Reconstituted HDL (Milano) Treatment Efficaciously Reverses Heart Failure with Preserved Ejection Fraction in Mice
Abstract
:1. Introduction
2. Results
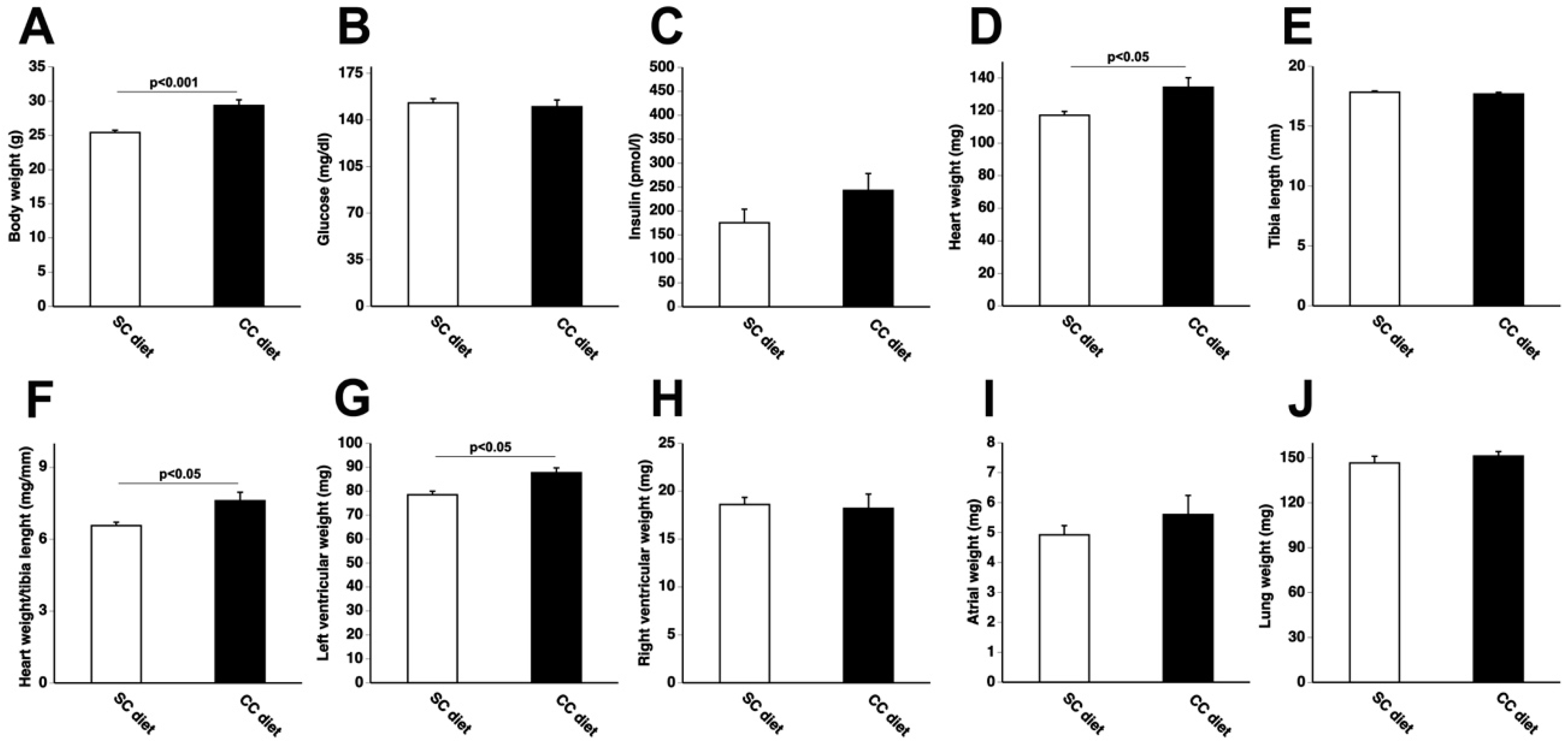
2.1. The 0.2% Cholesterol 10% Coconut Oil (CC) Diet Induces Cardiac Hypertrophy, Reduces Myocardial Capillary Density, and Increases Myocardial Fibrosis
2.2. Hemodynamic Deterioration in CC Diet Mice Is Consistent with Heart Failure with Preserved Ejection Fraction
2.3. Reconstituted HDLMilano Significantly Decreases Cardiac Hypertrophy, Increases Myocardial Capillary Density, and Decreases Myocardial Fibrosis in CC Diet Mice
2.4. Hemodynamic Function Is Restored in CC Diet Mice following Intervention with MDCO-216
2.5. MDCO-216 Normalizes Myocardial Acetyl-Coenzyme A (Acetyl-CoA) Carboxylase (ACC) Levels and DECREASES Myocardial Transforming Growth Factor (TGF)-β1 Levels in CC Diet Mice
2.6. MDCO-216 Significantly Improves Exercise Capacity in CC DIET mice
3. Discussion
4. Materials and Methods
4.1. Reconstituted HDLMilano
4.2. In Vivo Experiments and Study Design
4.3. In Vivo Hemodynamic Measurements
4.4. Lipoprotein and Murine Apo A-I Quantification in Plasma
4.5. Quantification of Myocardial Protein Levels by Western Blot
4.6. Histological Analyses
4.7. Exercise Treadmill Testing
4.8. Statistical Analysis
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| HFpEF | Heart failure with preserved ejection fraction |
| HFrEF | Heart failure with reduced ejection fraction |
| CC | 0.2% cholesterol 10% coconut oil |
| SC | standard chow |
| apo | apolipoprotein |
| HDL | high-density lipoprotein |
| LV | left ventricle |
| POPC | 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphatidylcholine |
| TAC | transverse aortic constriction |
| MPVS | Millar Pressure-Volume (PV) Loop System |
References
- Mosterd, A.; Hoes, A.W. Clinical epidemiology of heart failure. Heart 2007, 93, 1137–1146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rathi, S.; Deedwania, P.C. The epidemiology and pathophysiology of heart failure. Med. Clin. N. Am. 2012, 96, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Muthuramu, I.; Singh, N.; Amin, R.; De Geest, B. Role of lipids and lipoproteins in myocardial biology and in the development of heart failure. Clin. Lipidol. 2015, 10, 329–342. [Google Scholar] [CrossRef] [Green Version]
