekar, C.; Kim, Y.H.; Pulido, S.; Flickinger, C.J.; Herr, J.C. Validation of a testis specific serine/threonine kinase [TSSK] family and the substrate of TSSK1 & 2, TSKS, as contraceptive targets. Soc. Reprod. Fertil. Suppl. 2007, 63, 87–101. [Google Scholar] [PubMed]
- Zhang, Z.; Kostetskii, I.; Tang, W.; Haig-Ladewig, L.; Sapiro, R.; Wei, Z.; Patel, A.M.; Bennett, J.; Gerton, G.L.; Moss, S.B.; et al. Deficiency of SPAG16L causes male infertility associated with impaired sperm motility. Biol. Reprod. 2006, 74, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Cool, B.; Zinker, B.; Chiou, W.; Kifle, L.; Cao, N.; Perham, M.; Dickinson, R.; Adler, A.; Gagne, G.; Iyengar, R.; et al. Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metab. 2006, 3, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Hurtado de Llera, A.; Martin-Hidalgo, D.; Gil, M.C.; Garcia-Marin, L.J.; Bragado, M.J. AMPK up-activation reduces motility and regulates other functions of boar spermatozoa. Mol. Hum. Reprod. 2015, 21, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Calle-Guisado, V.; de Hurtado, L.A.; Gonzalez-Fernandez, L.; Bragado, M.J.; Garcia-Marin, L.J. Human sperm motility is downregulated by the AMPK activator A769662. Andrology 2017, 5, 1131–1140. [Google Scholar] [CrossRef] [PubMed]
- De Hurtado, L.A.; Martin-Hidalgo, D.; Gil, M.C.; Garcia-Marin, L.J.; Bragado, M.J. New insights into transduction pathways that regulate boar sperm function. Theriogenology 2016, 85, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, W.D.; Liu, X.Q.; Zhang, P.F.; Hao, Y.N.; Li, L.; Chen, L.; Shen, W.; Tang, X.F.; Min, L.J.; et al. Hydrogen Sulfide and/or Ammonia Reduces Spermatozoa Motility through AMPK/AKT Related Pathways. Sci. Rep. 2016, 6, 37884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertoldo, M.J.; Guibert, E.; Tartarin, P.; Guillory, V.; Ramé, C.; Nadal-Desbarats, L.; Foretz, M.; Viollet, B.; Dupont, J.; Froment, P. Effect of metformin on the fertilizing ability of mouse spermatozoa. Cryobiology 2014, 68, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Harayama, H. Roles of intracellular cyclic AMP signal transduction in the capacitation and subsequent hyperactivation of mouse and boar spermatozoa. J. Reprod. Dev. 2013, 59, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Yanagimachi, R. Mammalian fertilization. In The Physiology of Reproduction; Knobil, E., Neil, J.D., Eds.; Raven Press: New York, NY, USA, 1994; pp. 189–317. [Google Scholar]
- Martin-Hidalgo, D.; Hurtado de Llera, A.; Yeste, M.; Gil, M.C.; Bragado, M.J.; Garcia-Marin, L.J. Adenosine monophosphate-activated kinase, AMPK, is involved in the maintenance of the quality of extended boar semen during long-term storage. Theriogenology 2013. [Google Scholar] [CrossRef] [PubMed]
- Gadella, B.M.; Harrison, R.A. The capacitating agent bicarbonate induces protein kinase A-dependent changes in phospholipid transbilayer behavior in the sperm plasma membrane. Development 2000, 127, 2407–2420. [Google Scholar] [PubMed]
- Hurtado de Llera, A.; Martin-Hidalgo, D.; Gil, M.C.; Garcia-Marin, L.J.; Bragado, M.J. The calcium/CaMKKalpha/beta and the cAMP/PKA pathways are essential upstream regulators of AMPK activity in boar spermatozoa. Biol. Reprod. 2014, 90, 29. [Google Scholar] [CrossRef] [PubMed]
- Gadella, B.M.; van Gestel, R.A. Bicarbonate and its role in mammalian sperm function. Anim. Reprod. Sci. 2004, 82–83, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.A. Rapid PKA-catalysed phosphorylation of boar sperm proteins induced by the capacitating agent bicarbonate. Mol. Reprod. Dev. 2004, 67, 337–352. [Google Scholar] [CrossRef] [PubMed]
- Garrett, L.J.; Revell, S.G.; Leese, H.J. Adenosine triphosphate production by bovine spermatozoa and its relationship to semen fertilizing ability. J. Androl. 2008, 29, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Miki, K. Energy metabolism and sperm function. Soc. Reprod. Fertil. Suppl. 2007, 65, 309–325. [Google Scholar] [PubMed]
