Mouse Cardiac Pde1C Is a Direct Transcriptional Target of Pparα
Abstract
:1. Introduction
2. Results
2.1. Treatment with PPARα Agonist Leads to Increased Pde1C mRNA Expression in H9c2 Cardiomyocytes
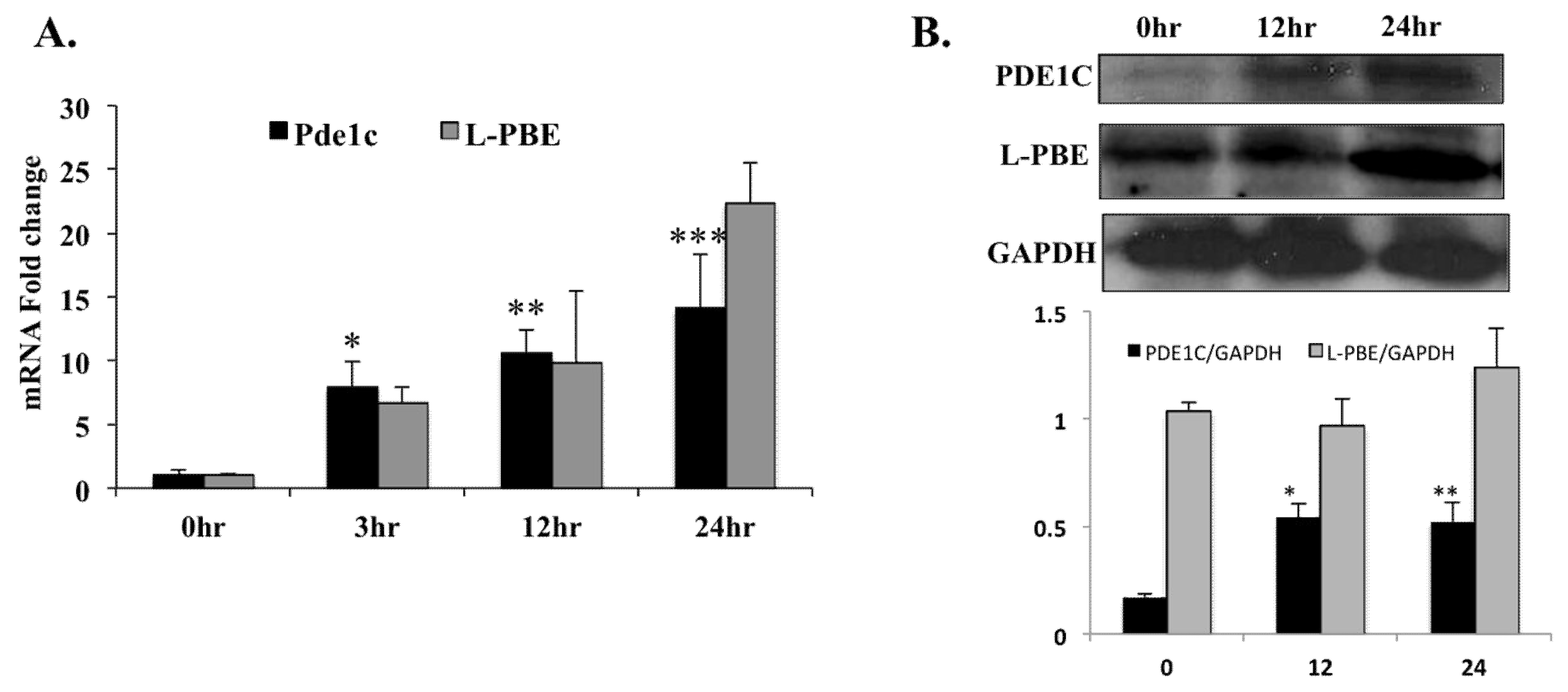
2.2. Treatment with PPARα Agonist Wy-14,643 Increased Pde1C mRNA and PDE1C Protein Expression in the Hearts of WT Mice
2.3. Treatment with PPARα Agonist Wy-14,643 Led to Increased mRNA Levels of Several Members of PDE Family in H9c2 Cardiomyocytes
2.4. Wy-14,643 Treatment did not Induce Pde1C mRNA Expression in the Liver of WT Mice
2.5. Cardiac Pde1C mRNA Levels did not Alter upon Wy-14,643 Injection of Pparα-/- and TmcsMed1-/- Mice
2.6. PPARα Binds at Two Sites of the Pde1C Promoter
2.7. Treatment with PPARα Agonist Wy-14,643 Led to Reduced Cyclic AMP Levels in Mouse Hearts and H9c2 Cardiomyocytes
2.8. Troponin I, an Indicator of Failing Heart, is Increased in WT Mouse Hearts upon Wy-14,643 Treatment
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Cardiomyoblast Cell Culture
4.3. Quantitative Real-Time PCR
4.4. Protein Expression Analysis
4.5. Pde1C Promoter Analysis
4.6. Chromatin Immunoprecipitation
4.7. Cyclic AMP Measurement
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zaccolo, M. Phosphodiesterases and compartmentalized cAMP signalling in the heart. Eur. J. Cell Biol. 2006, 85, 693–697. [Google Scholar] [CrossRef] [PubMed]
- Feldman, M.D.; Copelas, L.; Gwathmey, J.K.; Phillips, P.; Warren, S.E.; Schoen, F.J.; Grossman, W.; Morgan, J.P. Deficient production of cyclic AMP: Pharmacologic evidence of an important cause of contractile dysfunction in patients with end-stage heart failure. Circulation 1987, 75, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Katz, A.M. Cyclic adenosine monophosphate effects on the myocardium: A man who blows hot and cold with one breath. J. Am. Coll. Cardiol. 1983, 2, 143–149. [Google Scholar] [CrossRef]
- Goraya, T.A.; Cooper, D.M. Ca2+-calmodulin-dependent phosphodiesterase (PDE1): Current perspectives. Cell. Signal. 2005, 17, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Vandeput, F.; Wolda, S.L.; Krall, J.; Hambleton, R.; Uher, L.; McCaw, K.N.; Radwanski, P.B.; Florio, V.; Movsesian, M.A. Cyclic nucleotide phosphodiesterase PDE1C1 in human cardiac myocytes. J. Biol. Chem. 2007, 282, 32749–32757. [Google Scholar] [CrossRef] [PubMed]
- Bautista Nino, P.K.; Durik, M.; Danser, A.H.; de Vries, R.; Musterd-Bhaggoe, U.M.; Meima, M.E.; Kavousi, M.; Ghanbari, M.; Hoeijmakers, J.H.; O’Donnell, C.J.; et al. Phosphodiesterase 1 regulation is a key mechanism in vascular aging. Clin. Sci. 2015, 129, 1061–1075. [Google Scholar] [CrossRef] [PubMed]
- Knight, W.E.; Chen, S.; Zhang, Y.; Oikawa, M.; Wu, M.; Zhou, Q.; Miller, C.L.; Cai, Y.; Mickelsen, D.M.; Moravec, C.; et al. PDE1C deficiency antagonizes pathological cardiac remodeling and dysfunction. Proc. Natl. Acad. Sci. USA 2016, 113, E7116–E7125. [Google Scholar] [CrossRef] [PubMed]
