Receptor-Targeted Glial Brain Tumor Therapies
Abstract
:1. Glial Tumors
- Glial Tumors Overview
- ○
- Glial tumors are malignancies of supportive glial cells.
- ○
- Low Grade Gliomas (LGG) overview: morphological, radiographic, molecular, and genetic characteristics
- ○
- High Grade Gliomas (HGG) overview: morphological, radiographic, molecular, and genetic characteristics
- ○
- Four major subtypes of GBM: proneural, neural, classical, and mesenchymal
- ○
- Response Assessment in Neuro-Oncology (RANO) as a standard method used to assess treatment responses.
- ○
- Major challenges in treatment of GBM: poor penetration of drugs, blood-brain barrier, location of the tumor, infiltrative nature of cells, heterogeneity, and resistance to therapy
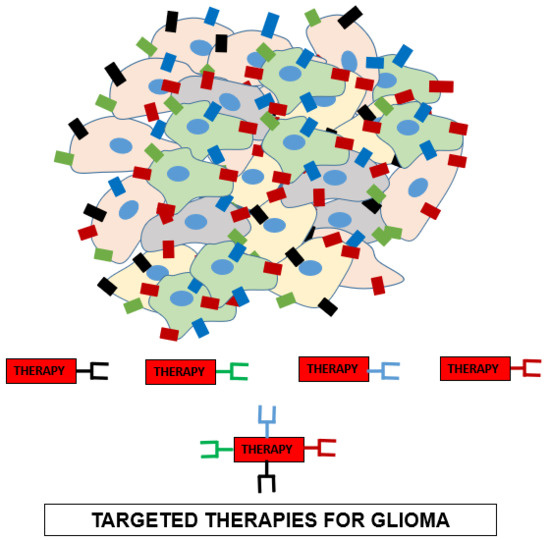
- Targeted Therapies for Glioma
- ○
- There is a need to specifically target glioma cells and spare normal cells.
- ○
- Tumor Specific Antigens/ Tumor Associated Antigens (TSA/TAA) are specific to tumor cells and can serve as excellent targets for therapy.
- ○
- Immunotherapy is the fourth pillar of cancer treatment in addition to surgery, chemotherapy and radiation therapy.
- Interleukin 13 Receptor Alpha 2 (IL-13RA2) in Glioma Treatment
- ○
- Overexpressed in up to 75% of patients, IL-13RA2 was discovered as a glioma-specific marker over two decades ago in the Debinski Laboratory.
- ○
- Over time, about 30 variations of targeted therapies have focused on IL-13RA2, including ICT 107 and IL-13-PE38QQR.
- ○
- IL-13RA2 belongs to cancer/testes antigen group.
- ○
- Over 200 cancer/testes antigens belonging to 70 different families have been identified so far.
- ○
- Other cancer/testes antigens found in Glioma: ACTL8, XAGE3, BORIS, OIP5, DDX43, CTAGE1, KNL1, MAGE-A12, BAGE1
- ○
- IL-13RA2 targeting:
- ○
- IL-13 ligand can target IL-13RA2.
- ○
- IL-13 based fusion/conjugated proteins, nanoparticles, liposomes, viruses, IL-13 conjugated radiotherapy agents, antibodies, peptide-pulsed dendritic cells, and CAR T-cells are used to target IL-13RA2.
- ○
- IL-13PE38QQR, or Cintredekin Besudotox (CB) is one of the most potent recombinant cytotoxin in preclinical and clinical trials to treat glioma.
- ○
- Optimizing the ligands for therapies:
- ○
- Mutations in amino acid positions 13, 66, 69 and 105 can enhance the specificity of IL-13 to the IL-13RA2.
- ○
- Addition of Pseudomonas exotoxin domain 2 (D2) and a nuclear localization signal (NLS) to IL-13 can directly transport IL-13 based cytotoxins to the nucleus of the glioma cells.
- ○
- IL-13 based drug delivery:
- ○
- IL-13 directed viruses: modified adeno and lentiviruses expressing IL-13 include LU-13 and MV Hcdelta18-IL-13.
- ○
- IL-13RA2 targeted radiotherapy agents: copper-64 radiolabeled Pep-1L and alpha particle emitter, actinium-225 radiolabeled Pep-1L have been used to target IL-13RA2.
- ○
- Peptide vaccines: multiple TSA/TAA including IL-13RA2, EGFRvIII, AIM-2, gp-100, HER-2, MAGE-1, EphA2, and survivin have been targeted using peptide vaccines.
- ○
- Dendritic cell-based vaccines: multiple TSA/TAA including IL-13RA2, EphA2, Wilms tumor 1 have been targeted using multiple antigen pulsed dendritic cells.
- ○
- CAR T-cell therapy: both first- and second-generation CAR T-cells have successfully targeted IL-13RA2 including a Phase I clinical trial for GBM at The City of Hope.
- Compromising a vastly interconnected tumor ecosystem with a single therapeutic approach is a great challenge. Hence, multiple targeting of various facets of the tumor ecosystem can enhance therapeutic potential and provide significant clinical benefit.
- Eph/ephrin receptor system in gliomas
- ○
- EphA2, EphA3, and EphB3 are overexpressed in gliomas and have been extensively utilized for targeted therapy.
- EGFR/EGF receptor system in gliomas
- ○
- EGFR/EGF is one of the most studied receptor systems in brain tumors.
- ○
- EGFR is amplified in 40-60% patients and 30-50% EGFR amplifications contain EGFRvIII mutations.
- ○
- EGFR targeted therapies include: siRNA, small molecule inhibitors, antibodies, and CAR T-cells.
- Other receptors/targeted therapies
- ○
- Other receptors used to target glioma include: VEGFR, PDGFR, TGFR, FGFR, c-Met receptor, uPAR, GPCR, LDL-R, LRP1, and FOLR.
- Immune checkpoint inhibitors
- ○
- GBM cells consistently work to maintain and generate an immunosuppressive environment.
- ○
- While the use of immune checkpoint inhibitors shows great promise, its clinical benefits are yet to be established in glioma.
- Viral/genetic therapies
- ○
- Over 20 oncolytic virus candidates including reovirus, oncolytic measles, oncolytic herpes simplex virus, and vesicular stomatitis virus have been tested in clinical trials to treat GBM.
- ○
- While the use of viral therapy in glioma shows great promise, its consistent clinical benefits are yet to be established.
- Conclusions
- ○
- Effective treatment of malignant gliomas is a great challenge in oncology.
- ○
- Notable advancements in antigen-specific targeting, viral and genetic silencing, immune checkpoint inhibitors, peptide and dendritic cell vaccines, CAR T-cell therapy, chemotherapy and radiation therapy, in conjugation with effective drug delivery systems like CED offer promise to tackle this grave challenge in glioma therapy.
- ○
- Targeting multiple facets of the tumor ecosystem, personalized antigen screening, and using state of the art ex vivo technologies can enhance the clinical benefits of current and future treatment options.
2. Targeted Therapies for Gliomas
3. Interleukin 13 Receptor Alpha 2 (IL-13RA2) in Glioma Treatment
3.1. IL-13RA2 Targeting
3.2. Optimizing the Ligand for Therapies
3.3. IL-13-Based Drug Delivery
3.3.1. IL-13-Directed Viruses
3.3.2. IL-13RA2-Targeted Radiotherapy Agents
3.4. Peptide Vaccines
3.5. Dendritic Cell-Based Vaccines
3.6. CAR T-Cell Therapy
4. Eph/Ephrin Receptor System in Gliomas
5. EGFR/EGF Receptor System in Gliomas
6. Other Receptors/Targeted Therapies
7. Immune Checkpoint Inhibitors
8. Viral/Genetic Therapies
9. Conclusions
Funding
Conflicts of Interest
Abbreviations
| 1-MT | 1-Methylthryptophan |
| ACT | Adoptive Cell Transfer |
| ACTL8 | Actin Like Protein 8 |
| AIM-2 | Absent In Melanoma 2 |
| ALL | Acute Lymphoblastic Leukemia |
| BAGE | B Melanoma Antigen |
| BBB | Blood-Brain Barrier |
| Bcl-XL | B-cell Lymphoma Extra-Large |
| BORIS | Brother Of The Regulator Of Imprinted Sites |
| CAds | Conditionally Replicating Adenoviruses |
| CAR T | Chimeric Antigen Receptor T-cells |
| CB | Cintredekin Besudotox |
| CCL2 | Cytokines Including Chemokine (C-C motif) Ligand 2 |
| CD | Cluster of Differentiation |
| CED | Convection-Enhanced Delivery |
| CNS | Central Nervous System |
| CT | Computerized Axial Tomography |
| CT 45 | Cancer/Testis Antigen 45 |
| CTAGE1 | Cutaneous T-cell Lymphoma Associated Antigen 1 |
| CTL-4 | Cytotoxic T-lymphocyte-Associated Protein 4 |
| D2 | Domain 2 |
| DARPins | Designed Ankyrin Repeat Proteins |
| DDX43 | Dead-Box Helicase 43 |
| E | Glutamic Acid |
| EGF | Endothelial Growth Factor |
| EGFR | Epidermal Growth Factor Receptor |
| Eph | Erythropoietin-Producing-Human |
| ephrin | Erythropoietin-Producing-Human Receptor Interacting Ligands |
| FasL | Fas ligand |
| FDA | Food and Drug Administration |
| FGFR | Fibroblast Growth Factor Receptor |
| FOLR | Folate Receptor |
| GAGE | G-Antigen |
| GBM | Glioblastoma |
| GFAP | Glial Fibrillary Acidic Protein |
| gp-100 | glycoprotein 100 |
| GPCR | G-Protein Coupled Receptors |
| HAGE | Helicase Antigen |
| HER-2 | Epidermal Growth Factor Receptor |
| HLA-G | Human Leukocyte Antigen G |
| HLAI | Human Leukocyte Antigen I |
| HMGB1 | High Mobility Group Box 1 |
| IDH1 | Isocitrate Dehydrogenase Gene 1 |
| IFN γ | Interferon γ |
| IL | Interleukin |
| IL-13RA1 | Interleukin-13 Receptor A1 |
| IL-13RA2 | Interleukin 13 Receptor Alpha 2 |
| IL-4RA | Interleukin-4 Receptor A |
| K | Lysine |
| KNL1 | Kinetochore Scaffold 1 |
| LAG-3 | Lymphocyte Activation Gene 3 |
| LDL-R | Low Density Lipoprotein Receptor |
| LGG | Lower Grade Glioma |
| LRP1 | Low Density Lipoprotein Receptor-Related Protein |
| MAGE | Melanoma Associated Antigen |
| MAPK | Mitogen-Activated Protein Kinase |
| MART | Melanoma-Associated Antigen Recognized by T cells |
| MDSC | Myeloid Derived Suppressor Cells |
| MGMT | Protein O6 Methylguanine-DNA Methyltransferase |
| MHC | Major Histocompatibility Complex |
| MHCI | Major Histocompatibility Complex Class I |
| MRI | Magnetic resonance Imaging |
| NLS | Nucleus Localizing Signal |
| OIP5 | Opa-Interacting Protein 5 |
| PD-1 | Programmed Death 1 |
| PDGFR | Platelet-Derived Growth Factor Receptor |
| PD-L1 | Programmed Death Ligand 1 |
| PD-L2 | Programmed Death Ligand 2 |
| PTEN | Phosphatase and Tensin Homolog |
| PTEN | Phosphatase And Tensin Homolog |
| RANO | Response Assessment in Neuro-Oncology |
| scFv | Small Chain Variable Fragment |
| SCID | Severe Combined Immunodeficiency |
| SP 17 | Sperm Protein 17 |
| SSX | Synovial Sarcoma X |
| STAT6 | Signal Transducer And Activator Of Transcription 6 |
| TAA | Tumor-Associated Antigens |
| TAM | Tumor-Associated Macrophages |
| TERT | Telomerase Reverse Transcriptase |
| TGFR | Transforming Growth Factor Receptor |
| TGFβ | Tumor Growth Factor β |
| TH2 | T Helper 2 |
| TIM-3 | T-cell Immunoglobulin And Mucin Domain 3 |
| TNFα | Tumor Necrosis Factor α |
| TP53 | Tumor Protein 53 |
| TRP-2 | Tyrosine-Related Protein 2 |
| TSA | Tumor-Specific Antigens |
| TT Fields | Tumor-Treating Fields |
| Upar | Low Density Lipoprotein Receptor |
| VEGFR | Vascular Endothelial Growth Factor Receptor |
| XAGE3 | X-Antigen Family Member 3 |
| Y | Tyrosine |
References
- Allen, N.J.; Barres, B.A. Neuroscience: Glia—More than just brain glue. Nature 2009, 457, 675–677. [Google Scholar] [CrossRef] [PubMed]