- Nguyen, T.M.; Duittoz, A.; Praud, C.; Combarnous, Y.; Blesbois, E. Calcium channels in chicken sperm regulate motility and the acrosome reaction. FEBS J. 2016, 283, 1902–1920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woods, A.; Johnstone, S.R.; Dickerson, K.; Leiper, F.C.; Fryer, L.G.; Neumann, D.; Schlattner, U.; Wallimann, T.; Carlson, M.; Carling, D. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr. Biol. 2003, 13, 2004–2008. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.P.; Reoma, J.L.; Gamm, D.M.; Uhler, M.D. LKB1, a novel serine/threonine protein kinase and potential tumour suppressor, is phosphorylated by cAMP-dependent protein kinase (PKA) and prenylated in vivo. Biochem. J. 2000, 345, 673–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sapkota, G.P.; Kieloch, A.; Lizcano, J.M.; Lain, S.; Arthur, J.S.; Williams, M.R.; Morrice, N.; Deak, M.; Alessi, D.R. Phosphorylation of the protein kinase mutated in Peutz-Jeghers cancer syndrome, LKB1/STK11, at Ser431 by p90(RSK) and cAMP-dependent protein kinase, but not its farnesylation at Cys(433), is essential for LKB1 to suppress cell vrowth. J. Biol. Chem. 2001, 276, 19469–19482. [Google Scholar] [CrossRef] [PubMed]
- Denison, F.C.; Smith, L.B.; Muckett, P.J.; O’Hara, L.; Carling, D.; Woods, A. LKB1 is an essential regulator of spermatozoa release during spermiation in the mammalian testis. PLoS ONE 2011, 6, e28306. [Google Scholar] [CrossRef] [PubMed]
- Omar, B.; Zmuda-Trzebiatowska, E.; Manganiello, V.; Goransson, O.; Degerman, E. Regulation of AMP-activated protein kinase by cAMP in adipocytes: Roles for phosphodiesterases, protein kinase B, protein kinase A, Epac and lipolysis. Cell Signal. 2009, 21, 760–766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Wang, L.; Li, Y.; Zhao, N.; Zhen, L.; Fu, J.; Yang, Q. Calcium regulates motility and protein phosphorylation by changing cAMP and ATP concentrations in boar sperm in vitro. Anim. Reprod. Sci. 2016, 172, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.M.; Combarnous, Y.; Praud, C.; Duittoz, A.; Blesbois, E. Ca2+/Calmodulin-Dependent Protein Kinase Kinases (CaMKKs) Effects on AMP-Activated Protein Kinase (AMPK) Regulation of Chicken Sperm Functions. PLoS ONE 2016, 11, e0147559. [Google Scholar] [CrossRef] [PubMed]
- Hawley, S.A.; Pan, D.A.; Mustard, K.J.; Ross, L.; Bain, J.; Edelman, A.M.; Frenguelli, B.G.; Hardie, D.G. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005, 2, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Woods, A.; Dickerson, K.; Heath, R.; Hong, S.P.; Momcilovic, M.; Johnstone, S.R.; Carlson, M.; Carling, D. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005, 2, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Breitbart, H.; Naor, Z. Protein kinases in mammalian sperm capacitation and the acrosome reaction. Rev. Reprod. 1999, 4, 151–159. [Google Scholar] [CrossRef] [PubMed]
- NagDas, S.K.; Winfrey, V.P.; Olson, G.E. Identification of ras and its downstream signaling elements and their potential role in hamster sperm motility. Biol. Reprod. 2002, 67, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Jungnickel, M.K.; Sutton, K.A.; Wang, Y.; Florman, H.M. Phosphoinositide-dependent pathways in mouse sperm are regulated by egg ZP3 and drive the acrosome reaction. Dev. Biol. 2007, 304, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Dong, Y.; Zhang, J.; Scholz, R.; Neumann, D.; Zou, M.H. Identification of the serine 307 of LKB1 as a novel phosphorylation site essential for its nucleocytoplasmic transport and endothelial cell angiogenesis. Mol. Cell. Biol. 2009, 29, 3582–3596. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Moriasi, C.M.; Zhang, M.; Zhao, Y.; Zou, M.H. Phosphorylation of Serine 399 in LKB1 Protein Short Form by Protein Kinase Czeta Is Required for Its Nucleocytoplasmic Transport and Consequent AMP-activated Protein Kinase (AMPK) Activation. J. Biol. Chem. 2013, 288, 16495–16505. [Google Scholar] [CrossRef] [PubMed]