- Escher, P.; Wahli, W. Peroxisome proliferator-activated receptors: Insight into multiple cellular functions. Mutat. Res. 2000, 448, 121–138. [Google Scholar] [CrossRef]
- Kersten, S.; Desvergne, B.; Wahli, W. Roles of PPARs in health and disease. Nature 2000, 405, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Reddy, J.K. Transcription coactivators for peroxisome proliferator-activated receptors. Biochim. Biophys. Acta 2007, 1771, 936–951. [Google Scholar] [CrossRef] [PubMed]
- Misra, P.; Reddy, J.K. Peroxisome proliferator-activated receptor-alpha activation and excess energy burning in hepatocarcinogenesis. Biochimie 2014, 98, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Gilde, A.J.; van der Lee, K.A.; Willemsen, P.H.; Chinetti, G.; van der Leij, F.R.; van der Vusse, G.J.; Staels, B.; van Bilsen, M. Peroxisome proliferator-activated receptor (PPAR) alpha and PPARbeta/delta, but not PPARgamma, modulate the expression of genes involved in cardiac lipid metabolism. Circ. Res. 2003, 92, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Barger, P.M.; Kelly, D.P. PPAR signaling in the control of cardiac energy metabolism. Trends Cardiovasc. Med. 2000, 10, 238–245. [Google Scholar] [CrossRef]
- Jia, Y.; Chang, H.C.; Schipma, M.J.; Liu, J.; Shete, V.; Liu, N.; Sato, T.; Thorp, E.B.; Barger, P.M.; Zhu, Y.J.; et al. Cardiomyocyte-Specific Ablation of Med1 Subunit of the Mediator Complex Causes Lethal Dilated Cardiomyopathy in Mice. PLoS ONE 2016, 11, e0160755. [Google Scholar]
- Malik, S.; Roeder, R.G. The metazoan Mediator coactivator complex as an integrative hub for transcriptional regulation. Nat. Rev. Genet. 2010, 11, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Viswakarma, N.; Reddy, J.K. Med1 subunit of the mediator complex in nuclear receptor-regulated energy metabolism, liver regeneration, and hepatocarcinogenesis. Gene Expr. 2014, 16, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Qi, C.; Kashireddi, P.; Surapureddi, S.; Zhu, Y.J.; Rao, M.S.; Le Roith, D.; Chambon, P.; Gonzalez, F.J.; Reddy, J.K. Transcription coactivator PBP, the peroxisome proliferator-activated receptor (PPAR)-binding protein, is required for PPARalpha-regulated gene expression in liver. J. Biol. Chem. 2004, 279, 24427–24434. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, S. The failing heart--an engine out of fuel. N. Engl. J. Med. 2007, 356, 1140–1151. [Google Scholar] [CrossRef] [PubMed]
- Drosatos, K.; Pollak, N.M.; Pol, C.J.; Ntziachristos, P.; Willecke, F.; Valenti, M.C.; Trent, C.M.; Hu, Y.; Guo, S.; Aifantis, I.; et al. Cardiac Myocyte KLF5 Regulates Ppara Expression and Cardiac Function. Circ. Res. 2016, 118, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Finck, B.N.; Lehman, J.J.; Leone, T.C.; Welch, M.J.; Bennett, M.J.; Kovacs, A.; Han, X.; Gross, R.W.; Kozak, R.; Lopaschuk, G.D.; et al. The cardiac phenotype induced by PPARalpha overexpression mimics that caused by diabetes mellitus. J. Clin. Investig. 