- Lathia, J.D.; Mack, S.C.; Mulkearns-Hubert, E.E.; Valentim, C.L.L.; Rich, J.N. Cancer stem cells in glioblastoma. Genes Dev. 2015, 29, 1203–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanai, N.; Alvarez-Buylla, A.; Berger, M.S. Neural Stem Cells and the Origin of Gliomas. N. Engl. J. Med. 2005, 353, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.A.; Cha, S.; Mayo, M.C.; Chen, M.-H.; Keles, E.; VandenBerg, S.; Berger, M.S. Relationship of glioblastoma multiforme to neural stem cell regions predicts invasive and multifocal tumor phenotype. Neuro Oncol. 2007, 9, 424–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goffart, N.; Kroonen, J.; Rogister, B. Glioblastoma-initiating cells: relationship with neural stem cells and the micro-environment. Cancers 2013, 5, 1049–1071. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Wesseling, P.; Kros, J.M.; Jeuken, J.W.M. The pathological diagnosis of diffuse gliomas: Towards a smart synthesis of microscopic and molecular information in a multidisciplinary context. Diagn. Histopathol. 2011, 17, 486–494. [Google Scholar] [CrossRef]
- Shaw, E.G.; Wang, M.; Coons, S.W.; Brachman, D.G.; Buckner, J.C.; Stelzer, K.J.; Barger, G.R.; Brown, P.D.; Gilbert, M.R.; Mehta, M.P. Randomized trial of radiation therapy plus procarbazine, lomustine, and vincristine chemotherapy for supratentorial adult low-grade glioma: Initial results of RTOG 9802. J. Clin. Oncol. 2012, 30, 3065–3070. [Google Scholar] [CrossRef] [PubMed]
- Buckner, J.C.; Shaw, E.G.; Pugh, S.L.; Chakravarti, A.; Gilbert, M.R.; Barger, G.R.; Coons, S.; Ricci, P.; Bullard, D.; Brown, P.D.; et al. Radiation plus Procarbazine, CCNU, and Vincristine in Low-Grade Glioma. N. Engl. J. Med. 2016, 374, 1344–1355. [Google Scholar] [CrossRef] [PubMed]
- Shaw, E.G.; Wisoff, J.H. Prospective clinical trials of intracranial low-grade glioma in adults and children. Neuro. Oncol. 2003, 5, 153–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guillevin, R.; Herpe, G.; Verdier, M.; Guillevin, C. Low-grade gliomas: The challenges of imaging. Diagn. Interv. Imaging 2014, 95, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.L.; Holmen, S.L.; Colman, H. IDH1 and IDH2 mutations in gliomas. Curr. Neurol. Neurosci. Rep. 2013, 13, 345. [Google Scholar] [CrossRef] [PubMed]
- Forst, D.A.; Nahed, B.V; Loeffler, J.S.; Batchelor, T.T. Low-grade gliomas. Oncologist 2014, 19, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Kamran, N.; Calinescu, A.; Candolfi, M.; Chandran, M.; Mineharu, Y.; Asad, A.S.; Koschmann, C.; Nunez, F.J.; Lowenstein, P.R.; Castro, M.G. Recent advances and future of immunotherapy for glioblastoma. Expert Opin. Biol. Ther. 2016, 16, 1245–1264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nuño, M.; Birch, K.; Mukherjee, D.; Sarmiento, J.M.; Black, K.L.; Patil, C.G. Survival and Prognostic Factors of Anaplastic Gliomas. Neurosurgery 2013, 73, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E. Glioblastoma: Overview of Disease and Treatment. Clin. J. Oncol. Nurs. 2016, 20, S2–S8. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.K.; Llaguno, S.R.A.; McKay, R.M.; Parada, L.F. Glioblastoma multiforme: A perspective on recent findings in human cancer and mouse models. BMB Rep. 2011, 44, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Rong, Y.; Durden, D.L.; Van Meir, E.G.; Brat, D.J. “Pseudopalisading” necrosis in glioblastoma: A familiar morphologic feature that links vascular pathology, hypoxia, and angiogenesis. J. Neuropathol. Exp. Neurol. 2006, 65, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Bastien, J.I.L.; Mcneill, K.A.; Fine, H.A. Molecular Characterizations of Glioblastoma, Targeted Therapy, and Clinical Results to Date. Cancer 2014, 121, 502–518. [Google Scholar] [CrossRef] [PubMed]
- von Deimling, A.; Nagel, J.; Bender, B.; Lenartz, D.; Schramm, J.; Louis, D.N.; Wiestler, O.D. Deletion mapping of chromosome 19 in human gliomas. Int. J. Cancer 1994, 57, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Idbaih, A.; Marie, Y.; Pierron, G.; Brennetot, C.; Hoang-Xuan, K.; Kujas, M.; Mokhtari, K.; Sanson, M.; Lejeune, J.; Aurias, A.; et al. Two types of chromosome 1p losses with opposite significance in gliomas. Ann. Neurol. 2005, 58, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Qi, C.; Maling, G.; Xiang, W.; Yanhui, L.; Ruofei, L.; Yunhe, M.; Jiewen, L.; Qing, M. TERT mutation in glioma: Frequency, prognosis and risk. J. Clin. Neurosci. 2016, 26, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Brennan, C.W.; Verhaak, R.G.W.; McKenna, A.; Campos, B.; Noushmehr, H.; Salama, S.R.; Zheng, S.; Chakravarty, D.; Sanborn, J.Z.; Berman, S.H.; et al. The Somatic Genomic Landscape of Glioblastoma. Cell 2013, 155, 462–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verhaak, R.G.W.; Hoadley, K.A.; Purdom, E.; Wang, V.; Qi, Y.; Wilkerson, M.D.; Miller, C.R.; Ding, L.; Golub, T.; Mesirov, J.P.; et al. Cancer Genome Atlas Research Network Integrated Genomic Analysis Identifies Clinically Relevant Subtypes of Glioblastoma Characterized by Abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010, 17, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Noushmehr, H.; Weisenberger, D.J.; Diefes, K.; Phillips, H.S.; Pujara, K.; Berman, B.P.; Pan, F.; Pelloski, C.E.; Sulman, E.P.; Bhat, K.P.; et al. Cancer Genome Atlas Research Network Identification of a CpG Island Methylator Phenotype that Defines a Distinct Subgroup of Glioma. Cancer Cell 2010, 17, 510–522. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.P.; Tirosh, I.; Trombetta, J.J.; Shalek, A.K.; Gillespie, S.M.; Wakimoto, H.; Cahill, D.P.; Nahed, B.V; Curry, W.T.; Martuza, R.L.; et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science 2014, 344, 1396–1401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellingson, B.M.; Wen, P.Y.; Cloughesy, T.F. Modified Criteria for Radiographic Response Assessment in Glioblastoma Clinical Trials. Neurotherapeutics 2017, 14, 307–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostrom, Q.T.; Gittleman, H.; de Blank, P.M.; Finlay, J.L.; Gurney, J.G.; McKean-Cowdin, R.; Stearns, D.S.; Wolff, J.E.; Liu, M.; Wolinsky, Y.; et al. American Brain Tumor Association Adolescent and Young Adult Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2008–2012. Neuro Oncol. 2016, 18, i1–i50. [Google Scholar] [CrossRef] [PubMed]
- Kitange, G.J.; Carlson, B.L.; Schroeder, M.A.; Grogan, P.T.; Lamont, J.D.; Decker, P.A.; Wu, W.; James, C.D.; Sarkaria, J.N. Induction of MGMT expression is associated with temozolomide resistance in glioblastoma xenografts. Neuro Oncol. 2009, 11, 281–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burgess, D.J. Cancer genetics: Initially complex, always heterogeneous. Nat. Rev. Cancer 2011, 11, 153. [Google Scholar] [CrossRef] [PubMed]
- Ellis, H.P.; Greenslade, M.; Powell, B.; Spiteri, I.; Sottoriva, A.; Kurian, K.M. Current Challenges in Glioblastoma: Intratumour Heterogeneity, Residual Disease, and Models to Predict Disease Recurrence. Front. Oncol. 2015, 5, 251. [Google Scholar] [CrossRef] [PubMed]
- Debinski, W. Drug cocktails for effective treatment of glioblastoma multiforme. Expert Rev. Neurother. 2008, 8, 515–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.R.; O’Neill, B.P. Glioblastoma survival in the United States before and during the temozolomide era. J. Neurooncol. 2012, 107, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.L.; Beroukhim, R. Beating the odds: Extreme long-term survival with glioblastoma. Neuro Oncol. 2014, 16, 1159–1160. [Google Scholar] [CrossRef] [PubMed]
- Malpass, K. Identification of novel glioblastoma-associated antigens reveals targets for immunotherapy. Nat. Rev. Neurol. 2012, 8, 240. [Google Scholar] [CrossRef] [PubMed]
- Dutoit, V.; Herold-Mende, C.; Hilf, N.; Schoor, O.; Beckhove, P.; Bucher, J.; Dorsch, K.; Flohr, S.; Fritsche, J.; Lewandrowski, P.; et al. Exploiting the glioblastoma peptidome to discover novel tumour-associated antigens for immunotherapy. Brain 2012, 135, 1042–1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatano, M.; Eguchi, J.; Tatsumi, T.; Kuwashima, N.; Dusak, J.E.; Kinch, M.S.; Pollack, I.F.; Hamilton, R.L.; Storkus, W.J.; Okada, H. EphA2 as a glioma-associated antigen: A novel target for glioma vaccines. Neoplasia 2005, 7, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Johanns, T.M.; Bowman-Kirigin, J.A.; Liu, C.; Dunn, G.P. Targeting Neoantigens in Glioblastoma: An Overview of Cancer Immunogenomics and Translational Implications. Neurosurgery 2017, 64, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Pearson, J.R.D.; Regad, T. Targeting cellular pathways in glioblastoma multiforme. Signal Transduct. Target. Ther. 2017, 2, 17040. [Google Scholar] [CrossRef] [PubMed]
- Yaghi, N.K.; Wei, J.; Hashimoto, Y.; Kong, L.-Y.; Gabrusiewicz, K.; Nduom, E.K.; Ling, X.; Huang, N.; Zhou, S.; Kerrigan, B.C.P.; et al. Immune modulatory nanoparticle therapeutics for intracerebral glioma. Neuro Oncol. 2017, 19, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Pico De Coañ, Y.; Choudhury, A.; Kiessling, R. Checkpoint blockade for cancer therapy: Revitalizing a suppressed immune system. Trends Mol. Med. 2015, 21, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swartz, A.M.; Batich, K.A.; Fecci, P.E.; Sampson, J.H. Peptide vaccines for the treatment of glioblastoma. J. Neurooncol. 2015, 123, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Müller, I.; Altherr, D.; Eyrich, M.; Flesch, B.; Friedmann, K.S.; Ketter, R.; Oertel, J.; Schwarz, E.C.; Technau, A.; Urbschat, S.; et al. Tumor antigen–specific T cells for immune monitoring of dendritic cell–treated glioblastoma patients. Cytotherapy 2016, 18, 1146–1161. [Google Scholar] [CrossRef] [PubMed]
- Migliorini, D.; Dietrich, P.-Y.; Stupp, R.; Linette, G.P.; Posey, A.D.; June, C.H. CAR T-Cell Therapies in Glioblastoma: A First Look. Clin. Cancer Res. 2018, 24, 535–540. [Google Scholar] [CrossRef] [PubMed]
- FDA Approval Brings First Gene Therapy to the United States. Available online: https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm574058.htm (accessed on 5 September 2018).
