Characterization of Ecklonia cava Alginate Films Containing Cinnamon Essential Oils
Abstract
:1. Introduction
2. Results and Discussion
2.1. Attenuated Total Reflectance-Fourier Transform Infrared (ATR-FTIR) Analysis of ECA
2.2. Physical Properties of ECA Films with CaCl2
2.3. Physical Properties of ECA Films Containing EOs
2.4. Optical Properties of ECA Films Containing EOs
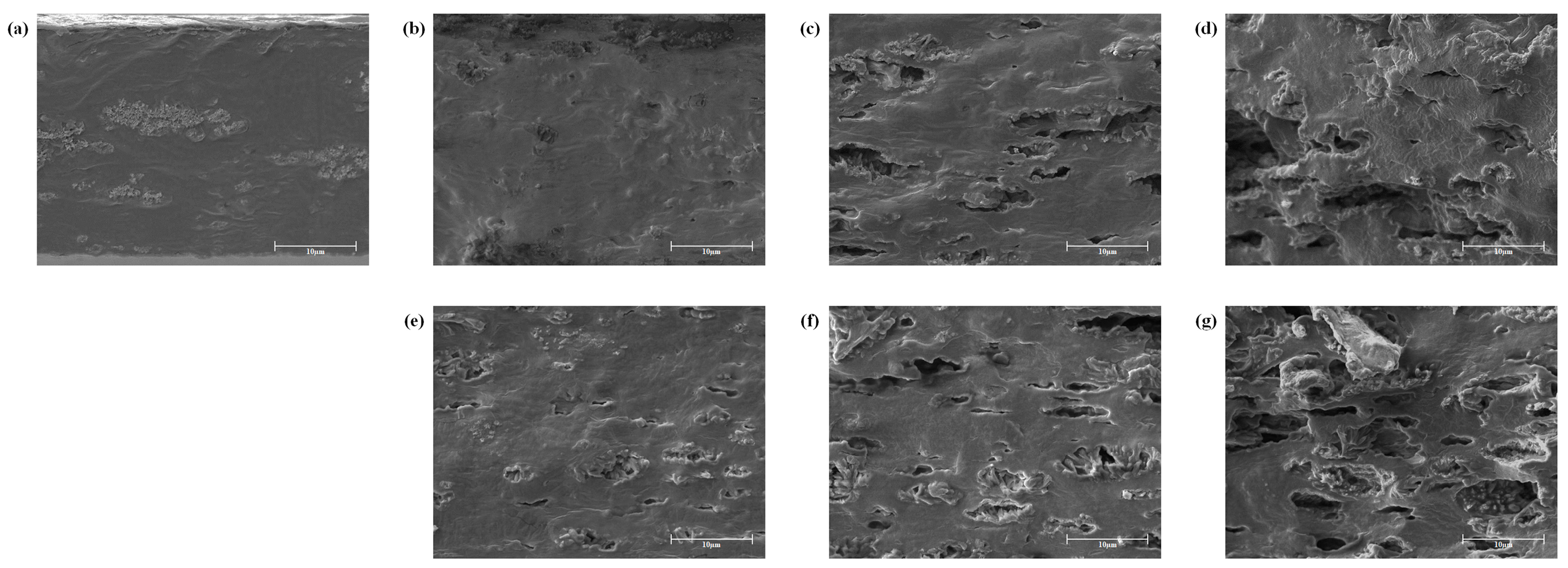
2.5. Scanning Electron Microscopy (SEM) Analysis
2.6. Thermal Analysis
2.7. Antioxidant Activity
2.8. Antimicrobial Activity
3. Materials and Methods
3.1. Materials
3.2. Alginate Extraction
3.3. ATR-FTIR Spectroscopy
3.4. Preparation of ECA Films Containing EOs
3.5. Physical Properties
3.6. Optical Properties
3.7. SEM Analysis
3.8. Thermal Property Analysis
3.9. Antioxidant Activity
3.10. Antimicrobial Activity
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Atarés, L.; Chiralt, A. Essential oils as additives in biodegradable films and coatings for active food packaging. Trends Food Sci. Technol. 2016, 48, 51–62. [Google Scholar] [CrossRef]
- Khalil, H.A.; Tye, Y.Y.; Saurabh, C.K.; Leh, C.P.; Lai, T.K.; Chong, E.W.N.; Syakir, M.I. Biodegradable polymer films from seaweed polysaccharides: A review on cellulose as a reinforcement material. Express Polym. Lett. 2017, 11, 244–265. [Google Scholar] [CrossRef]
- Tavassoli-Kafrani, E.; Shekarchizadeh, H.; Masoudpour-Behabadi, M. Development of edible films and coatings from alginates and carrageenans. Carbohydr. Polym. 2016, 137, 360–374. [Google Scholar] [CrossRef] [PubMed]
- Wijesinghe, W.A.J.P.; Jeon, Y.J. Exploiting biological activities of brown seaweed Ecklonia cava for potential industrial applications: A review. Int. J. Food Sci. Nutr. 2012, 63, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.H. Seasonal variation in the chemical composition of brown algae with special reference to alginic acid. Korean J. Fish. Aquat. Sci. 1969, 2, 71–82. [Google Scholar]
- Jost, V.; Reinelt, M. Effect of Ca2+ induced crosslinking on the mechanical and barrier properties of cast alginate films. J. Appl. Polym. Sci. 2018, 135, 45754. [Google Scholar] [CrossRef]
- Fawzy, M.A.; Gomaa, M.; Hifney, A.F.; Abdel-Gawad, K.M. Optimization of alginate alkaline extraction technology from Sargassum latifolium and its potential antioxidant and emulsifying properties. Carbohydr. Polym. 2017, 157, 1903–1912. [Google Scholar] [CrossRef] [PubMed]
- Sellimi, S.; Younes, I.; Ayed, H.B.; Maalej, H.; Montero, V.; Rinaudo, M.; Nasri, M. Structural, physicochemical and antioxidant properties of sodium alginate isolated from a Tunisian brown seaweed. Int. J. Biol. Macromol. 2015, 72, 1358–1367. [Google Scholar] [CrossRef] [PubMed]