2002, 109, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Aubert, G.; Vega, R.B.; Kelly, D.P. Perturbations in the gene regulatory pathways controlling mitochondrial energy production in the failing heart. Biochim. Biophys. Acta 2013, 1833, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Zungu, M.; Young, M.E.; Stanley, W.C.; Essop, M.F. Chronic treatment with the peroxisome proliferator-activated receptor alpha agonist Wy-14,643 attenuates myocardial respiratory capacity and contractile function. Mol. Cell. Biochem. 2009, 330, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Reddy, J.K.; Krishnakantha, T.P. Hepatic peroxisome proliferation: Induction by two novel compounds structurally unrelated to clofibrate. Science 1975, 190, 787–789. [Google Scholar] [CrossRef] [PubMed]
- Reddy, J.K.; Rao, M.S.; Azarnoff, D.L.; Sell, S. Mitogenic and carcinogenic effects of a hypolipidemic peroxisome proliferator, [4-chloro-6-(2,3-xylidino)-2-pyrimidinylthio]acetic acid (Wy-14,643), in rat and mouse liver. Cancer Res. 1979, 39, 152–161. [Google Scholar] [PubMed]
- Johnson, T.E.; Holloway, M.K.; Vogel, R.; Rutledge, S.J.; Perkins, J.J.; Rodan, G.A.; Schmidt, A. Structural requirements and cell-type specificity for ligand activation of peroxisome proliferator-activated receptors. J. Steroid Biochem. Mol. Biol. 1997, 63, 1–8. [Google Scholar] [CrossRef]
- Desvergne, B.; Wahli, W. Peroxisome proliferator-activated receptors: Nuclear control of metabolism. Endocr. Rev. 1999, 20, 649–688. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.E.; Kass, D.A. Cardiac Phosphodiesterases and Their Modulation for Treating Heart Disease. Handb. Exp. Pharmacol. 2017, 243, 249–269. [Google Scholar] [PubMed]
- Bender, A.T.; Beavo, J.A. Cyclic nucleotide phosphodiesterases: Molecular regulation to clinical use. Pharmacol. Rev. 2006, 58, 488–520. [Google Scholar] [CrossRef] [PubMed]
- Lazar, M.A. Becoming fat. Gene Develop. 2002, 16, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvares, K.; Fan, C.; Dadras, S.S.; Yeldandi, A.V.; Rachubinski, R.A.; Capone, J.P.; Subramani, S.; Iannaccone, P.M.; Rao, M.S.; Reddy, J.K. An upstream region of the enoyl-coenzyme A hydratase/3-hydroxyacyl-coenzyme A dehydrogenase gene directs luciferase expression in liver in response to peroxisome proliferators in transgenic mice. Cancer Res. 1994, 54, 2303–2306. [Google Scholar] [PubMed]
- Cai, Y.; Nagel, D.J.; Zhou, Q.; Cygnar, K.D.; Zhao, H.; Li, F.; Pi, X.; Knight, P.A.; Yan, C. Role of cAMP-phosphodiesterase 1C signaling in regulating growth factor receptor stability, vascular smooth muscle cell growth, migration, and neointimal hyperplasia. Circ. Res. 2015, 116, 1120–1132. [Google Scholar] [CrossRef] [PubMed]
- Satoh, K.; Kikuchi, N.; Kurosawa, R.; Shimokawa, H. PDE1C negatively regulates growth factor receptor degradation and promotes VSMC proliferation. Circ. Res. 2015, 116, 1098–1100. [Google Scholar] [CrossRef] [PubMed]