- Hunter, P. The fourth pillar. EMBO Rep. 2017, 18, 1889–1892. [Google Scholar] [CrossRef] [PubMed]
- Janiczek, M.; Szylberg, Ł.; Kasperska, A.; Kowalewski, A.; Parol, M.; Antosik, P.; Radecka, B.; Marszałek, A. Immunotherapy as a Promising Treatment for Prostate Cancer: A Systematic Review. J. Immunol. Res. 2017, 2017, 4861570. [Google Scholar] [CrossRef] [PubMed]
- Okada, H.; Butterfield, L.H.; Hamilton, R.L.; Hoji, A.; Sakaki, M.; Ahn, B.J.; Kohanbash, G.; Drappatz, J.; Engh, J.; Amankulor, N.; et al. Induction of robust type-I CD8+ T-cell responses in WHO grade 2 low-grade glioma patients receiving peptide-based vaccines in combination with poly-ICLC. Clin. Cancer Res. 2015, 21, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Wilson, E.H.; Weninger, W.; Hunter, C.A. Trafficking of immune cells in the central nervous system. J. Clin. Investig. 2010, 120, 1368–1379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, I.; Han, S.J.; Kaur, G.; Crane, C.; Parsa, A.T. The role of microglia in central nervous system immunity and glioma immunology. J. Clin. Neurosci. 2010, 17, 6–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wendeln, A.-C.; Degenhardt, K.; Kaurani, L.; Gertig, M.; Ulas, T.; Jain, G.; Wagner, J.; Häsler, L.M.; Wild, K.; Skodras, A.; et al. Innate immune memory in the brain shapes neurological disease hallmarks. Nature 2018, 556, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.; Xia, Y.; Bettegowda, C.; Weller, M. Current state of immunotherapy for glioblastoma. Nat. Rev. Clin. Oncol. 2018, 15, 422–442. [Google Scholar] [CrossRef] [PubMed]
- Filley, A.C.; Henriquez, M.; Dey, M. Recurrent glioma clinical trial, CheckMate-143: The game is not over yet. Oncotarget 2017, 8, 91779–91794. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.E.; Alizadeh, D.; Starr, R.; Weng, L.; Wagner, J.R.; Naranjo, A.; Ostberg, J.R.; Blanchard, M.S.; Kilpatrick, J.; Simpson, J.; et al. Regression of Glioblastoma after Chimeric Antigen Receptor T-Cell Therapy. N. Engl. J. Med. 2016, 375, 2561–2569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.J.; Kang, W.Y.; Yoon, Y.; Jin, J.Y.; Song, H.J.; Her, J.H.; Kang, S.M.; Hwang, Y.K.; Kang, K.J.; Joo, K.M.; et al. Natural killer (NK) cells inhibit systemic metastasis of glioblastoma cells and have therapeutic effects against glioblastomas in the brain. BMC Cancer 2015, 15, 1011. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Burger, M.C.; Jennewein, L.; Genßler, S.; Schönfeld, K.; Zeiner, P.; Hattingen, E.; Harter, P.N.; Mittelbronn, M.; Tonn, T.; et al. ErbB2/HER2-Specific NK Cells for Targeted Therapy of Glioblastoma. JNCI J. Natl. Cancer Inst. 2016, 108. [Google Scholar] [CrossRef] [PubMed]
- Kmiecik, J.; Zimmer, J.; Chekenya, M. Natural killer cells in intracranial neoplasms: Presence and therapeutic efficacy against brain tumours. J. Neurooncol. 2014, 116, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Cancer Institute, Blue Ribbon Panel Report 2016. Available online: https://www.cancer.gov/research/key-initiatives/moonshot-cancer-initiative/blue-ribbon-panel/blue-ribbon-panel-report-2016.pdf (accessed on 14 September 2018).
- Debinski, W.; Obiri, N.I.; Powers, S.K.; Pastan, I.; Puri, R.K. Human glioma cells overexpress receptors for interleukin 13 and are extremely sensitive to a novel chimeric protein composed of interleukin 13 and pseudomonas exotoxin. Clin. Cancer Res. 1995, 1, 1253–1258. [Google Scholar] [PubMed]
- Debinski, W.; Gibo, D.M.; Hulet, S.W.; Connor, J.R.; Gillespie, G.Y. Receptor for interleukin 13 is a marker and therapeutic target for human high-grade gliomas. Clin. Cancer Res. 1999, 5, 985–990. [Google Scholar] [PubMed]
- Sattiraju, A.; Sai, K.K.S.; Xuan, A.; Pandya, D.N.; Almaguel, F.G.; Wadas, T.J.; Herpai, D.M.; Debinski, W.; Mintz, A. IL13RA2 targeted alpha particle therapy against glioblastomas. Oncotarget 2017, 8, 42997–43007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thaci, B.; Brown, C.E.; Binello, E.; Werbaneth, K.; Sampath, P.; Sengupta, S. Significance of interleukin-13 receptor alpha 2-targeted glioblastoma therapy. Neuro Oncol. 2014, 16, 1304–1312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunwar, S. Convection enhanced delivery of IL13-PE38QQR for treatment of recurrent malignant glioma: Presentation of interim findings from ongoing phase 1 studies. Acta Neurochir. Suppl. 2003, 88, 105–111. [Google Scholar] [PubMed]
- Scanlan, M.J.; Gure, A.O.; Jungbluth, A.A.; Old, L.J.; Chen, Y.-T. Cancer/testis antigens: an expanding family of targets for cancer immunotherapy. Immunol. Rev. 2002, 188, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Van der Bruggen, P.; Traversari, C.; Chomez, P.; Lurquin, C.; De Plaen, E.; Van den Eynde, B.; Knuth, A.; Boon, T. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science 1991, 254, 1643–1647. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-H.; Wong, E.W.; Cheng, C.Y. Cancer/testis (CT) antigens, carcinogenesis and spermatogenesis. Spermatogenesis 2011, 1, 209–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freitas, M.R.P.; Malheiros, S.M.F.; Stávale, J.N.; Biassi, T.P.; Zamunér, F.T.; Begnami, M.D.F.S.; Soares, F.A.; Vettore, A.L.; Freitas, M.R.P.; Malheiros, S.M.F.; et al. Expression of Cancer/Testis Antigens is Correlated with Improved Survival in Glioblastoma. Oncotarget 2013, 4, 636–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akiyama, Y.; Komiyama, M.; Miyata, H.; Yagoto, M.; Ashizawa, T.; Iizuka, A.; Oshita, C.; Kume, A.; Nogami, M.; Ito, I.; et al. Novel cancer-testis antigen expression on glioma cell lines derived from high-grade glioma patients. Oncol. Rep. 2014, 31, 1683–1690. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, G.J.; Bancroft, A.; Grencis, R.K.; McKenzie, A.N. A distinct role for interleukin-13 in Th2-cell-mediated immune responses. Curr. Biol. 1998, 8, 339–342. [Google Scholar] [CrossRef] [Green Version]
- Deepak, P.; Kumar, S.; Acharya, A. Overexpression of Interleukin-13 in a Murine T-Cell Lymphoma: A Possible Factor of DL-Induced Immunosuppression and Tumor Progression. Cancer Invest. 2009, 27, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Saigusa, S.; Tanaka, K.; Inoue, Y.; Toiyama, Y.; Okugawa, Y.; Iwata, T.; Mohri, Y.; Kusunoki, M. Low serum interleukin-13 levels correlate with poorer prognoses for colorectal cancer patients. Int. Surg. 2014, 99, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Sai, K.K.S.; Sattiraju, A.; Almaguel, F.G.; Xuan, A.; Rideout, S.; Krishnaswamy, R.S.; Zhang, J.; Herpai, D.M.; Debinski, W.; Mintz, A. Peptide-based PET imaging of the tumor restricted IL13RA2 biomarker. Oncotarget 2017, 8, 50997–51007. [Google Scholar] [CrossRef] [PubMed]
- Mintz, A.; Gibo, D.M.; Slagle-Webb, B.; Christensen, N.D.; Debinski, W. IL-13Ralpha2 is a glioma-restricted receptor for interleukin-13. Neoplasia 2002, 4, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Mintz, A.; Gibo, D.M.; MadhanKumar, A.B.; Cladel, N.M.; Christensen, N.D.; Debinski, W. Protein- and DNA-Based Active Immunotherapy Targeting Interleukin-13 Receptor Alpha2. Cancer Biother. Radiopharm. 2008, 23, 581–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debinski, W.; Dickinson, P.; Rossmeisl, J.H.; Robertson, J.; Gibo, D.M. New Agents for Targeting of IL-13RA2 Expressed in Primary Human and Canine Brain Tumors. PLoS ONE 2013, 8, e77719. [Google Scholar] [CrossRef] [PubMed]
- Pandya, H.; Gibo, D.M.; Garg, S.; Kridel, S.; Debinski, W. An interleukin 13 receptor α 2–specific peptide homes to human Glioblastoma multiforme xenografts. Neuro. Oncol. 2012, 14, 6–18. [Google Scholar] [CrossRef] [PubMed]
- Pandya, H.; Gibo, D.M.; Debinski, W. Molecular targeting of intracellular compartments specifically in cancer cells. Genes Cancer 2010, 1, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Mintz, A.; Gibo, D.M.; Madhankumar, A.B.; Debinski, W. Molecular targeting with recombinant cytotoxins of interleukin-13 receptor alpha2-expressing glioma. J. Neurooncol. 2003, 64, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Arima, K.; Sato, K.; Tanaka, G.; Kanaji, S.; Terada, T.; Honjo, E.; Kuroki, R.; Matsuo, Y.; Izuhara, K. Characterization of the Interaction between Interleukin-13 and Interleukin-13 Receptors. J. Biol. Chem. 2005, 280, 24915–24922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsi, L.C.; Kundu, S.; Palomo, J.; Xu, B.; Ficco, R.; Vogelbaum, M.A.; Cathcart, M.K. Silencing IL-13Rα2 promotes glioblastoma cell death via endogenous signaling. Mol. Cancer Ther. 2011, 10, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.E.; Warden, C.D.; Starr, R.; Deng, X.; Badie, B.; Yuan, Y.-C.; Forman, S.J.; Barish, M.E. Glioma IL13Rα2 is associated with mesenchymal signature gene expression and poor patient prognosis. PLoS ONE 2013, 8, e77769. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.; Conyers, J.M.; Zhu, D.; Gibo, D.M.; Dorsey, J.F.; Debinski, W.; Mintz, A. IL-13Rα2-Targeted Therapy Escapees: Biologic and Therapeutic Implications. Transl. Oncol. 2011, 4, 390–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bregy, A.; Wong, T.M.; Shah, A.H.; Goldberg, J.M.; Komotar, R.J. Active immunotherapy using dendritic cells in the treatment of glioblastoma multiforme. Cancer Treat. Rev. 2013, 39, 891–907. [Google Scholar] [CrossRef] [PubMed]
- Ahn, B.J.; Pollack, I.F.; Okada, H. Immune-checkpoint blockade and active immunotherapy for glioma. Cancers 2013, 5, 1379–1412. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, D.T.; Fong, C.; Yew, A.; Spasic, M.; Garcia, H.M.; Kruse, C.A.; Yang, I. Passive immunotherapeutic strategies for the treatment of malignant gliomas. Neurosurg. Clin. N. Am. 2012, 23, 481–495. [Google Scholar] [CrossRef] [PubMed]
- Schuster, M.; Nechansky, A.; Kircheis, R. Cancer immunotherapy. Biotechnol. J. 2006, 1, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Coley, W.B., II. Contribution to the Knowledge of Sarcoma. Ann. Surg. 1891, 14, 199–220. [Google Scholar] [CrossRef] [PubMed]
- Debinski, W.; Obiri, N.I.; Pastan, I.; Puri, R.K. A Novel Chimeric Protein Composed of Interleukin 13 and Pseudomonas Exotoxin Is Highly Cytotoxic to Human Carcinoma Cells Expressing Receptors for Interleukin 13 and Interleukin 4. J. Biol. Chem. 1995, 270, 16775–16780. [Google Scholar] [CrossRef] [PubMed]
- Debinski, W. An immune regulatory cytokine receptor and glioblastoma multiforme: An unexpected link. Crit. Rev. Oncog. 1998, 9, 255–268. [Google Scholar] [PubMed]
- Mut, M.; Sherman, J.H.; Shaffrey, M.E.; Schiff, D. Cintredekin besudotox in treatment of malignant glioma. Expert Opin. Biol. Ther. 2008, 8, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Kunwar, S.; Prados, M.D.; Chang, S.M.; Berger, M.S.; Lang, F.F.; Piepmeier, J.M.; Sampson, J.H.; Ram, Z.; Gutin, P.H.; Gibbons, R.D.; et al. Cintredekin Besudotox Intraparenchymal Study Group Direct Intracerebral Delivery of Cintredekin Besudotox (IL13-PE38QQR) in Recurrent Malignant Glioma: A Report by the Cintredekin Besudotox Intraparenchymal Study Group. J. Clin. Oncol. 2007, 25, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Vogelbaum, M.A.; Sampson, J.H.; Kunwar, S.; Chang, S.M.; Shaffrey, M.; Asher, A.L.; Lang, F.F.; Croteau, D.; Parker, K.; Grahn, A.Y.; et al. Convection-enhanced delivery of cintredekin besudotox (interleukin-13-PE38QQR) followed by radiation therapy with and without themozolomide in newly diagnosed malignant gliomas. Neurosurgery 2007, 61, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Kunwar, S.; Chang, S.M.; Prados, M.D.; Berger, M.S.; Sampson, J.H.; Croteau, D.; Sherman, J.W.; Grahn, A.Y.; Shu, V.S.; Dul, J.L.; et al. Safety of intraparenchymal convection-enhanced delivery of cintredekin besudotox in early-phase studies. Neurosurg. Focus 2006, 20, E15. [Google Scholar] [PubMed]