- Kuorwel, K.K.; Cran, M.J.; Orbell, J.D.; Buddhadasa, S.; Bigger, S.W. Review of mechanical properties, migration, and potential applications in active food packaging systems containing nanoclays and nanosilver. Compr. Rev. Food Sci. F. 2015, 14, 411–430. [Google Scholar] [CrossRef]
- Ranasinghe, P.; Pigera, S.; Premakumara, G.S.; Galappaththy, P.; Constantine, G.R.; Katulanda, P. Medicinal properties of ‘true’cinnamon (Cinnamomum zeylanicum): A systematic review. BMC Complement Altern. Med. 2013, 13, 275. [Google Scholar] [CrossRef] [PubMed]
- Sangal, A. Role of cinnamon as beneficial antidiabetic food adjunct: A review. Adv. Appl. Sci. Res. 2011, 2, 440–450. [Google Scholar]
- Mahmood, A.; Bano, S.; Kim, S.G.; Lee, K.H. Water–methanol separation characteristics of annealed SA/PVA complex membranes. J. Memb. Sci. 2012, 415, 360–367. [Google Scholar] [CrossRef]
- Falkeborg, M.; Paitaid, P.; Shu, A.N.; Pérez, B.; Guo, Z. Dodecenyl succinylated alginate as a novel material for encapsulation and hyperactivation of lipases. Carbohydr. Polym. 2015, 133, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.; Sousa, A.; Coelho, H.; Amado, A.M.; Ribeiro-Claro, P.J. Use of FTIR, FT-Raman and 13 C-NMR spectroscopy for identification of some seaweed phycocolloids. Biomol. Eng. 2003, 20, 223–228. [Google Scholar] [CrossRef]
- Benavides, S.; Villalobos-Carvajal, R.; Reyes, J.E. Physical, mechanical and antibacterial properties of alginate film: Effect of the crosslinking degree and oregano essential oil concentration. J. Food Eng. 2012, 110, 232–239. [Google Scholar] [CrossRef]
- Zactiti, E.M.; Kieckbusch, T.G. Potassium sorbate permeability in biodegradable alginate films: Effect of the antimicrobial agent concentration and crosslinking degree. J. Food Eng. 2006, 77, 462–467. [Google Scholar] [CrossRef]
- Zactiti, E.M.; Kieckbusch, T.G. Release of potassium sorbate from active films of sodium alginate crosslinked with calcium chloride. Packag. Technol. Sci. 2009, 22, 349–358. [Google Scholar] [CrossRef]
- Han, Y.; Yu, M.; Wang, L. Physical and antimicrobial properties of sodium alginate/carboxymethyl cellulose films incorporated with cinnamon essential oil. Food Packag. Shelf Life 2018, 15, 35–42. [Google Scholar] [CrossRef]
- Pranoto, Y.; Salokhe, V.M.; Rakshit, S.K. Physical and antibacterial properties of alginate-based edible film incorporated with garlic oil. Food Res. Int. 2005, 38, 267–272. [Google Scholar] [CrossRef]
- Abdollahi, M.; Rezaei, M.; Farzi, G. Improvement of active chitosan film properties with rosemary essential oil for food packaging. Int. J. Food Sci. Technol. 2012, 47, 847–853. [Google Scholar] [CrossRef]
- Shojaee-Aliabadi, S.; Hosseini, H.; Mohammadifar, M.A.; Mohammadi, A.; Ghasemlou, M.; Hosseini, S.M.; Khaksar, R. Characterization of κ-carrageenan films incorporated plant essential oils with improved antimicrobial activity. Carbohydr. Polym. 2014, 101, 582–591. [Google Scholar] [CrossRef] [PubMed]