- Mosterd, A.; Hoes, A.W.; de Bruyne, M.C.; Deckers, J.W.; Linker, D.T.; Hofman, A.; Grobbee, D.E. Prevalence of heart failure and left ventricular dysfunction in the general population; The Rotterdam Study. Eur. Heart J. 1999, 20, 447–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roger, V.L.; Weston, S.A.; Redfield, M.M.; Hellermann-Homan, J.P.; Killian, J.; Yawn, B.P.; Jacobsen, S.J. Trends in heart failure incidence and survival in a community-based population. JAMA 2004, 292, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Luscher, T.F. Heart failure: The cardiovascular epidemic of the 21st century. Eur. Heart J. 2015, 36, 395–397. [Google Scholar] [CrossRef] [PubMed]
- Owan, T.E.; Hodge, D.O.; Herges, R.M.; Jacobsen, S.J.; Roger, V.L.; Redfield, M.M. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N. Engl. J. Med. 2006, 355, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Zile, M.R.; Baicu, C.F.; Gaasch, W.H. Diastolic heart failure--abnormalities in active relaxation and passive stiffness of the left ventricle. N. Engl. J. Med. 2004, 350, 1953–1959. [Google Scholar] [CrossRef] [PubMed]
- Borlaug, B.A. The pathophysiology of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 2014, 11, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Velagaleti, R.S.; Massaro, J.; Vasan, R.S.; Robins, S.J.; Kannel, W.B.; Levy, D. Relations of lipid concentrations to heart failure incidence: The Framingham Heart Study. Circulation 2009, 120, 2345–2351. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.S.; Tan, L.; Lu Long, J.; Davidson, W.S. The proteomic diversity of high density lipoproteins: Our emerging understanding of its importance in lipid transport and beyond. J. Lipid Res. 2013. [Google Scholar] [CrossRef] [PubMed]
- Gordts, S.C.; Singh, N.; Muthuramu, I.; De Geest, B. Pleiotropic effects of HDL: Towards new therapeutic areas for HDL-targeted interventions. Curr. Mol. Med. 2014, 14, 481–503. [Google Scholar] [CrossRef] [PubMed]
- Muthuramu, I.; Amin, R.; De Geest, B. New perspectives on biological HDL-targeted therapies. Expert Opin. Biol. Ther. 2017, 17, 793–796. [Google Scholar] [CrossRef] [PubMed]
- Van Linthout, S.; Frias, M.; Singh, N.; De Geest, B. Therapeutic potential of HDL in cardioprotection and tissue repair. Handb. Exp. Pharmacol. 2015, 224, 527–565. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Liu, X.; Xu, J.; Weng, L.; Ren, J.; Ge, J.; Zou, Y. High-density lipoprotein inhibits mechanical stress-induced cardiomyocyte autophagy and cardiac hypertrophy through angiotensin II type 1 receptor-mediated PI3K/Akt pathway. J. Cell. Mol. Med. 2015, 19, 1929–1938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, L.; Gong, H.; Ge, J.; Jiang, G.; Zhou, N.; Li, L.; Ye, Y.; Zhang, G.; Ge, J.; Zou, Y. High density lipoprotein downregulates angiotensin II type 1 receptor and inhibits angiotensin II-induced cardiac hypertrophy. Biochem. Biophys. Res. Commun. 2011, 404, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Amin, R.; Muthuramu, I.; Aboumsallem, J.P.; Mishra, M.; Jacobs, F.; De Geest, B. Selective HDL-Raising Human Apo A-I Gene Therapy Counteracts Cardiac Hypertrophy, Reduces Myocardial Fibrosis, and Improves Cardiac Function in Mice with Chronic Pressure Overload. Int. J. Mol. Sci. 2017, 18, 2012. [Google Scholar] [CrossRef] [PubMed]