- Kunwar, S.; Chang, S.; Westphal, M.; Vogelbaum, M.; Sampson, J.; Barnett, G.; Shaffrey, M.; Ram, Z.; Piepmeier, J.; Prados, M.; et al. PRECISE Study Group Phase III randomized trial of CED of IL13-PE38QQR vs Gliadel wafers for recurrent glioblastoma. Neuro Oncol. 2010, 12, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Sampson, J.H.; Archer, G.; Pedain, C.; Wembacher-Schröder, E.; Westphal, M.; Kunwar, S.; Vogelbaum, M.A.; Coan, A.; Herndon, J.E.; Raghavan, R.; et al. Investigators, P. T. Poor drug distribution as a possible explanation for the results of the PRECISE trial. J. Neurosurg. 2010, 113, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Madhankumar, A.B.; Mintz, A.; Debinski, W. Alanine-scanning Mutagenesis of α-Helix D Segment of Interleukin-13 Reveals New Functionally Important Residues of the Cytokine. J. Biol. Chem. 2002, 277, 43194–43205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debinski, W. Recombinant cytotoxins specific for cancer cells. Ann. N. Y. Acad. Sci. 1999, 886, 297–299. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.P.; Debinski, W. Mutants of interleukin 13 with altered reactivity toward interleukin 13 receptors. J. Biol. Chem. 1999, 274, 29944–29950. [Google Scholar] [CrossRef] [PubMed]
- Madhankumar, A.B.; Mintz, A.; Debinski, W. Interleukin 13 mutants of enhanced avidity toward the glioma-associated receptor, IL13Ralpha2. Neoplasia 2004, 6, 15–22. [Google Scholar] [CrossRef]
- Sonawane, P.; Choi, Y.A.; Pandya, H.; Herpai, D.M.; Fokt, I.; Priebe, W.; Debinski, W. Novel Molecular Multilevel Targeted Antitumor Agents. Cancer Transl. Med. 2017, 3, 69–79. [Google Scholar] [PubMed]
- Balyasnikova, I.V; Wainwright, D.A.; Solomaha, E.; Lee, G.; Han, Y.; Thaci, B.; Lesniak, M.S. Characterization and immunotherapeutic implications for a novel antibody targeting interleukin (IL)-13 receptor α2. J. Biol. Chem. 2012, 287, 30215–30227. [Google Scholar] [CrossRef] [PubMed]
- Madhankumar, A.B.; Slagle-Webb, B.; Mintz, A.; Sheehan, J.M.; Connor, J.R. Interleukin-13 receptor-targeted nanovesicles are a potential therapy for glioblastoma multiforme. Mol. Cancer Ther. 2006, 5, 3162–3169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madhankumar, A.B.; Slagle-Webb, B.; Wang, X.; Yang, Q.X.; Antonetti, D.A.; Miller, P.A.; Sheehan, J.M.; Connor, J.R. Efficacy of interleukin-13 receptor-targeted liposomal doxorubicin in the intracranial brain tumor model. Mol. Cancer Ther. 2009, 8, 648–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Shi, W.; Song, W.; Wang, L.; Liu, X.; Chen, J.; Huang, R. Tumor cell targeted delivery by specific peptide-modified mesoporous silica nanoparticles. J. Mater. Chem. 2012, 22, 14608. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, K.; Zhao, J.; Liu, X.; Bu, J.; Yan, X.; Huang, R. Multifunctional Mesoporous Silica-Coated Graphene Nanosheet Used for Chemo-Photothermal Synergistic Targeted Therapy of Glioma. J. Am. Chem. Soc. 2013, 135, 4799–4804. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.F.; Conrad, C.; Gomez-Manzano, C.; Yung, W.K.A.; Sawaya, R.; Weinberg, J.S.; Prabhu, S.S.; Rao, G.; Fuller, G.N.; Aldape, K.D.; et al. Phase I Study of DNX-2401 (Delta-24-RGD) Oncolytic Adenovirus: Replication and Immunotherapeutic Effects in Recurrent Malignant Glioma. J. Clin. Oncol. 2018, 36, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Ulasov, I.V.; Tyler, M.A.; Han, Y.; Glasgow, J.N.; Lesniak, M.S. Novel Recombinant Adenoviral Vector That Targets The Interleukin-13 Receptor α2 Chain Permits Effective Gene Transfer to Malignant Glioma. Hum. Gene Ther. 2007, 18, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Ou, W.; Marino, M.P.; Suzuki, A.; Joshi, B.; Husain, S.R.; Maisner, A.; Galanis, E.; Puri, R.K.; Reiser, J. Specific Targeting of Human Interleukin (IL)-13 Receptor α2-Positive Cells with Lentiviral Vectors Displaying IL-13. Hum. Gene Ther. Methods 2012, 23, 137–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, G.; Roizman, B. Construction and properties of a herpes simplex virus 1 designed to enter cells solely via the IL-13alpha2 receptor. Proc. Natl. Acad. Sci. USA. 2006, 103, 5508–5513. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, K.; Hanauer, J.R.; Prüfer, S.; Münch, R.C.; Völker, I.; Filippis, C.; Jost, C.; Hanschmann, K.-M.; Cattaneo, R.; Peng, K.-W.; et al. DARPin-targeting of measles virus: Unique bispecificity, effective oncolysis, and enhanced safety. Mol. Ther. 2013, 21, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Candolfi, M.; Xiong, W.; Yagiz, K.; Liu, C.; Muhammad, A.K.M.G.; Puntel, M.; Foulad, D.; Zadmehr, A.; Ahlzadeh, G.E.; Kroeger, K.M.; et al. Therapy-mediated delivery of targeted cytotoxins for glioma therapeutics. Proc. Natl. Acad. Sci. USA 2010, 107, 20021–20026. [Google Scholar] [CrossRef] [PubMed]
- Phuphanich, S.; Wheeler, C.J.; Rudnick, J.D.; Mazer, M.; Wang, H.; Nuño, M.A.; Richardson, J.E.; Fan, X.; Ji, J.; Chu, R.M.; et al. Phase I trial of a multi-epitope-pulsed dendritic cell vaccine for patients with newly diagnosed glioblastoma. Cancer Immunol. Immunother. 2013, 62, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Del Vecchio, C.A.; Giacomini, C.P.; Vogel, H.; Jensen, K.C.; Florio, T.; Merlo, A.; Pollack, J.R.; Wong, A.J. EGFRvIII gene rearrangement is an early event in glioblastoma tumorigenesis and expression defines a hierarchy modulated by epigenetic mechanisms. Oncogene 2013, 32, 2670–2681. [Google Scholar] [CrossRef] [PubMed]
- Schuster, J.; Lai, R.K.; Recht, L.D.; Reardon, D.A.; Paleologos, N.A.; Groves, M.D.; Mrugala, M.M.; Jensen, R.; Baehring, J.M.; Sloan, A.; et al. A phase II, multicenter trial of rindopepimut (CDX-110) in newly diagnosed glioblastoma: The ACT III study. Neuro Oncol. 2015, 17, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Zussman, B.M.; Engh, J.A. Outcomes of the ACT III Study. Neurosurgery 2015, 76, N17. [Google Scholar] [CrossRef] [PubMed]
- Pollack, I.F.; Jakacki, R.I.; Butterfield, L.H.; Hamilton, R.L.; Panigrahy, A.; Normolle, D.P.; Connelly, A.K.; Dibridge, S.; Mason, G.; Whiteside, T.L.; et al. Immune responses and outcome after vaccination with glioma-associated antigen peptides and poly-ICLC in a pilot study for pediatric recurrent low-grade gliomas. Neuro Oncol. 2016, 18, 1157–1168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pollack, I.F.; Jakacki, R.I.; Butterfield, L.H.; Hamilton, R.L.; Panigrahy, A.; Potter, D.M.; Connelly, A.K.; Dibridge, S.A.; Whiteside, T.L.; Okada, H. Antigen-Specific Immune Responses and Clinical Outcome After Vaccination With Glioma-Associated Antigen Peptides and Polyinosinic-Polycytidylic Acid Stabilized by Lysine and Carboxymethylcellulose in Children with Newly Diagnosed Malignant Brainstem and Nonbrainstem Gliomas. J. Clin. Oncol. 2014, 32, 2050–2058. [Google Scholar] [PubMed]
- Saka, M.; Amano, T.; Kajiwara, K.; Yoshikawa, K.; Ideguchi, M.; Nomura, S.; Fujisawa, H.; Kato, S.; Fujii, M.; Ueno, K.; et al. Vaccine therapy with dendritic cells transfected with Il13ra2 mRNA for glioma in mice. J. Neurosurg. 2010, 113, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Muul, L.M.; Solomon, D.; Rosenberg, S.A. Expansion of human tumor infiltrating lymphocytes for use in immunotherapy trials. J. Immunol. Methods 1987, 102, 127–141. [Google Scholar] [CrossRef]
- Morgan, R.A.; Dudley, M.E.; Wunderlich, J.R.; Hughes, M.S.; Yang, J.C.; Sherry, R.M.; Royal, R.E.; Topalian, S.L.; Kammula, U.S.; Restifo, N.P.; et al. Cancer Regression in Patients After Transfer of Genetically Engineered Lymphocytes. Science 2006, 314, 126–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gross, G.; Waks, T.; Eshhar, Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc. Natl. Acad. Sci. USA 1989, 86, 10024–10028. [Google Scholar] [CrossRef] [PubMed]
- Eshhar, Z.; Gross, G. Chimeric T cell receptor which incorporates the anti-tumour specificity of a monoclonal antibody with the cytolytic activity of T cells: A model system for immunotherapeutical approach. Br. J. Cancer Suppl. 1990, 10, 27–29. [Google Scholar] [PubMed]
- Almåsbak, H.; Aarvak, T.; Vemuri, M.C. CAR T Cell Therapy: A Game Changer in Cancer Treatment. J. Immunol. Res. 2016, 2016, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Golubovskaya, V. CAR-T Cell Therapy: From the Bench to the Bedside. Cancers 2017, 9, 150. [Google Scholar] [CrossRef] [PubMed]
- FDA Approves CAR-T Cell Therapy to Treat Adults with Certain Types of Large B-Cell Lymphoma. Available online: https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm581216.htm (accessed on 5 September 2018).
- Brown, C.E.; Starr, R.; Aguilar, B.; Brito, A.; Chang, B.; Sarkissian, A.; Weng, L.; Jensen, M.; Barish, M.E.; Badie, B.; et al. Clinical development of IL13Ra2-targeting CAR T cells for the treatment of glioblastoma. J. Immuno. Ther. Cancer 2015, 3, 114. [Google Scholar] [CrossRef]
- Brown, C.E.; Badie, B.; Barish, M.E.; Weng, L.; Ostberg, J.R.; Chang, W.-C.; Naranjo, A.; Starr, R.; Wagner, J.; Wright, C.; et al. Bioactivity and Safety of IL13R 2-Redirected Chimeric Antigen Receptor CD8+ T Cells in Patients with Recurrent Glioblastoma. Clin. Cancer Res. 2015, 21, 4062–4072. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.E.; Aguilar, B.; Starr, R.; Yang, X.; Chang, W.-C.; Weng, L.; Chang, B.; Sarkissian, A.; Brito, A.; Sanchez, J.F.; et al. Optimization of IL13Rα2-Targeted Chimeric Antigen Receptor T Cells for Improved Anti-tumor Efficacy against Glioblastoma. Mol. Ther. 2018, 26, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Krebs, S.; Chow, K.K.H.; Yi, Z.; Rodriguez-Cruz, T.; Hegde, M.; Gerken, C.; Ahmed, N.; Gottschalk, S. T cells redirected to interleukin-13Rα2 with interleukin-13 mutein–chimeric antigen receptors have anti-glioma activity but also recognize interleukin-13Rα1. Cytotherapy 2014, 16, 1121–1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagley, S.J.; Desai, A.S.; Linette, G.P.; June, C.H.; O’Rourke, D.M. CAR T-cell therapy for glioblastoma: Recent clinical advances and future challenges. Neuro Oncol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Kahlon, K.S.; Brown, C.; Cooper, L.J.N.; Raubitschek, A.; Forman, S.J.; Jensen, M.C. Specific recognition and killing of glioblastoma multiforme by interleukin 13-zetakine redirected cytolytic T cells. Cancer Res. 2004, 64, 9160–9166. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Aiyama, H.; Ishikawa, E. CAR-T cell in vivo tracking method using PET scan with the reporter gene and new investigational tracer [18 F] FHBG. Transl. Cancer Res. 2017, 6, S1003–S1005. [Google Scholar] [CrossRef]
- Yaghoubi, S.S.; Jensen, M.C.; Satyamurthy, N.; Budhiraja, S.; Paik, D.; Czernin, J.; Gambhir, S.S. Noninvasive detection of therapeutic cytolytic T cells with 18F-FHBG PET in a patient with glioma. Nat. Clin. Pract. Oncol. 2009, 6, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Berger, C.; Jensen, M.C.; Lansdorp, P.M.; Gough, M.; Elliott, C.; Riddell, S.R. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J. Clin. Invest. 2008, 118, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Phase I Study of Cellular Immunotherapy for Recurrent/Refractory Malignant Glioma Using Intratumoral Infusions of GRm13Z40-2, An Allogeneic CD8+ Cytolitic T-Cell Line Genetically Modified to Express the IL 13-Zetakine and HyTK and to be Resistant to Glucocorticoids, in Combination with Interleukin-2. Available online: https://clinicaltrials.gov/ct2/show/NCT01082926 (accessed on 5 September 2018).