- Tongnuanchan, P.; Benjakul, S.; Prodpran, T. Properties and antioxidant activity of fish skin gelatin film incorporated with citrus essential oils. Food Chem. 2012, 134, 1571–1579. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, Q.; Critzer, F.; Davidson, P.M.; Zhong, Q. Physical and antibacterial properties of alginate films containing cinnamon bark oil and soybean oil. LWT Food Sci. Technol. 2015, 64, 423–430. [Google Scholar] [CrossRef]
- Atarés, L.; De-Jesús, C.; Talens, P.; Chiralt, A. Characterization of SPI-based edible films incorporated with cinnamon or ginger essential oils. J. Food Eng. 2010, 99, 384–391. [Google Scholar] [CrossRef]
- Peng, Y.; Li, Y. Combined effects of two kinds of essential oils on physical, mechanical and structural properties of chitosan films. Food Hydrocoll. 2014, 36, 287–293. [Google Scholar] [CrossRef]
- Wu, J.; Sun, X.; Guo, X.; Ge, S.; Zhang, Q. Physicochemical properties, antimicrobial activity and oil release of fish gelatin films incorporated with cinnamon essential oil. Aquac. Fish. 2017, 2, 185–192. [Google Scholar] [CrossRef]
- Yadav, M.; Rhee, K.Y.; Park, S.J. Synthesis and characterization of graphene oxide/carboxymethylcellulose/alginate composite blend films. Carbohydr. Polym. 2014, 110, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Wang, L.F.; Rhim, J.W. Preparations and characterization of alginate/silver composite films: Effect of types of silver particles. Carbohydr. Polym. 2016, 146, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.P.; Santos, J.E.; Chierice, G.O.; Cavalheiro, E.T.G. Thermal behavior of alginic acid and its sodium salt. Eclética Química 2004, 29, 57–64. [Google Scholar] [CrossRef]
- Pongjanyakul, T.; Priprem, A.; Puttipipatkhachorn, S. Investigation of novel alginate−magnesium aluminum silicate microcomposite films for modified-release tablets. J. Control Release. 2005, 107, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Naidu, B.V.K.; Sairam, M.; Raju, K.V.; Aminabhavi, T.M. Thermal, viscoelastic, solution and membrane properties of sodium alginate/hydroxyethylcellulose blends. Carbohydr. Polym. 2005, 61, 52–60. [Google Scholar]
- Tongnuanchan, P.; Benjakul, S.; Prodpran, T. Structural, morphological and thermal behavior characterizations of fish gelatin film incorporated with basil and citronella essential oils as affected by surfactants. Food Hydrocoll. 2014, 41, 33–43. [Google Scholar] [CrossRef]