- Bragado, M.J.; Aparicio, I.M.; Gil, M.C.; Garcia-Marin, L.J. Protein kinases A and C and phosphatidylinositol 3 kinase regulate glycogen synthase kinase-3A serine 21 phosphorylation in boar spermatozoa. J. Cell. Biochem. 2010, 109, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Harayama, H.; Miyake, M. A cyclic adenosine 3′,5′-monophosphate-dependent protein kinase C activation is involved in the hyperactivation of boar spermatozoa. Mol. Reprod. Dev. 2006, 73, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Emerling, B.M.; Weinberg, F.; Snyder, C.; Burgess, Z.; Mutlu, G.M.; Viollet, B.; Budinger, G.R.; Chandel, N.S. Hypoxic activation of AMPK is dependent on mitochondrial ROS but independent of an increase in AMP/ATP ratio. Free Radic. Biol. Med. 2009, 46, 1386–1391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Lamirande, E.; Lamothe, G.; Villemure, M. Control of superoxide and nitric oxide formation during human sperm capacitation. Free Radic. Biol. Med. 2009, 46, 1420–1427. [Google Scholar] [CrossRef] [PubMed]
- Zmijewski, J.W.; Banerjee, S.; Bae, H.; Friggeri, A.; Lazarowski, E.R.; Abraham, E. Exposure to hydrogen peroxide induces oxidation and activation of AMP-activated protein kinase. J. Biol. Chem. 2010, 285, 33154–33164. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, Y.; Fu, J.; Zhen, L.; Zhao, N.; Yang, Q.; Li, S.; Li, X. Cadmium inhibits mouse sperm motility through inducing tyrosine phosphorylation in a specific subset of proteins. Reprod. Toxicol. 2016, 63, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.Y.; Wu, R.Y.; Zhao, Y.; Xu, C.S.; Zhang, W.D.; Ge, W.; Liu, J.; Sun, Z.Y.; Zou, S.H.; Shen, W. Ochratoxin A exposure decreased sperm motility via the AMPK and PTEN signaling pathways. Toxicol. Appl. Pharmacol. 2018, 340, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, E.; Giwercman, A.; Keiding, N.; Skakkebaek, N.E. Evidence for decreasing quality of semen during past 50 years. BMJ 1992, 305, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Levine, H.; Jorgensen, N.; Martino-Andrade, A.; Mendiola, J.; Weksler-Derri, D.; Mindlis, I.; Pinotti, R.; Swan, S.H. Temporal trends in sperm count: A systematic review and meta-regression analysis. Hum. Reprod. Update 2017, 23, 646–659. [Google Scholar] [CrossRef] [PubMed]
- Swan, S.H.; Elkin, E.P.; Fenster, L. The question of declining sperm density revisited: An analysis of 101 studies published 1934-1996. Environ. Health Perspect. 2000, 108, 961–966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, A.; Durairajanayagam, D.; du Plessis, S.S. Utility of antioxidants during assisted reproductive techniques: An evidence based review. Reprod. Biol. Endocrinol. 2014, 12, 112. [Google Scholar] [CrossRef] [PubMed]
- Knox, R.V. Artificial insemination in pigs today. Theriogenology 2016, 85, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Gogol, P.; Szczesniak-Fabianczyk, B.; Wierzchos-Hilczer, A. The photon emission, ATP level and motility of boar spermatozoa during liquid storage. Reprod. Biol. 2009, 9, 39–49. [Google Scholar] [CrossRef]
- Nguyen, Q.T.; Wallner, U.; Schmicke, M.; Waberski, D.; Henning, H. Energy metabolic state in hypothermically stored boar spermatozoa using a revised protocol for efficient ATP extraction. Biol. Open 2016, 5, 1743–1751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurtado de Llera, A.; Martin-Hidalgo, D.; Garcia-Marin, L.J.; Bragado, M.J. Metformin blocks mitochondrial membrane potential and inhibits sperm motility in fresh and refrigerated boar spermatozoa. Reprod. Domest. Anim. 2018, 53, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Fryer, L.G.; Parbu-Patel, A.; Carling, D. The Anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J. Biol. Chem. 2002, 277, 25226–25232. [Google Scholar] [CrossRef] [PubMed]