- Kong, S.; Sengupta, S.; Tyler, B.; Bais, A.J.; Ma, Q.; Doucette, S.; Zhou, J.; Sahin, A.; Carter, B.S.; Brem, H.; et al. Suppression of Human Glioma Xenografts with Second-Generation IL13R-Specific Chimeric Antigen Receptor-Modified T Cells. Clin. Cancer Res. 2012, 18, 5949–5960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watson, D.C.; Moysi, E.; Valentin, A.; Bergamaschi, C.; Devasundaram, S.; Fortis, S.P.; Bear, J.; Chertova, E.; Bess, J.; Sowder, R.; et al. Treatment with native heterodimeric IL-15 increases cytotoxic lymphocytes and reduces SHIV RNA in lymph nodes. PLoS Pathog. 2018, 14, e1006902. [Google Scholar]
- Xu, X.-J.; Song, D.-G.; Poussin, M.; Ye, Q.; Sharma, P.; Rodríguez-García, A.; Tang, Y.-M.; Powell, D.J. Multiparameter comparative analysis reveals differential impacts of various cytokines on CART cell phenotype and function ex vivo and in vivo. Oncotarget 2016, 7, 82354–82368. [Google Scholar] [CrossRef] [PubMed]
- Hurton, L.V.; Singh, H.; Najjar, A.M.; Switzer, K.C.; Mi, T.; Maiti, S.; Olivares, S.; Rabinovich, B.; Huls, H.; Forget, M.-A.; et al. Tethered IL-15 augments antitumor activity and promotes a stem-cell memory subset in tumor-specific T cells. Proc. Natl. Acad. Sci. USA 2016, 113, E7788–E7797. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Wang, J.; Huang, H.; Zhao, Y. Novel immunotherapies for adult patients with B-lineage acute lymphoblastic leukemia. J. Hematol. Oncol. 2017, 10, 150. [Google Scholar] [CrossRef] [PubMed]
- Bonifant, C.L.; Jackson, H.J.; Brentjens, R.J.; Curran, K.J. Toxicity and management in CAR T-cell therapy. Mol. Ther. Oncolytics 2016, 3. [Google Scholar] [CrossRef] [PubMed]
- Maude, S.L.; Barrett, D.; Teachey, D.T.; Grupp, S.A. Managing cytokine release syndrome associated with novel T cell-engaging therapies. Cancer J. 2014, 20, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Au, R. Immunooncology: Can the Right Chimeric Antigen Receptors T-Cell Design Be Made to Cure All Types of Cancers and Will It Be Covered? J. Pharm. 2017, 2017, 7513687. [Google Scholar] [CrossRef] [PubMed]
- Mata, M.; Gerken, C.; Nguyen, P.; Krenciute, G.; Spencer, D.M.; Gottschalk, S. Inducible Activation of MyD88 and CD40 in CAR T Cells Results in Controllable and Potent Antitumor Activity in Preclinical Solid Tumor Models. Cancer Discov. 2017, 7, 1306–1319. [Google Scholar] [CrossRef] [PubMed]
- Engineering CD20-Specific Chimeric Receptor-Redirected T Cells with Inducible Co-Expression of a Caspase-9 Based Suicide Switch for Adoptive Immunotherapy of CD20 Positive Lymphoma. Mol. Ther. 2012, 20, S76. [CrossRef]
- O’Rourke, D.M.; Nasrallah, M.P.; Desai, A.; Melenhorst, J.J.; Mansfield, K.; Morrissette, J.J.D.; Martinez-Lage, M.; Brem, S.; Maloney, E.; Shen, A.; et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci. Transl. Med. 2017, 9, eaaa0984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, H.J.; Brentjens, R.J. Overcoming Antigen Escape with CAR T-cell Therapy. Cancer Discov. 2015, 5, 1238–1240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hegde, M.; Mukherjee, M.; Grada, Z.; Pignata, A.; Landi, D.; Navai, S.A.; Wakefield, A.; Fousek, K.; Bielamowicz, K.; Chow, K.K.H.; et al. Tandem CAR T cells targeting HER2 and IL13Rα2 mitigate tumor antigen escape. J. Clin. Invest. 2016, 126, 3036–3052. [Google Scholar] [CrossRef] [PubMed]
- Krenciute, G.; Prinzing, B.L.; Yi, Z.; Wu, M.-F.; Liu, H.; Dotti, G.; Balyasnikova, I.V; Gottschalk, S. Transgenic Expression of IL15 Improves Antiglioma Activity of IL13Rα2-CAR T Cells but Results in Antigen Loss Variants. Cancer Immunol. Res. 2017, 5, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Debinski, W. When better still might not be good enough. Transl. Cancer Res. 2017, 6, S1244–S1247. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Hu-Lieskovan, S.; Wargo, J.A.; Ribas, A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017, 168, 707–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benitez, J.A.; Ma, J.; D’Antonio, M.; Boyer, A.; Camargo, M.F.; Zanca, C.; Kelly, S.; Khodadadi-Jamayran, A.; Jameson, N.M.; Andersen, M.; et al. PTEN regulates glioblastoma oncogenesis through chromatin-associated complexes of DAXX and histone H3.3. Nat. Commun. 2017, 8, 15223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, P.C.; Lu, G.; Deng, Y.; Wang, C.D.; Su, X.W.; Zhou, J.Y.; Chan, T.M.; Hu, X.; Poon, W.S. Inhibition of NF-κB Pathway and Modulation of MAPK Signaling Pathways in Glioblastoma and Implications for Lovastatin and Tumor Necrosis Factor-Related Apoptosis Inducing Ligand (TRAIL) Combination Therapy. PLoS ONE 2017, 12, e0171157. [Google Scholar] [CrossRef] [PubMed]
- Gieryng, A.; Kaminska, B. Myeloid-derived suppressor cells in gliomas. Contemp. Oncol. (Poznan, Poland) 2016, 20, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Hu, B.; Hu, X.; Kim, H.; Squatrito, M.; Scarpace, L.; deCarvalho, A.C.; Lyu, S.; Li, P.; Li, Y.; et al. Tumor Evolution of Glioma-Intrinsic Gene Expression Subtypes Associates with Immunological Changes in the Microenvironment. Cancer Cell 2017, 32, 42–56. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Jun, Y.; Kim, S.H.; Hoang, H.-H.; Jung, Y.; Kim, S.; Kim, J.; Austin, R.H.; Lee, S.; Park, S. Rapid emergence and mechanisms of resistance by U87 glioblastoma cells to doxorubicin in an in vitro tumor microfluidic ecology. Proc. Natl. Acad. Sci. USA 2016, 113, 14283–14288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, R.; Pan, Y.; Gutmann, D.H. The power of the few. Genes Dev. 2017, 31, 1177–1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unified nomenclature for Eph family receptors and their ligands, the ephrins. Eph Nomenclature Committee. Cell 1997, 90, 403–404. [CrossRef]
- Klein, R. Eph/ephrin signalling during development. Development 2012, 139, 4105–4109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatzizacharias, N.A.; Giaginis, C.T.; Agapitos, E.; Theocharis, S.E. The role of ephrins’ receptors and ephrins’ ligands in normal placental development and disease. Expert Opin. Ther. Targets 2014, 18, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Baumann, G.; Travieso, L.; Liebl, D.J.; Theus, M.H. Pronounced hypoxia in the subventricular zone following traumatic brain injury and the neural stem/progenitor cell response. Exp. Biol. Med. 2013, 238, 830–841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frugier, T.; Conquest, A.; McLean, C.; Currie, P.; Moses, D.; Goldshmit, Y. Expression and Activation of EphA4 in the Human Brain after Traumatic Injury. J. Neuropathol. Exp. Neurol. 2012, 71, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Cavodeassi, F.; Ivanovitch, K.; Wilson, S.W. Eph/Ephrin signalling maintains eye field segregation from adjacent neural plate territories during forebrain morphogenesis. Development 2013, 140, 4193–4202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bochenek, M.L.; Dickinson, S.; Astin, J.W.; Adams, R.H.; Nobes, C.D. Ephrin-B2 regulates endothelial cell morphology and motility independently of Eph-receptor binding. J. Cell Sci. 2010, 123, 1235–1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xi, H.-Q.; Wu, X.-S.; Wei, B.; Chen, L. Eph receptors and ephrins as targets for cancer therapy. J. Cell. Mol. Med. 2012, 16, 2894–2909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.; Park, P.J.; Lai, W.; Maher, E.; Chakravarti, A.; Durso, L.; Jiang, X.; Yu, Y.; Brosius, A.; Thomas, M.; et al. Genome-Wide Screen Reveals Functional Gene Clusters in the Cancer Genome and Identifies EphA2 as a Mitogen in Glioblastoma. Cancer Res. 2006, 66, 10815–10823. [Google Scholar] [CrossRef] [PubMed]
- Wykosky, J.; Gibo, D.M.; Stanton, C.; Debinski, W. EphA2 as a novel molecular marker and target in glioblastoma multiforme. Mol. Cancer Res. 2005, 3, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Wykosky, J.; Debinski, W. The EphA2 receptor and ephrinA1 ligand in solid tumors: Function and therapeutic targeting. Mol. Cancer Res. 2008, 6, 1795–1806. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; He, S.; Fu, J.; Li, G.; Xu, R.; Lu, H.; Deng, J. Expression of EphrinB2 and EphB4 in glioma tissues correlated to the progression of glioma and the prognosis of glioblastoma patients. Clin. Transl. Oncol. 2012, 14, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-F.; Fokas, E.; Juricko, J.; You, A.; Rose, F.; Pagenstecher, A.; Engenhart-Cabillic, R.; An, H.-X. Increased expression of EphA7 correlates with adverse outcome in primary and recurrent glioblastoma multiforme patients. BMC Cancer 2008, 8, 79. [Google Scholar] [CrossRef] [PubMed]
- Teng, L.; Nakada, M.; Furuyama, N.; Sabit, H.; Furuta, T.; Hayashi, Y.; Takino, T.; Dong, Y.; Sato, H.; Sai, Y.; et al. Ligand-dependent EphB1 signaling suppresses glioma invasion and correlates with patient survival. Neuro. Oncol. 2013, 15, 1710–1720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferluga, S.; Tomé, C.M.L.; Herpai, D.M.; D’Agostino, R.; Debinski, W.; Ferluga, S.; Tomé, C.M.L.; Herpai, D.M.; D’Agostino, R.; Debinski, W. Simultaneous targeting of Eph receptors in glioblastoma. Oncotarget 2016, 7, 59860–59876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiari, R.; Hames, G.; Stroobant, V.; Texier, C.; Maillère, B.; Boon, T.; Coulie, P.G.; Baer, M.; Palath, V.; Bebbington, C.; et al. Identification of a tumor-specific shared antigen derived from an Eph receptor and presented to CD4 T cells on HLA class II molecules. Cancer Res. 2000, 60, 4855–4863. [Google Scholar] [PubMed]
- Day, B.W.; Stringer, B.W.; Al-Ejeh, F.; Ting, M.J.; Wilson, J.; Ensbey, K.S.; Jamieson, P.R.; Bruce, Z.C.; Lim, Y.C.; Offenhäuser, C.; et al. EphA3 Maintains Tumorigenicity and Is a Therapeutic Target in Glioblastoma Multiforme. Cancer Cell 2013, 23, 238–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wykosky, J.; Gibo, D.M.; Debinski, W. A novel, potent, and specific ephrinA1-based cytotoxin against EphA2 receptor expressing tumor cells. Mol. Cancer Ther. 2007, 6, 3208–3218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossmeisl, J.H.; Herpai, D.; Robertson, J.L.; Dickinson, P.J.; Tatter, S.B.; Debinski, W. P08.12 Tolerability and initial efficacy of convection-enhanced delivery of combinatorial IL-13RA2 and EphA2 targeted cytotoxins to dogs with spontaneous intracranial malignant gliomas. Neuro Oncol. 2017, 19. [Google Scholar] [CrossRef]