- Muthuramu, I.; Amin, R.; Aboumsallem, J.P.; Mishra, M.; Robinson, E.L.; De Geest, B. Hepatocyte-Specific SR-BI Gene Transfer Corrects Cardiac Dysfunction in Scarb1-Deficient Mice and Improves Pressure Overload-Induced Cardiomyopathy. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 2028–2040. [Google Scholar] [CrossRef] [PubMed]
- Kallend, D.G.; Reijers, J.A.; Bellibas, S.E.; Bobillier, A.; Kempen, H.; Burggraaf, J.; Moerland, M.; Wijngaard, P.L. A single infusion of MDCO-216 (ApoA-1 Milano/POPC) increases ABCA1-mediated cholesterol efflux and pre-beta 1 HDL in healthy volunteers and patients with stable coronary artery disease. Eur. Heart J. Cardiovasc. Pharmacother. 2016, 2, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Kempen, H.J.; Asztalos, B.F.; Moerland, M.; Jeyarajah, E.; Otvos, J.; Kallend, D.G.; Bellibas, S.E.; Wijngaard, P.L. High-Density Lipoprotein Subfractions and Cholesterol Efflux Capacities After Infusion of MDCO-216 (Apolipoprotein A-IMilano/Palmitoyl-Oleoyl-Phosphatidylcholine) in Healthy Volunteers and Stable Coronary Artery Disease Patients. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 736–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kempen, H.J.; Gomaraschi, M.; Simonelli, S.; Calabresi, L.; Moerland, M.; Otvos, J.; Jeyarajah, E.; Kallend, D.; Wijngaard, P.L.J. Persistent changes in lipoprotein lipids after a single infusion of ascending doses of MDCO-216 (apoA-IMilano/POPC) in healthy volunteers and stable coronary artery disease patients. Atherosclerosis 2016, 255, 17–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reijers, J.A.A.; Kallend, D.G.; Malone, K.E.; Jukema, J.W.; Wijngaard, P.L.J.; Burggraaf, J.; Moerland, M. MDCO-216 Does Not Induce Adverse Immunostimulation, in Contrast to Its Predecessor ETC-216. Cardiovasc. Drugs Ther. 2017, 31, 381–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aboumsallem, J.P.; Mishra, M.; Amin, R.; Muthuramu, I.; Kempen, H.; De Geest, B. Successful treatment of established heart failure in mice with recombinant HDL (Milano). Br. J. Pharmacol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Muthuramu, I.; Amin, R.; Postnov, A.; Mishra, M.; Aboumsallem, J.P.; Dresselaers, T.; Himmelreich, U.; Van Veldhoven, P.P.; Gheysens, O.; Jacobs, F.; et al. Cholesterol-Lowering Gene Therapy Counteracts the Development of Non-ischemic Cardiomyopathy in Mice. Mol. Ther. 2017, 25, 2513–2525. [Google Scholar] [CrossRef] [PubMed]