- Yeste, M. Sperm cryopreservation update: Cryodamage, markers, and factors affecting the sperm freezability in pigs. Theriogenology 2016, 85, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Amidi, F.; Pazhohan, A.; Shabani, N.M.; Khodarahmian, M.; Nekoonam, S. The role of antioxidants in sperm freezing: A review. Cell Tissue Bank. 2016, 17, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Shabani, N.M.; Nekoonam, S.; Naji, M.; Bakhshalizadeh, S.; Amidi, F. Cryoprotective effect of resveratrol on DNA damage and crucial human sperm messenger RNAs, possibly through 5’ AMP-activated protein kinase activation. Cell Tissue Bank. 2018, 19, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Martin-Hidalgo, D.; Hurtado de Llera, A.; Henning, H.; Wallner, U.; Waberski, D.; Bragado, M.J.; Gil, M.C.; Garcia-Marin, L.J. The Effect of Resveratrol on the Quality of Extended Boar Semen during Storage at 17 °C. J. Agric. Sci. 2013, 5, 231–242. [Google Scholar]
- Nguyen, T.M.; Seigneurin, F.; Froment, P.; Combarnous, Y.; Blesbois, E. The 5’-AMP-Activated Protein Kinase (AMPK) Is Involved in the Augmentation of Antioxidant Defenses in Cryopreserved Chicken Sperm. PLoS ONE 2015, 10, e0134420. [Google Scholar] [CrossRef] [PubMed]
- Bridges, H.R.; Jones, A.J.; Pollak, M.N.; Hirst, J. Effects of metformin and other biguanides on oxidative phosphorylation in mitochondria. Biochem. J. 2014, 462, 475–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Specie | ART/Stress | Sperm Effects | Reference |
|---|---|---|---|
| Boar | Preserved (17 °C) | 1. At short period (<4 days): Improves the % of motile sperm 2. At long period (≥4 days): Decreases sperm MMP Decreases acrosome membrane integrity Increases plasma membrane disorganization | [77] |
| Stallion | Cryopreserved (−196 °C) | No effect described | [59] |
| Chicken | Cryopreserved (−196 °C) | Decreases motility and antioxidant capacity | [119] |
| Stallion | Preserved (RT) | Reduces sperm motility | [60] |
| Human | Cryopreserved (−196 °C) | Deleterious effect in sperm motility and mitochondria Increases apoptotic-like spermatozoa | [64] |
| Compound | Specie | ART/Stress | Sperm Effects | Ref. |
|---|---|---|---|---|
| Resveratrol * | Boar | Preserved (17 °C) | Decreases sperm motility, MMP and ATP | [118] |
| Metformin * | Stallion | Cryopreserved | No effect described | [59] |
| AICAR * | Stallion | Cryopreserved | No effect described | [59] |
| Metformin | Mouse | Cryopreserved | Increases sperm motility and viability Increases fertility rate and quality of embryos | [74] |
| AICAR | Chicken | Cryopreserved | Protects against ROS and lipid peroxidation Improves motility and % rapid spermatozoa | [119] |
| Metformin | Chicken | Cryopreserved | Protects against ROS and lipid peroxidation Improves motility and % rapid spermatozoa | [119] |
| Rosiglitazone | Stallion | Preserved (RT) | Improves % motile and % rapid spermatozoa Shifts to glycolytic metabolism, increases glucose uptake and reduces ROS | [60] |
| Resveratrol | Human | Cryopreserved | Reduces ROS and apoptosis-like spermatozoa | [64] |
| Resveratrol | Human | Cryopreserved | Protects against apoptotic-like spermatozoa and MMP damage | [117] |
| Metformin * | Boar | Preserved (17 °C) | Reduces sperm motility and MMP | [113] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin-Hidalgo, D.; Hurtado de Llera, A.; Calle-Guisado, V.; Gonzalez-Fernandez, L.; Garcia-Marin, L.; Bragado, M.J. AMPK Function in Mammalian Spermatozoa. Int. J. Mol. Sci. 2018, 19, 3293. https://doi.org/10.3390/ijms19113293
Martin-Hidalgo D, Hurtado de Llera A, Calle-Guisado V, Gonzalez-Fernandez L, Garcia-Marin L, Bragado MJ. AMPK Function in Mammalian Spermatozoa. International Journal of Molecular Sciences. 2018; 19(11):3293. https://doi.org/10.3390/ijms19113293
Chicago/Turabian StyleMartin-Hidalgo, David, Ana Hurtado de Llera, Violeta Calle-Guisado, Lauro Gonzalez-Fernandez, Luis Garcia-Marin, and M. Julia Bragado. 2018. "AMPK Function in Mammalian Spermatozoa" International Journal of Molecular Sciences 19, no. 11: 3293. https://doi.org/10.3390/ijms19113293