- Yi, Z.; Prinzing, B.L.; Cao, F.; Gottschalk, S.; Krenciute, G. Optimizing EphA2-CAR T Cells for the Adoptive Immunotherapy of Glioma. Mol. Ther. Methods Clin. Dev. 2018, 9, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Debinski, W.; Priebe, W.; Tatter, S.B. Maximizing Local Access to Therapeutic Deliveries in Glioblastoma. Part I: Targeted Cytotoxic Therapy; Codon Publications: Brisbane, Australia, 2017; ISBN 9780994438126. [Google Scholar]
- Konecny, G.E.; Glas, R.; Dering, J.; Manivong, K.; Qi, J.; Finn, R.S.; Yang, G.R.; Hong, K.-L.; Ginther, C.; Winterhoff, B.; et al. Activity of the multikinase inhibitor dasatinib against ovarian cancer cells. Br. J. Cancer 2009, 101, 1699–1708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tryfonopoulos, D.; Walsh, S.; Collins, D.M.; Flanagan, L.; Quinn, C.; Corkery, B.; McDermott, E.W.; Evoy, D.; Pierce, A.; O’Donovan, N.; et al. Src: A potential target for the treatment of triple-negative breast cancer. Ann. Oncol. 2011, 22, 2234–2240. [Google Scholar] [CrossRef] [PubMed]
- Yeddula, N.; Xia, Y.; Ke, E.; Beumer, J.; Verma, I.M.; Berns, A.; Jacks, T.; Mccormick, F. Screening for tumor suppressors: Loss of ephrin receptor A2 cooperates with oncogenic KRas in promoting lung adenocarcinoma. Proc. Natl. Acad. Sci. USA 2015, 47, E6476–E6485. [Google Scholar] [CrossRef] [PubMed]
- Petty, A.; Myshkin, E.; Qin, H.; Guo, H.; Miao, H.; Tochtrop, G.P.; Hsieh, J.-T.; Page, P.; Liu, L.; Lindner, D.J.; et al. A Small Molecule Agonist of EphA2 Receptor Tyrosine Kinase Inhibits Tumor Cell Migration In Vitro and Prostate Cancer Metastasis In Vivo. PLoS ONE 2012, 7, e42120. [Google Scholar] [CrossRef] [PubMed]
- Rundle-Thiele, D.; Head, R.; Cosgrove, L.; Martin, J.H. Repurposing some older drugs that cross the blood-brain barrier and have potential anticancer activity to provide new treatment options for glioblastoma. Br. J. Clin. Pharmacol. 2016, 81, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Pasquale, E.B. Erratum: Eph receptor signalling casts a wide net on cell behaviour. Nat. Rev. Mol. Cell Biol. 2005, 6, 462–475. [Google Scholar] [CrossRef] [PubMed]
- Ridley, A.J. Rho GTPase signalling in cell migration. Curr. Opin. Cell Biol. 2015, 36, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Ridley, A. Blebbing: Motility research moves in a new direction. Nat. Rev. Mol. Cell Biol. 2009, 10, 164. [Google Scholar] [CrossRef]
- Montano, N.; Cenci, T.; Martini, M.; D’Alessandris, Q.G.; Pelacchi, F.; Ricci-Vitiani, L.; Maira, G.; De Maria, R.; Larocca, L.M.; Pallini, R. Expression of EGFRvIII in glioblastoma: Prognostic significance revisited. Neoplasia 2011, 13, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Heimberger, A.B.; Suki, D.; Yang, D.; Shi, W.; Aldape, K. The natural history of EGFR and EGFRvIII in glioblastoma patients. J. Transl. Med. 2005, 3, 38. [Google Scholar] [CrossRef] [PubMed]
- Voldborg, B.R.; Damstrup, L.; Spang-Thomsen, M.; Poulsen, H.S. Epidermal growth factor receptor (EGFR) and EGFR mutations, function and possible role in clinical trials. Ann. Oncol. 1997, 8, 1197–1206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ennis, B.W.; Lippman, M.E.; Dickson, R.B. The EGF receptor system as a target for antitumor therapy. Cancer Invest. 1991, 9, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Libermann, T.A.; Nusbaum, H.R.; Razon, N.; Kris, R.; Lax, I.; Soreq, H.; Whittle, N.; Waterfield, M.D.; Ullrich, A.; Schlessinger, J. Amplification, enhanced expression and possible rearrangement of EGF receptor gene in primary human brain tumours of glial origin. Nature 1985, 313, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Bonavia, R.; Inda, M.M.; Vandenberg, S.; Cheng, S.-Y.; Nagane, M.; Hadwiger, P.; Tan, P.; Sah, D.W.Y.; Cavenee, W.K.; Furnari, F.B. EGFRvIII promotes glioma angiogenesis and growth through the NF-κB, interleukin-8 pathway. Oncogene 2012, 31, 4054–4066. [Google Scholar] [CrossRef] [PubMed]
- Latha, K.; Li, M.; Chumbalkar, V.; Gururaj, A.; Hwang, Y.; Dakeng, S.; Sawaya, R.; Aldape, K.; Cavenee, W.K.; Bogler, O.; et al. Nuclear EGFRvIII-STAT5b complex contributes to glioblastoma cell survival by direct activation of the Bcl-XL promoter. Int. J. Cancer 2013, 132, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Resnier, P.; David, S.; Lautram, N.; Delcroix, G.J.-R.; Clavreul, A.; Benoit, J.-P.; Passirani, C. EGFR siRNA lipid nanocapsules efficiently transfect glioma cells in vitro. Int. J. Pharm. 2013, 454, 748–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.; Park, J.O.; Zhang, M. Treatment of glioblastoma multiforme using a combination of small interfering RNA targeting epidermal growth factor receptor and β-catenin. J. Gene Med. 2013, 15, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.D.; Krishnan, S.; Sarkaria, J.N.; Wu, W.; Jaeckle, K.A.; Uhm, J.H.; Geoffroy, F.J.; Arusell, R.; Kitange, G.; Jenkins, R.B.; et al. North Central Cancer Treatment Group Study N0177 Phase I/II Trial of Erlotinib and Temozolomide With Radiation Therapy in the Treatment of Newly Diagnosed Glioblastoma Multiforme: North Central Cancer Treatment Group Study N0177. J. Clin. Oncol. 2008, 26, 5603–5609. [Google Scholar] [PubMed]
- Gallego, O.; Cuatrecasas, M.; Benavides, M.; Segura, P.P.; Berrocal, A.; Erill, N.; Colomer, A.; Quintana, M.J.; Balaña, C.; Gil, M.; et al. Efficacy of erlotinib in patients with relapsed gliobastoma multiforme who expressed EGFRVIII and PTEN determined by immunohistochemistry. J. Neurooncol. 2014, 116, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Custodio, A.; Calles, A.; Pérez-Segura, P. Response to erlotinib in recurrent glioblastoma multiforme showing coexpression of EGFRvIII and PTEN. Clin. Transl. Oncol. 2010, 12, 310–314. [Google Scholar] [PubMed]
- Uhm, J.H.; Ballman, K.V; Wu, W.; Giannini, C.; Krauss, J.C.; Buckner, J.C.; James, C.D.; Scheithauer, B.W.; Behrens, R.J.; Flynn, P.J.; et al. Phase II evaluation of gefitinib in patients with newly diagnosed Grade 4 astrocytoma: Mayo/North Central Cancer Treatment Group Study N0074. Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Fukai, J.; Nishio, K.; Itakura, T.; Koizumi, F. Antitumor activity of cetuximab against malignant glioma cells overexpressing EGFR deletion mutant variant III. Cancer Sci. 2008, 99, 2062–2069. [Google Scholar] [CrossRef] [PubMed]
- Vredenburgh, J.J.; Desjardins, A.; Herndon, J.E.; Marcello, J.; Reardon, D.A.; Quinn, J.A.; Rich, J.N.; Sathornsumetee, S.; Gururangan, S.; Sampson, J.; et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J. Clin. Oncol. 2007, 25, 4722–4729. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Peng, Y.; Liao, Y.; Jiang, W.; Wei, R.; Huo, L.; Han, Z.; Duan, C.; Zhong, M. Nimotuzumab prolongs survival in patients with malignant gliomas: A phase I/II clinical study of concomitant radiochemotherapy with or without nimotuzumab. Exp. Ther. Med. 2012, 4, 151–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sampson, J.H.; Akabani, G.; Archer, G.E.; Berger, M.S.; Coleman, R.E.; Friedman, A.H.; Friedman, H.S.; Greer, K.; Herndon, J.E.; Kunwar, S.; et al. Intracerebral infusion of an EGFR-targeted toxin in recurrent malignant brain tumors. Neuro. Oncol. 2008, 10, 320–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, B.D.; O’Rourke, D.M.; Maus, M.V. Engineering Chimeric Antigen Receptor T cells to Treat Glioblastoma. J. Target. Ther. Cancer 2017, 6, 22–25. [Google Scholar] [PubMed]
- Rosenberg, S.A. Cell transfer immunotherapy for metastatic solid cancer—What clinicians need to know. Nat. Rev. Clin. Oncol. 2011, 8, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Brawley, V.; Hegde, M.; Bielamowicz, K.; Kalra, M.; Landi, D.; Robertson, C.; Gray, T.L.; Diouf, O.; Wakefield, A.; et al. HER2-Specific Chimeric Antigen Receptor–Modified Virus-Specific T Cells for Progressive Glioblastoma. JAMA Oncol. 2017, 3, 1094. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Brawley, V.; Hegde, M.; Bielamowicz, K.; Wakefield, A.; Ghazi, A.; Ashoori, A.; Diouf, O.; Gerken, C.; Landi, D.; et al. Autologous HER2 CMV bispecific CAR T cells are safe and demonstrate clinical benefit for glioblastoma in a Phase I trial. J. Immunother. Cancer 2015, 3, O11. [Google Scholar] [CrossRef]
- Reardon, D.A.; Turner, S.; Peters, K.B.; Desjardins, A.; Gururangan, S.; Sampson, J.H.; McLendon, R.E.; Herndon, J.E.; Jones, L.W.; Kirkpatrick, J.P.; et al. A review of VEGF/VEGFR-targeted therapeutics for recurrent glioblastoma. J. Natl. Compr. Canc. Netw. 2011, 9, 414–427. [Google Scholar] [CrossRef] [PubMed]
- Nazarenko, I.; Hede, S.-M.; He, X.; Hedrén, A.; Thompson, J.; Lindström, M.S.; Nistér, M. PDGF and PDGF receptors in glioma. Ups. J. Med. Sci. 2012, 117, 99–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joseph, J.V.; Balasubramaniyan, V.; Walenkamp, A.; Kruyt, F.A.E. TGF-β as a therapeutic target in high grade gliomas—Promises and challenges. Biochem. Pharmacol. 2013, 85, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Chae, Y.K.; Ranganath, K.; Hammerman, P.S.; Vaklavas, C.; Mohindra, N.; Kalyan, A.; Matsangou, M.; Costa, R.; Carneiro, B.; Villaflor, V.M.; et al. Inhibition of the fibroblast growth factor receptor (FGFR) pathway: The current landscape and barriers to clinical application. Oncotarget 2017, 8, 16052–16074. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.J.; Burns, T.C.; Zhang, Y.; Abounader, R. Targeting MET for glioma therapy. Neurosurg. Focus 2014, 37, E10. [Google Scholar] [CrossRef] [PubMed]
- Joshi, B.H.; Leland, P.; Silber, J.; Kreitman, R.J.; Pastan, I.; Berger, M.; Puri, R.K. IL-4 receptors on human medulloblastoma tumours serve as a sensitive target for a circular permuted IL-4-Pseudomonas exotoxin fusion protein. Br. J. Cancer 2002, 86, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Rustamzadeh, E.; Li, C.; Doumbia, S.; Hall, W.A.; Vallera, D.A. Targeting the over-expressed urokinase-type plasminogen activator receptor on glioblastoma multiforme. J. Neurooncol. 2003, 65, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Cherry, A.E.; Stella, N. G protein-coupled receptors as oncogenic signals in glioma: Emerging therapeutic avenues. Neuroscience 2014, 278, 222–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, T.; Wu, H.; Li, Y.; Huang, Y.; Yao, L.; Chen, X.; Han, X.; Zhou, Y.; Du, Z. Targeting transferrin receptor delivery of temozolomide for a potential glioma stem cell-mediated therapy. Oncotarget 2017, 8, 74451–74465. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Ahsan, S.M.; Kumar, J.M.; Kondapi, A.K.; Rao, N.M. Overcoming blood brain barrier with a dual purpose Temozolomide loaded Lactoferrin nanoparticles for combating glioma (SERP-17-12433). Sci. Rep. 2017, 7, 6602. [Google Scholar] [CrossRef] [PubMed]