- Muthuramu, I.; Amin, R.; Postnov, A.; Mishra, M.; Jacobs, F.; Gheysens, O.; Van Veldhoven, P.P.; De Geest, B. Coconut Oil Aggravates Pressure Overload-Induced Cardiomyopathy without Inducing Obesity, Systemic Insulin Resistance, or Cardiac Steatosis. Int. J. Mol. Sci. 2017, 18, 1565. [Google Scholar] [CrossRef] [PubMed]
- Kass, D.A. Ventricular arterial stiffening: Integrating the pathophysiology. Hypertension 2005, 46, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Starling, M.R. Left ventricular-arterial coupling relations in the normal human heart. Am. Heart J. 1993, 125, 1659–1666. [Google Scholar] [CrossRef]
- Zymlinski, R.; Biegus, J.; Sokolski, M.; Siwolowski, P.; Nawrocka-Millward, S.; Todd, J.; Jankowska, E.A.; Banasiak, W.; Cotter, G.; Cleland, J.G.; et al. Increased blood lactate is prevalent and identifies poor prognosis in patients with acute heart failure without overt peripheral hypoperfusion. Eur. J. Heart Fail. 2018, 20, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Adamo, L.; Nassif, M.E.; Novak, E.; LaRue, S.J.; Mann, D.L. Prevalence of lactic acidaemia in patients with advanced heart failure and depressed cardiac output. Eur. J. Heart Fail. 2017, 19, 1027–1033. [Google Scholar] [CrossRef] [PubMed]
- Grodin, J.L.; Tang, W.H.W. I will take my heart failure ‘lactate-free’ please. Eur. J. Heart Fail. 2018, 20, 1019–1020. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Van Craeyveld, E.; Jacobs, F.; Lievens, J.; Snoeys, J.; De Geest, B. Wild-type apo A-I and apo A-I(Milano) gene transfer reduce native and transplant arteriosclerosis to a similar extent. J. Mol. Med. 2009, 87, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Saddik, M.; Gamble, J.; Witters, L.A.; Lopaschuk, G.D. Acetyl-CoA carboxylase regulation of fatty acid oxidation in the heart. J. Biol. Chem. 1993, 268, 25836–25845. [Google Scholar] [PubMed]
- Kolwicz, S.C., Jr.; Olson, D.P.; Marney, L.C.; Garcia-Menendez, L.; Synovec, R.E.; Tian, R. Cardiac-specific deletion of acetyl CoA carboxylase 2 prevents metabolic remodeling during pressure-overload hypertrophy. Circ. Res. 2012, 111, 728–738. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Ma, C.; Yang, H.; Zhang, P.Y. Transforming growth factor beta and its role in heart disease. Exp. Ther. Med. 2017, 13, 2123–2128. [Google Scholar] [CrossRef] [PubMed]
- Spillmann, F.; De Geest, B.; Muthuramu, I.; Amin, R.; Miteva, K.; Pieske, B.; Tschope, C.; Van Linthout, S. Apolipoprotein A-I gene transfer exerts immunomodulatory effects and reduces vascular inflammation and fibrosis in ob/ob mice. J. Inflamm. (Lond.) 2016, 13, 25. [Google Scholar] [CrossRef] [PubMed]