- Sharma, U.K.; Sharma, A.K.; Pandey, A.K. Medicinal attributes of major phenylpropanoids present in cinnamon. BMC Complement Altern. Med. 2016, 16, 156. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Maurya, S.; Catalan, C.A. A comparison of chemical, antioxidant and antimicrobial studies of cinnamon leaf and bark volatile oils, oleoresins and their constituents. Food Chem. Toxicol. 2007, 45, 1650–1661. [Google Scholar] [CrossRef] [PubMed]
- Sanla-Ead, N.; Jangchud, A.; Chonhenchob, V.; Suppakul, P. Antimicrobial Activity of cinnamaldehyde and eugenol and their activity after incorporation into cellulose-based packaging films. Packag. Technol. Sci. 2012, 25, 7–17. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Song, K.B. Inhibitory effect of plant essential oil nanoemulsions against Listeria monocytogenes, Escherichia coli O157: H7, and Salmonella Typhimurium on red mustard leaves. Innov. Food Sci. Emerg. Technol. 2018, 45, 447–454. [Google Scholar] [CrossRef]
- Cho, M.; Yoon, S.J.; Kim, Y.B. The nutritional composition and antioxidant activity from Undariopsis peterseniana. Ocean Polar Res. 2013, 35, 273–280. [Google Scholar] [CrossRef]
- You, B.J.; Jeong, I.H.; Lee, K.H. Effect extraction conditions on bile acids binding capacity in vitro of alginate extracted from sea tangle (Laminaria spp.). Korean J. Fish. Aquat. Sci. 1997, 30, 31–38. [Google Scholar]
- Lee, K.Y.; Song, K.B. Preparation and characterization of an olive flounder (Paralichthys olivaceus) skin gelatin and polylactic acid bilayer film. J. Food Sci. 2017, 82, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.Y.; Lee, K.Y.; Beak, S.E.; Kim, H.; Song, K.B. Antimicrobial activity of gelatin films based on duck feet containing cinnamon leaf oil and their applications in packaging of cherry tomatoes. Food Sci. Biotechnol. 2017, 26, 1429–1435. [Google Scholar] [CrossRef] [PubMed]
- Shojaee-Aliabadi, S.; Hosseini, H.; Mohammadifar, M.A.; Mohammadi, A.; Ghasemlou, M.; Ojagh, S.M.; Khaksar, R. Characterization of antioxidant-antimicrobial κ-carrageenan films containing Satureja hortensis essential oil. Int. J. Biol. Macromol. 2013, 52, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Bitencourt, C.M.; Fávaro-Trindade, C.S.; Sobral, P.J.A.; Carvalho, R.A. Gelatin-based films additivated with curcuma ethanol extract: Antioxidant activity and physical properties of films. Food Hydrocoll. 2014, 40, 145–152. [Google Scholar] [CrossRef]





| CaCl2 (%) | Thickness (µm) | Tensile Strength (MPa) | Elongation at Break (%) | Water Vapor Permeability (×10−9 g /m s Pa) |
|---|---|---|---|---|
| 0 | 34.60 ± 0.86 d | 10.49 ± 1.38 d | 9.30 ± 0.89 bc | 1.89 ± 0.13 a |
| 1 | 35.00 ± 0.81 cd | 14.78 ± 2.74 b | 9.90 ± 0.56 ab | 1.86 ± 0.16 a |
| 2 | 35.96 ± 1.42 bc | 12.99 ± 1.65 bc | 9.96 ± 0.52 ab | 1.85 ± 0.15 a |
| 3 | 36.68 ± 0.61 b | 17.82 ± 1.05 a | 10.36 ± 0.27 a | 1.78 ± 0.02 a |
| 5 | 37.12 ± 0.64 b | 15.26 ± 1.80 b | 9.69 ± 0.60 ab | 1.83 ± 0.10 a |