- Maletínská, L.; Blakely, E.A.; Bjornstad, K.A.; Deen, D.F.; Knoff, L.J.; Forte, T.M. Human glioblastoma cell lines: Levels of low-density lipoprotein receptor and low-density lipoprotein receptor-related protein. Cancer Res. 2000, 60, 2300–2303. [Google Scholar] [PubMed]
- Guo, J.; Schlich, M.; Cryan, J.F.; O’Driscoll, C.M. Targeted Drug Delivery via Folate Receptors for the Treatment of Brain Cancer: Can the Promise Deliver? J. Pharm. Sci. 2017, 106, 3413–3420. [Google Scholar] [CrossRef] [PubMed]
- Paolillo, M.; Serra, M.; Schinelli, S. Integrins in glioblastoma: Still an attractive target? Pharmacol. Res. 2016, 113, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.H.; Shen, Y.L.; Keegan, P.; Pazdur, R. FDA Drug Approval Summary: Bevacizumab (Avastin(R)) as Treatment of Recurrent Glioblastoma Multiforme. Oncologist 2009, 14, 1131–1138. [Google Scholar] [CrossRef] [PubMed]
- Soffietti, R.; Trevisan, E.; Bertero, L.; Cassoni, P.; Morra, I.; Fabrini, M.G.; Pasqualetti, F.; Lolli, I.; Castiglione, A.; Ciccone, G.; et al. Bevacizumab and fotemustine for recurrent glioblastoma: A phase II study of AINO (Italian Association of Neuro-Oncology). J. Neurooncol. 2014, 116, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Balaña, C.; Estival, A.; Pineda, E.; Sepúlveda, J.; Mesía, C.; del Barco, S.; Gil-Gil, M.; Hardy, M.; Indacoechea, A.; Cardona, A.F. Prolonged survival after bevacizumab rechallenge in glioblastoma patients with previous response to bevacizumab. Neuro Oncol. Pract. 2016, 4, 15–23. [Google Scholar] [CrossRef]
- Stupp, R.; Hegi, M.E.; Neyns, B.; Goldbrunner, R.; Schlegel, U.; Clement, P.M.J.; Grabenbauer, G.G.; Ochsenbein, A.F.; Simon, M.; Dietrich, P.-Y.; et al. Phase I/IIa Study of Cilengitide and Temozolomide With Concomitant Radiotherapy Followed by Cilengitide and Temozolomide Maintenance Therapy in Patients With Newly Diagnosed Glioblastoma. J. Clin. Oncol. 2010, 28, 2712–2718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reardon, D.A.; Neyns, B.; Weller, M.; Tonn, J.C.; Nabors, L.B.; Stupp, R. Cilengitide: An RGD pentapeptide ανβ3 and ανβ5 integrin inhibitor in development for glioblastoma and other malignancies. Future Oncol. 2011, 7, 339–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timotheadou, E. New agents targeting angiogenesis in glioblastoma. Chemother. Res. Pract. 2011, 2011, 878912. [Google Scholar] [CrossRef] [PubMed]
- Batchelor, T.T.; Mulholland, P.; Neyns, B.; Nabors, L.B.; Campone, M.; Wick, A.; Mason, W.; Mikkelsen, T.; Phuphanich, S.; Ashby, L.S.; et al. Phase III Randomized Trial Comparing the Efficacy of Cediranib As Monotherapy, and in Combination With Lomustine, Versus Lomustine Alone in Patients With Recurrent Glioblastoma. J. Clin. Oncol. 2013, 31, 3212–3218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zustovich, F.; Landi, L.; Lombardi, G.; Porta, C.; Galli, L.; Fontana, A.; Amoroso, D.; Galli, C.; Andreuccetti, M.; Falcone, A.; et al. Sorafenib plus daily low-dose temozolomide for relapsed glioblastoma: A phase II study. Anticancer Res. 2013, 33, 3487–3494. [Google Scholar] [CrossRef] [PubMed]
- Wetmore, C.; Daryani, V.M.; Billups, C.A.; Boyett, J.M.; Leary, S.; Tanos, R.; Goldsmith, K.C.; Stewart, C.F.; Blaney, S.M.; Gajjar, A. Phase II evaluation of sunitinib in the treatment of recurrent or refractory high-grade glioma or ependymoma in children: A children’s Oncology Group Study ACNS1021. Cancer Med. 2016, 5, 1416–1424. [Google Scholar] [CrossRef] [PubMed]
- Haberler, C.; Gelpi, E.; Marosi, C.; Rössler, K.; Birner, P.; Budka, H.; Hainfellner, J.A. Immunohistochemical Analysis of Platelet-derived Growth Factor Receptor-α, -β, c-kit, c-abl, and Arg Proteins in Glioblastoma: Possible Implications for Patient Selection for Imatinib Mesylate Therapy. J. Neurooncol. 2006, 76, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, E.; Stupp, R.; van den Bent, M.J.; van Herpen, C.; Laigle Donadey, F.; Gorlia, T.; Hegi, M.; Lhermitte, B.; Strauss, L.C.; Allgeier, A.; et al. EORTC 26083 phase I/II trial of dasatinib in combination with CCNU in patients with recurrent glioblastoma. Neuro Oncol. 2012, 14, 1503–1510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, E.; Walters, I.B.; Hanahan, D. Brivanib, a dual FGF/VEGF inhibitor, is active both first and second line against mouse pancreatic neuroendocrine tumors developing adaptive/evasive resistance to VEGF inhibition. Clin. Cancer Res. 2011, 17, 5299–5310. [Google Scholar] [CrossRef] [PubMed]
- Weaver, M.; Laske, D.W. Transferrin receptor ligand-targeted toxin conjugate (Tf-CRM107) for therapy of malignant gliomas. J. Neurooncol. 2003, 65, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Han, H.; He, X.; Li, S.; Wu, C.; Yu, C.; Wang, S. Expression of the galectin-9-Tim-3 pathway in glioma tissues is associated with the clinical manifestations of glioma. Oncol. Lett. 2016, 11, 1829–1834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Didenko, V.V; Ngo, H.N.; Minchew, C.; Baskin, D.S. Apoptosis of T lymphocytes invading glioblastomas multiforme: A possible tumor defense mechanism. J. Neurosurg. 2002, 96, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Fecci, P.E.; Ochiai, H.; Mitchell, D.A.; Grossi, P.M.; Sweeney, A.E.; Archer, G.E.; Cummings, T.; Allison, J.P.; Bigner, D.D.; Sampson, J.H. Systemic CTLA-4 blockade ameliorates glioma-induced changes to the CD4+ T cell compartment without affecting regulatory T-cell function. Clin. Cancer Res. 2007, 13, 2158–2167. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.; Song, G.; Yu, J. The prognostic significance of PD-L1 expression in patients with glioma: A meta-analysis. Sci. Rep. 2017, 7, 4231. [Google Scholar] [CrossRef] [PubMed]
- Nduom, E.K.; Weller, M.; Heimberger, A.B. Immunosuppressive mechanisms in glioblastoma. Neuro. Oncol. 2015, 17, vii9–vii14. [Google Scholar]
- Zagzag, D.; Salnikow, K.; Chiriboga, L.; Yee, H.; Lan, L.; Ali, M.A.; Garcia, R.; Demaria, S.; Newcomb, E.W. Downregulation of major histocompatibility complex antigens in invading glioma cells: Stealth invasion of the brain. Lab. Investig. 2005, 85, 328–341. [Google Scholar] [CrossRef] [PubMed]
- Silk, A.W.; Bassetti, M.F.; West, B.T.; Tsien, C.I.; Lao, C.D. Ipilimumab and radiation therapy for melanoma brain metastases. Cancer Med. 2013, 2, 899–906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vom Berg, J.; Vrohlings, M.; Haller, S.; Haimovici, A.; Kulig, P.; Sledzinska, A.; Weller, M.; Becher, B. Intratumoral IL-12 combined with CTLA-4 blockade elicits T cell-mediated glioma rejection. J. Exp. Med. 2013, 210, 2803–2811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwalla, P.; Barnard, Z.; Fecci, P.; Dranoff, G.; Curry, W.T. Sequential Immunotherapy by Vaccination with GM-CSF–expressing Glioma Cells and CTLA-4 Blockade Effectively Treats Established Murine Intracranial Tumors. J. Immunother. 2012, 35, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Ott, P.A.; Hodi, F.S.; Buchbinder, E.I. Inhibition of Immune Checkpoints and Vascular Endothelial Growth Factor as Combination Therapy for Metastatic Melanoma: An Overview of Rationale, Preclinical Evidence, and Initial Clinical Data. Front. Oncol. 2015, 5, 202. [Google Scholar] [CrossRef] [PubMed]
- Prasad, V.; Kaestner, V. Nivolumab and pembrolizumab: Monoclonal antibodies against programmed cell death-1 (PD-1) that are interchangeable. Semin. Oncol. 2017, 44, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Omuro, A.; Vlahovic, G.; Lim, M.; Sahebjam, S.; Baehring, J.; Cloughesy, T.; Voloschin, A.; Ramkissoon, S.H.; Ligon, K.L.; Latek, R.; et al. Nivolumab with or without ipilimumab in patients with recurrent glioblastoma: Results from exploratory phase I cohorts of CheckMate 143. Neuro. Oncol. 2018, 20, 674–686. [Google Scholar] [CrossRef] [PubMed]
- Momtaz, P.; Postow, M.A. Immunologic checkpoints in cancer therapy: Focus on the programmed death-1 (PD-1) receptor pathway. Pharmgenomics Pers. Med. 2014, 7, 357–365. [Google Scholar] [PubMed]
- Seidel, J.A.; Otsuka, A.; Kabashima, K. Anti-PD-1 and Anti-CTLA-4 Therapies in Cancer: Mechanisms of Action, Efficacy, and Limitations. Front. Oncol. 2018, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.C.; Joller, N.; Kuchroo, V.K. Lag-3, Tim-3, and TIGIT: Co-inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity 2016, 44, 989–1004. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Feng, S.; Xu, L.; Shi, W.; Wang, X.; Wang, H.; Yu, C.; Dong, T.; Xu, M.; Liang, G. Tim-3 on Peripheral CD4+ and CD8+ T Cells Is Involved in the Development of Glioma. DNA Cell Biol. 2014, 33, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Ngiow, S.F.; von Scheidt, B.; Akiba, H.; Yagita, H.; Teng, M.W.L.; Smyth, M.J. Anti-TIM3 Antibody Promotes T Cell IFN-Mediated Antitumor Immunity and Suppresses Established Tumors. Cancer Res. 2011, 71, 3540–3551. [Google Scholar] [CrossRef] [PubMed]
- Andrews, L.P.; Marciscano, A.E.; Drake, C.G.; Vignali, D.A.A. LAG3 (CD223) as a cancer immunotherapy target. Immunol. Rev. 2017, 276, 80–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujii, M.; Sugamura, K.; Sano, K.; Nakai, M.; Sugita, K.; Hinuma, Y. High-affinity receptor-mediated internalization and degradation of interleukin 2 in human T cells. J. Exp. Med. 1986, 163, 550–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barzon, L.; Pacenti, M.; Franchin, E.; Colombo, F.; Palù, G. HSV-tk/IL-2 Gene Therapy for Glioblastoma Multiforme. Methods Mol. Biol. 2009, 542, 529–549. [Google Scholar] [PubMed]