- Spillmann, F.; Miteva, K.; Pieske, B.; Tschope, C.; Van Linthout, S. High-Density Lipoproteins Reduce Endothelial-to-Mesenchymal Transition. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1774–1777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sodek, J.; Ganss, B.; McKee, M.D. Osteopontin. Crit. Rev. Oral Biol. Med. 2000, 11, 279–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rangaswami, H.; Bulbule, A.; Kundu, G.C. Osteopontin: Role in cell signaling and cancer progression. Trends Cell Biol. 2006, 16, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Singh, M.; Singh, K. Osteopontin modulates myocardial hypertrophy in response to chronic pressure overload in mice. Hypertension 2004, 44, 826–831. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.R.; Schnee, J.; Wang, W.; Kim, S.; Fishbein, M.C.; Bruemmer, D.; Law, R.E.; Nicholas, S.; Ross, R.S.; Hsueh, W.A. Osteopontin modulates angiotensin II-induced fibrosis in the intact murine heart. J. Am. Coll. Cardiol. 2004, 43, 1698–1705. [Google Scholar] [CrossRef] [PubMed]
- Caparon, M.H.; Rust, K.J.; Hunter, A.K.; McLaughlin, J.K.; Thomas, K.E.; Herberg, J.T.; Shell, R.E.; Lanter, P.B.; Bishop, B.F.; Dufield, R.L.; et al. Integrated solution to purification challenges in the manufacture of a soluble recombinant protein in E. coli. Biotechnol. Bioeng. 2010, 105, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Kempen, H.J.; Gomaraschi, M.; Bellibas, S.E.; Plassmann, S.; Zerler, B.; Collins, H.L.; Adelman, S.J.; Calabresi, L.; Wijngaard, P.L. Effect of repeated apoA-IMilano/POPC infusion on lipids, (apo)lipoproteins, and serum cholesterol efflux capacity in cynomolgus monkeys. J. Lipid Res. 2013, 54, 2341–2353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Junqueira, L.C.; Bignolas, G.; Brentani, R.R. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem. J. 1979, 11, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, I.; Minamino, T.; Toko, H.; Okada, S.; Ikeda, H.; Yasuda, N.; Tateno, K.; Moriya, J.; Yokoyama, M.; Nojima, A.; et al. Excessive cardiac insulin signaling exacerbates systolic dysfunction induced by pressure overload in rodents. J. Clin. Investig. 2010, 120, 1506–1514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Craeyveld, E.; Jacobs, F.; Gordts, S.C.; De Geest, B. Low-density lipoprotein receptor gene transfer in hypercholesterolemic mice improves cardiac function after myocardial infarction. Gene Ther. 2012, 19, 860–871. [Google Scholar] [CrossRef] [PubMed]