| 7 | 38.80 ± 1.20 a | 10.79 ± 1.55 cd | 8.85 ± 0.49 c | 1.74 ± 0.07 a |
| EOs (%) | Thickness (µm) | Tensile Strength (MPa) | Elongation at Break (%) | Water Vapor Permeability (×10−9 g /m s Pa) | |
|---|---|---|---|---|---|
| Control | 0 | 36.68 ± 0.61 d | 17.82 ± 1.05 a | 10.36 ± 0.27 c | 1.78 ± 0.02 d |
| CLO | 0.4 | 40.12 ± 0.73 c | 17.40 ± 0.87 ab | 17.94 ± 2.59 ab | 2.01 ± 0.05 c |
| 0.7 | 44.12 ± 0.67 b | 15.10 ± 1.88 cd | 18.25 ± 3.32 ab | 2.35 ± 0.06 b | |
| 1.0 | 49.80 ± 0.60 a | 14.94 ± 1.22 cd | 17.28 ± 1.40 ab | 2.56 ± 0.05 a | |
| CBO | 0.4 | 40.40 ± 1.14 c | 16.02 ± 0.80 bc | 16.92 ± 1.73 ab | 1.99 ± 0.03 c |
| 0.7 | 44.08 ± 0.61 b | 15.45 ± 1.32 c | 18.65 ± 2.06 a | 2.30 ± 0.07 b | |
| 1.0 | 49.68 ± 0.46 a | 13.58 ± 0.92 d | 15.61 ± 1.77 b | 2.54 ± 0.09 a | |
| EOs (%) | L * | a * | b * | ΔE * | Opacity (A/mm) | |
|---|---|---|---|---|---|---|
| Control | 0 | 72.03 ± 0.27 a | 5.16 ± 0.10 e | 49.19 ± 0.34 ab | - | 9.77 ± 0.31 f |
| CLO | 0.4 | 68.52 ± 0.40 b | 7.71 ± 0.26 d | 49.76 ± 0.32 a | 4.39 ± 0.48 d | 13.43 ± 0.51 d |
| 0.7 | 66.40 ± 0.43 d | 8.53 ± 0.19 b | 45.21 ± 1.08 d | 7.75 ± 0.35 b | 22.64 ± 0.53 b | |
| 1.0 | 64.73 ± 1.11 e | 8.96 ± 0.66 a | 44.55 ± 0.77 d | 9.48 ± 1.20 a | 25.12 ± 0.37 a | |
| CBO | 0.4 | 68.48 ± 0.33 b | 7.52 ± 0.19 d | 49.00 ± 0.97 ab | 4.37 ± 0.36 d | 12.12 ± 0.19 e |
| 0.7 | 67.12 ± 1.10 c | 8.17 ± 0.24 c | 48.52 ± 0.81 b | 5.86 ± 1.10 c | 20.85 ± 0.34 c | |
| 1.0 | 64.01 ± 0.67 f | 8.59 ± 0.42 b | 46.87 ± 1.29 c | 9.11 ± 0.79 a | 22.54 ± 0.54 b | |
| EOs (%) | Inhibition Zone (mm) | ||||
|---|---|---|---|---|---|
| E. coli O157:H7 | S. Typhimurium | S. aureus | L. monocytogenes | ||
| Control | 0 | ND * | ND | ND | ND |
| CLO | 0.4 | ND | ND | ND | ND |
| 0.7 | 12.04 ± 0.10 c | 11.49 ± 0.14 c | 10.77 ± 0.07 b | 13.17 ± 0.49 b | |
| 1.0 | 14.50 ± 0.51 a | 15.37 ± 0.16 a | 14.28 ± 0.25 a | 15.02 ± 0.17 a | |
| CBO | 0.4 | ND | ND | ND | ND |
| 0.7 | 10.88 ± 0.08 d | 10.77 ± 0.09 d | 10.35 ± 0.52 b | 12.03 ± 0.66 c | |
| 1.0 | 13.48 ± 0.17 b | 12.76 ± 0.22 b | 13.93 ± 0.36 a | 14.48 ± 0.13 a | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baek, S.-K.; Kim, S.; Song, K.B. Characterization of Ecklonia cava Alginate Films Containing Cinnamon Essential Oils. Int. J. Mol. Sci. 2018, 19, 3545. https://doi.org/10.3390/ijms19113545
Baek S-K, Kim S, Song KB. Characterization of Ecklonia cava Alginate Films Containing Cinnamon Essential Oils. International Journal of Molecular Sciences. 2018; 19(11):3545. https://doi.org/10.3390/ijms19113545
Chicago/Turabian StyleBaek, Su-Kyoung, Sujin Kim, and Kyung Bin Song. 2018. "Characterization of Ecklonia cava Alginate Films Containing Cinnamon Essential Oils" International Journal of Molecular Sciences 19, no. 11: 3545. https://doi.org/10.3390/ijms19113545