- Okada, H.; Giezeman-Smits, K.M.; Tahara, H.; Attanucci, J.; Fellows, W.K.; Lotze, M.T.; Chambers, W.H.; Bozik, M.E. Effective cytokine gene therapy against an intracranial glioma using a retrovirally transduced IL-4 plus HSVtk tumor vaccine. Gene Ther. 1999, 6, 219–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawaji, H.; Tokuyama, T.; Yamasaki, T.; Amano, S.; Sakai, N.; Namba, H. Interferon-β and temozolomide combination therapy for temozolomide monotherapy-refractory malignant gliomas. Mol. Clin. Oncol. 2015, 3, 909–913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boorjian, S.A.; Milowsky, M.I.; Kaplan, J.; Albert, M.; Cobham, M.V.; Coll, D.M.; Mongan, N.P.; Shelton, G.; Petrylak, D.; Gudas, L.J.; et al. Phase 1/2 Clinical Trial of Interferon α2b and Weekly Liposome-encapsulated All-trans Retinoic Acid in Patients with Advanced Renal Cell Carcinoma. J. Immunother. 2007, 30, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Mancone, S.; Lycan, T.; Ahmed, T.; Topaloglu, U.; Dothard, A.; Petty, W.J.; Strowd, R.E. Severe neurologic complications of immune checkpoint inhibitors: A single-center review. J. Neurol. 2018, 265, 1636–1642. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowska, A.; Nandhu, M.S.; Behera, P.; Chiocca, E.A.; Viapiano, M.S. Strategies in gene therapy for glioblastoma. Cancers 2013, 5, 1271–1305. [Google Scholar] [CrossRef] [PubMed]
- Fukuhara, H.; Ino, Y.; Todo, T. Oncolytic virus therapy: A new era of cancer treatment at dawn. Cancer Sci. 2016, 107, 1373–1379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liikanen, I.; Koski, A.; Merisalo-Soikkeli, M.; Hemminki, O.; Oksanen, M.; Kairemo, K.; Joensuu, T.; Kanerva, A.; Hemminki, A. Serum HMGB1 is a predictive and prognostic biomarker for oncolytic immunotherapy. Oncoimmunology 2015, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, L.; Ge, K.; Zheng, Z. [Suicide gene therapy for glioma using HSV-tk/GCV system]. Zhonghua Zhong Liu Za Zhi 1997, 19, 353–357. [Google Scholar] [PubMed]
- Rainov, N.G. A Phase III Clinical Evaluation of Herpes Simplex Virus Type 1 Thymidine Kinase and Ganciclovir Gene Therapy as an Adjuvant to Surgical Resection and Radiation in Adults with Previously Untreated Glioblastoma Multiforme. Hum. Gene Ther. 2000, 11, 2389–2401. [Google Scholar] [CrossRef] [PubMed]
- King, G.D.; Kroeger, K.M.; Bresee, C.J.; Candolfi, M.; Liu, C.; Manalo, C.M.; Muhammad, A.G.; Pechnick, R.N.; Lowenstein, P.R.; Castro, M.G. Flt3L in Combination With HSV1-TK-mediated Gene Therapy Reverses Brain Tumor–induced Behavioral Deficits. Mol. Ther. 2008, 16, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Samson, A.; Scott, K.J.; Taggart, D.; West, E.J.; Wilson, E.; Nuovo, G.J.; Thomson, S.; Corns, R.; Mathew, R.K.; Fuller, M.J.; et al. Intravenous delivery of oncolytic reovirus to brain tumor patients immunologically primes for subsequent checkpoint blockade. Sci. Transl. Med. 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.; Paraskevakou, G.; Liu, C.; Iankov, I.D.; Msaouel, P.; Zollman, P.; Myers, R.; Peng, K.W.; Russell, S.J.; Galanis, E. Oncolytic measles virus strains in the treatment of gliomas. Expert Opin. Biol. Ther. 2008, 8, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Aoki, H.; Kühnel, F.; Kondo, Y.; Kubicka, S.; Wirth, T.; Iwado, E.; Iwamaru, A.; Fujiwara, K.; Hess, K.R.; et al. Autophagic Cell Death of Malignant Glioma Cells Induced by a Conditionally Replicating Adenovirus. JNCI J. Natl. Cancer Inst. 2006, 98, 625–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ning, J.; Wakimoto, H. Oncolytic herpes simplex virus-based strategies: Toward a breakthrough in glioblastoma therapy. Front. Microbiol. 2014, 5, 303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiocca, E.A.; Abbed, K.M.; Tatter, S.; Louis, D.N.; Hochberg, F.H.; Barker, F.; Kracher, J.; Grossman, S.A.; Fisher, J.D.; Carson, K.; et al. A Phase I Open-Label, Dose-Escalation, Multi-Institutional Trial of Injection with an E1B-Attenuated Adenovirus, ONYX-015, into the Peritumoral Region of Recurrent Malignant Gliomas, in the Adjuvant Setting. Mol. Ther. 2004, 10, 958–966. [Google Scholar] [CrossRef] [PubMed]
- Panek, W.K.; Kane, J.R.; Young, J.S.; Rashidi, A.; Kim, J.W.; Kanojia, D.; Lesniak, M.S. Hitting the nail on the head: Combining oncolytic adenovirus-mediated virotherapy and immunomodulation for the treatment of glioma. Oncotarget 2017, 8, 89391–89405. [Google Scholar] [CrossRef] [PubMed]
- Cary, Z.D.; Willingham, M.C.; Lyles, D.S. Oncolytic vesicular stomatitis virus induces apoptosis in U87 glioblastoma cells by a type II death receptor mechanism and induces cell death and tumor clearance in vivo. J. Virol. 2011, 85, 5708–5717. [Google Scholar] [CrossRef] [PubMed]
- Wollmann, G.; Ozduman, K.; van den Pol, A.N. Oncolytic virus therapy for glioblastoma multiforme: Concepts and candidates. Cancer J. 2012, 18, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Msaouel, P.; Dispenzieri, A.; Galanis, E. Clinical testing of engineered oncolytic measles virus strains in the treatment of cancer: An overview. Curr. Opin. Mol. Ther. 2009, 11, 43–53. [Google Scholar] [PubMed]
- Viral Therapy in Treating Patients with Recurrent Glioblastoma Multiforme. Available online: https://clinicaltrials.gov/ct2/show/NCT00390299 (accessed on 5 September 2018).
- Gagan, J.; Van Allen, E.M. Next-generation sequencing to guide cancer therapy. Genome Med. 2015, 7, 80. [Google Scholar] [CrossRef] [PubMed]
- Kravchenko-Balasha, N.; Wang, J.; Remacle, F.; Levine, R.D.; Heath, J.R. Glioblastoma cellular architectures are predicted through the characterization of two-cell interactions. Proc. Natl. Acad. Sci. USA 2014, 111, 6521–6526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, W.; Shin, Y.S.; Xue, M.; Matsutani, T.; Masui, K.; Yang, H.; Ikegami, S.; Gu, Y.; Herrmann, K.; Johnson, D.; et al. Single-Cell Phosphoproteomics Resolves Adaptive Signaling Dynamics and Informs Targeted Combination Therapy in Glioblastoma. Cancer Cell 2016, 29, 563–573. [Google Scholar] [CrossRef] [PubMed]


| Type of Therapy | Therapy | TSA/TAA Target (s) | Reference (s) |
|---|---|---|---|
| Peptide vaccines | Poly ICLC | IL-13RA2, Wilms Tumor 1, Survivin | [50] |
| CDX-110 | EGFRvIII | [116] | |
| Poly ICLC | IL-13RA2, EphA2, Survivin | [119] | |
| Cilengitide | Integrin | [226] | |
| Liposomes/Nanoparticles/Nanosheets | IL-13 based Liposome | IL-13RA2 | [104,105] |
| IL-13 based Nanoparticles | IL-13RA2 | [106,107] | |
| Transferrin based Nanoparticles | Transferrin receptor | [218] | |
| Lactoferrin based Nanoparticles | Lactoferrin receptor | [219] | |
| Radiotherapy | EphA3 mAb(IIIA4) Radiolabelled with (177 Lu) | EphA3 | [176] |
| [64Cu]Pep-1L | IL-13RA2 | [63,74] | |
| Antigen pulsed dendritic cells | Multiple Epitope Pulsed Dendritic Cells (ICT-107) | IL-13RA2, MAGE-1, TRP-2, gp-100, EGFR, AIM-2 | [114] |
| Antibodies | Cetuximab | EGFRvIII | [202] |
| Bevacizumab and Irinotecan | VEGF | [203] | |
| Nimotuzumab | EGFR | [204] | |
| Bevacizumab | VEGF | [223] | |
| Immunotoxins | Transferrin-CRM 107 | Transferrin | [235] |
| ephrin A1 based Cytotoxin | EphA2 | [177] | |
| TGF-α based Cytotoxin | EGFR | [205] | |
| IL-4 based Cytotoxin | IL-4RA | [215] | |
| Amino-terminal Fragment of uPA based Cytotoxin | uPAR | [216] | |
| IL-13 and ephrin A1 based Cytotoxins | IL-13RA2, EphA2 | [178] | |
| ephrin A5 based Cytotoxins | EphA2, EphA3, EphB2 | [174] | |
| IL-13 based Cytotoxin | IL-13RA2 | [65] | |
| CAR T-cell therapy | CAR T-cells targeting EGFRvIII | EGFRvIII | [206] |
| CAR T-cells targeting HER2 | HER2 | [208,209] | |
| CAR T-cells targeting EphA2 | EphA2 | [179] | |
| IL-13.E13K.R109K CAR T cells | IL-13RA2 | [138] | |
| TanCARs | HER2, IL-13RA2 | [150] | |
| GRm13Z40-2 | IL-13RA2 | [56,129,137] | |
| IL-13(E13Y)-zetakine CAR T cells | IL-13RA2 | [125] | |
| IL-13BBζ-CAR T cells | IL-13RA2 | [130] | |
| IL-13 (E13K, E13Y, E13K.K105R, E13Y.K105R) based CAR T-cells | IL-13RA2 | [131] | |
| IL-13RA2 with Herpes Simplex Virus and PET reporter gene | IL-13RA2 | [134,135] | |
| Viral/Genetic Therapy | Delta-24-RGD (DNX-2401) Oncolytic Adenovirus | αvβ3 and αvβ5 Integrins | [108] |
| Ad.mhIL-4.TRE.mnhIL-13-PE) Adenovirus | IL-13RA2 | [113] | |
| R5141, Recombinant Herpes Simplex Virus-1 | IL-13RA3 | [111] | |
| DARPin-targeted Measles Virus | HER2 | [112] | |
| LU-13, Adenovirus | IL-13RA2 | [109] | |
| IL-13 based Lentiviral Vectors | IL-13RA2 | [110] | |
| Oncolytic Measles Virus | CD46 | [267,274,275] | |
| Reovirus | N/A | [266] | |
| Conditional Replicating Adenovirus (CAd) | Coxsackie and adenovirus receptor | [268] | |
| Oncolytic Herpes Simplex Virus | CD133 and CD111 | [269] | |
| ONYX-015, Recombinant Adenovirus | Coxsackie and adenovirus receptor | [270] | |
| Oncolytic Vesicular Stomatitis Virus | Death receptor | [272] | |
| IL-4 and Herpes Simplex Virus Thymidine Kinase | CD133 and CD111 | [256] | |
| Herpes Simplex Virus-Thymidine Kinase Ganciclovir (HSV-TK/GCV) | CD133 and CD111 | [263] | |
| Immune checkpoint inhibitors | Ipilimumab | CTLA-4 on T-cells | [242] |
| Nivolumab and Ipilumumab | PD-1 and CTLA-4 on T-cells respectively | [247] | |
| Anti TIM-3 | TIM-3 on T-cells | [252] | |
| Anti LAG-3 and Nivolumab | LAG-3 and PD-1 on T-cells respectively | [55] | |
| anti-CTLA-4 mAb (9D9, IgG2b) | CTLA-4 on T-cells | [243] | |
| ani-CTLA-4mAb and Granulocyte-Macrophage Colony Stimulating Factor (GMCSF) | CTLA-4 on T-cells | [244] | |
| Tyrosine kinase inhibitors | Cediranib | VEGFR | [229] |
| Sorafenib | VEGFR | [230] | |
| Sunitinib | VEGFR | [231] | |
| Vandetanib | VEGFR | [228] | |
| Imatinib | PDGFR | [232] | |
| Desatinib | PDGFR | [233] | |
| Brivanib | FGFR | [234] | |
| siRNA based Therapy | Lipid Nanocapsules | EGFR | [196] |
| Transfection of siRNA | EGFR and β-catenin | [197] | |
| Small molecule inhibitors | Erlotinib | EGFR | [198] |
| Geftinib | EGFR | [201] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, P.; Debinski, W. Receptor-Targeted Glial Brain Tumor Therapies. Int. J. Mol. Sci. 2018, 19, 3326. https://doi.org/10.3390/ijms19113326
Sharma P, Debinski W. Receptor-Targeted Glial Brain Tumor Therapies. International Journal of Molecular Sciences. 2018; 19(11):3326. https://doi.org/10.3390/ijms19113326
Chicago/Turabian StyleSharma, Puja, and Waldemar Debinski. 2018. "Receptor-Targeted Glial Brain Tumor Therapies" International Journal of Molecular Sciences 19, no. 11: 3326. https://doi.org/10.3390/ijms19113326