- Gordts, S.C.; Muthuramu, I.; Nefyodova, E.; Jacobs, F.; Van Craeyveld, E.; De Geest, B. Beneficial effects of selective HDL-raising gene transfer on survival, cardiac remodelling and cardiac function after myocardial infarction in mice. Gene Ther. 2013, 20, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Bayat, H.; Swaney, J.S.; Ander, A.N.; Dalton, N.; Kennedy, B.P.; Hammond, H.K.; Roth, D.M. Progressive heart failure after myocardial infarction in mice. Basic Res. Cardiol. 2002, 97, 206–213. [Google Scholar] [CrossRef] [PubMed]








| Standard Chow (n = 15) | CC Diet (n = 22) | |
|---|---|---|
| Heart rate (bpm) | 588 ± 11 | 572 ± 9 |
| Pmax (mm Hg) | 98.2 ± 1.2 | 81.8 ± 2.1 **** |
| Pes (mm Hg) | 96.3 ± 1.0 | 74.7 ± 2.1 **** |
| dP/dtmax (mmHg/ms) | 9.57 ± 0.67 | 8.00 ± 0.67 * |
| PRSW (mmHg) | 82.7 ± 7.9 | 65.4 ± 2.9 * |
| Ees (mmHg/μL) | 8.48 ± 0.61 | 3.97 ± 0.33 **** |
| Pmin (mm Hg) | −0.528 ± 0.830 | 1.41 ± 0.36 * |
| Ped (mm Hg) | 1.90 ± 0.47 | 4.43 ± 0.30 **** |
| dP/dtmin (mmHg/ms) | −9.94 ± 0.71 | −7.95 ± 0.38 * |
| Tau (ms) | 5.39 ± 0.19 | 6.77 ± 0.26 *** |
| Slope EDPVR (mmHg/μL) | 0.259 ± 0.039 | 0.765 ± 0.176 * |
| EDV (μL) | 27.0 ± 2.0 | 23.3 ± 1.3 |
| ESV (μL) | 10.5 ± 1.3 | 10.4 ± 1.2 |
| Stroke volume (μL) | 16.5 ± 1.0 | 12.9 ± 0.9 * |
| Ejection fraction (%) | 62.2 ± 2.8 | 56.5 ± 3.4 |
| Cardiac output (ml/min) | 9.67 ± 0.62 | 7.40 ± 0.55 * |
| Stroke work (mmHg·μL) | 1290 ± 80 | 856 ± 74 *** |
| dV/dtmax (μL/s) | 773 ± 66 | 579 ± 43 * |
| dV/dtmin (μL/s) | −821 ± 63 | −607 ± 42 ** |
| Ea (mmHg/μL) | 6.33 ± 0.55 | 6.10 ± 0.37 |
| Ea/Ees | 0.809 ± 0.105 | 2.03 ± 0.38 **** |
| Reference SC Diet (n = 10) | Buffer SC Diet (n = 10) | MDCO-216 SC Diet (n = 10) | Reference CC Diet (n = 10) | Buffer CC Diet (n = 10) | MDCO-216 CC Diet (n = 10) | |
|---|---|---|---|---|---|---|
| Total cholesterol | 1.62 ± 0.08 | 1.61 ±0.08 | 1.64 ±0.10 | 1.67 ± 0.08 | 1.69 ±0.09 | 1.61 ±0.10 |
| Non-HDL cholesterol | 0.325 ± 0.048 | 0.365 ± 0.047 | 0.389 ± 0.052 | 0.409 ± 0.060 | 0.416 ± 0.040 | 0.331 ± 0.052 |
| HDL cholesterol | 1.29 ± 0.07 | 1.24 ± 0.10 | 1.25 ± 0.12 | 1.26 ±0.06 | 1.27 ± 0.06 | 1.28 ± 0.12 |
| Reference SC Diet (n = 15) | Buffer SC Diet (n = 11) | MDCO-216 SC Diet (n = 10) | Reference CC Diet (n = 22) | Buffer CC Diet (n = 13) | MDCO-216 CC Diet (n = 14) | |
|---|---|---|---|---|---|---|
| Heart rate (bpm) | 588 ± 11 | 584 ± 22 | 594 ± 13 | 572 ± 9 | 579 ± 20 | 587 ± 19 |
| Pmax (mm Hg) | 98.2 ± 1.2 | 96.6 ±3.7 | 100 ± 1 | 81.8 ± 2.1 !!!! | 83.3 ± 4.2 ! | 97.7 ± 3.7 §§* |
| Pes (mm Hg) | 96.3 ± 1.0 | 93.0 ±3.6 | 95.5 ± 1.3 | 74.7 ± 2.1 !!!! | 80.5 ± 4.0 ! | 93.3 ± 3.2 §§§* |
| dP/dtmax (mmHg/ms) | 9.57 ± 0.67 | 10.3 ± 0.4 | 10.2 ± 0.5 | 8.00 ± 0.67 ! | 8.22 ± 0.31 !!! | 12.2 ± 0.6 §§§** |
| PRSW (mmHg) | 82.7 ± 7.9 | 80.0 ± 7.3 | 79.5 ± 4.7 | 65.4 ± 2.9 ! | 64.0 ± 2.4 ! | 77.6 ± 4.2 §* |
| Ees (mmHg/μL) | 8.48 ± 0.61 | 8.23 ± 0.74 | 8.23 ± 0.74 | 3.97 ± 0.33 !!!! | 4.58 ± 0.42 !!! | 8.31 ± 0.63 §§*** |
| Pmin (mm Hg) | −0.528 ± 0.830 | 1.58 ± 0.41 | 0.0839 ± 0.648 | 1.41 ± 0.36 ! | 2.38 ± 0.36 | 0.601 ± 0.534* |
| Ped (mm Hg) | 1.90 ± 0.47 | 2.64 ± 0.51 | 1.92 ± 0.54 | 4.43 ± 0.30 !!!! | 4.30 ± 0.34 ! | 3.80 ± 0.47 |
| dP/dtmin (mmHg/ms) | −9.94 ± 0.71 | −9.98 ± 0.31 | −10.3 ± 0.5 | −7.95 ± 0.38 ! | −8.01 ± 0.33 !!! | −9.99 ± 0.32 §§§** |
| Tau (ms) | 5.39 ± 0.19 | 5.38 ± 0.25 | 5.05 ± 0.27 | 6.77 ± 0.26 !!! | 7.67 ± 0.44 !!! | 5.23 ± 0.24 §§§*** |
| Slope EDPVR (mmHg/μL) | 0.259 ± 0.039 | 0.314 ± 0.052 | 0.237 ± 0.035 | 0.765 ± 0.176 ! | 0.617 ± 0.138 | 0.336 ± 0.061 |
| EDV (μL) | 27.0 ± 2.0 | 25.0 ±0.8 | 26.2 ± 1.2 | 23.3 ± 1.3 | 24.5 ± 1.2 | 26.8 ± 1.4 |
| ESV (μL) | 10.5 ± 1.3 | 9.56 ± 0.41 | 9.58 ± 1.29 | 10.4 ± 1.2 | 11.1 ± 0.9 | 9.76 ± 1.11 |
| Stroke volume (μL) | 16.5 ± 1.0 | 15.4 ± 0.7 | 16.6 ± 0.8 | 12.9 ± 0.9 ! | 13.4 ± 0.6 ! | 17.0 ± 0.9 §§* |
| Ejection fraction (%) | 62.2 ± 2.8 | 61.5 ±1.6 | 64.3 ± 3.8 | 56.5 ± 3.4 | 55.3 ± 2.0 | 64.4 ± 2.9* |
| Cardiac output (ml/min) | 9.67 ± 0.62 | 9.00 ± 0.56 | 9.84 ± 0.49 | 7.40 ± 0.55 ! | 7.84 ± 0.55 | 10.0 ± 0.7 §§* |
| Stroke work (mmHg·μL) | 1290 ± 80 | 1180 ± 80 | 1330 ± 70 | 856 ± 74 !!! | 909 ± 78 ! | 1330 ± 100 §§§** |
| dV/dtmax (μL/s) | 773 ± 66 | 725 ± 44 | 778 ± 87 | 579 ± 43 ! | 536 ± 51 ! | 774 ± 90 * |
| dV/dtmin (μL/s) | −821 ± 63 | −766 ± 31 | −829 ± 64 | −607 ± 42 !! | −538 ± 67 !! | −717 ± 80 |
| Ea (mmHg/μL) | 6.33 ± 0.55 | 6.15 ± 0.34 | 5.89 ± 0.33 | 6.10 ± 0.37 | 6.04 ± 0.26 | 5.70 ± 0.36 |
| Ea/Ees | 0.809 ± 0.105 | 0.820 ± 0.091 | 0.752 ± 0.078 | 2.03 ± 0.38 !!!! | 1.51 ± 0.21 !! | 0.740 ± 0.074 §§§*** |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mishra, M.; Muthuramu, I.; Aboumsallem, J.P.; Kempen, H.; De Geest, B. Reconstituted HDL (Milano) Treatment Efficaciously Reverses Heart Failure with Preserved Ejection Fraction in Mice. Int. J. Mol. Sci. 2018, 19, 3399. https://doi.org/10.3390/ijms19113399
Mishra M, Muthuramu I, Aboumsallem JP, Kempen H, De Geest B. Reconstituted HDL (Milano) Treatment Efficaciously Reverses Heart Failure with Preserved Ejection Fraction in Mice. International Journal of Molecular Sciences. 2018; 19(11):3399. https://doi.org/10.3390/ijms19113399
Chicago/Turabian StyleMishra, Mudit, Ilayaraja Muthuramu, Joseph Pierre Aboumsallem, Herman Kempen, and Bart De Geest. 2018. "Reconstituted HDL (Milano) Treatment Efficaciously Reverses Heart Failure with Preserved Ejection Fraction in Mice" International Journal of Molecular Sciences 19, no. 11: 3399. https://doi.org/10.3390/ijms19113399