- Rowther, F.B.; Wei, W.; Dawson, T.P.; Ashton, K.; Singh, A.; Madiesse-Timchou, M.P.; Thomas, D.G.; Darling, J.L.; Warr, T. Cyclic nucleotide phosphodiesterase-1C (PDE1C) drives cell proliferation, migration and invasion in glioblastoma multiforme cells in vitro. Mol. Carcinog. 2016, 55, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.L.; Oikawa, M.; Cai, Y.; Wojtovich, A.P.; Nagel, D.J.; Xu, X.; Xu, H.; Florio, V.; Rybalkin, S.D.; Beavo, J.A.; et al. Role of Ca2+/calmodulin-stimulated cyclic nucleotide phosphodiesterase 1 in mediating cardiomyocyte hypertrophy. Circ. Res. 2009, 105, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Rybalkin, S.D.; Bornfeldt, K.E.; Sonnenburg, W.K.; Rybalkina, I.G.; Kwak, K.S.; Hanson, K.; Krebs, E.G.; Beavo, J.A. Calmodulin-stimulated cyclic nucleotide phosphodiesterase (PDE1C) is induced in human arterial smooth muscle cells of the synthetic, proliferative phenotype. J. Clin. Investig. 1997, 100, 2611–2621. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Knight, W.; Chen, S.; Mohan, A.; Yan, C. A Multiprotein Complex with TRPC, PDE1C, and A2R Plays a Critical Role in Regulating Cardiomyocyte cAMP and Survival. Circulation 2018. [Google Scholar] [CrossRef] [PubMed]
- Medina, A.E. Therapeutic utility of phosphodiesterase type I inhibitors in neurological conditions. Front. Neurosci. 2011, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, T.E.; Meinke, P.T.; Berger, J.P. PPAR ligands: Potential therapies for metabolic syndrome. Curr. Diabetes Rep. 2005, 5, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Young, M.E.; Laws, F.A.; Goodwin, G.W.; Taegtmeyer, H. Reactivation of peroxisome proliferator-activated receptor alpha is associated with contractile dysfunction in hypertrophied rat heart. J. Biol. Chem. 2001, 276, 44390–44395. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.C.; Kovacs, A.; Ford, D.A.; Hsu, F.F.; Garcia, R.; Herrero, P.; Saffitz, J.E.; Schaffer, J.E. A novel mouse model of lipotoxic cardiomyopathy. J. Clin. Investig. 2001, 107, 813–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.T.; Grayburn, P.; Karim, A.; Shimabukuro, M.; Higa, M.; Baetens, D.; Orci, L.; Unger, R.H. Lipotoxic heart disease in obese rats: Implications for human obesity. Proc. Natl. Acad. Sci. USA 2000, 97, 1784–1789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baranowski, M.; Blachnio-Zabielska, A.; Gorski, J. Peroxisome proliferator-activated receptor alpha activation induces unfavourable changes in fatty acid composition of myocardial phospholipids. J. Physiol. Pharmacol. 2009, 60, 13–20. [Google Scholar] [PubMed]
- Tien, E.S.; Hannon, D.B.; Thompson, J.T.; Vanden Heuvel, J.P. Examination of Ligand-Dependent Coactivator Recruitment by Peroxisome Proliferator-Activated Receptor-alpha (PPARalpha). PPAR Res. 2006, 2006, 69612. [Google Scholar] [CrossRef] [PubMed]





| WT LIVER | WT HEART | |||||
|---|---|---|---|---|---|---|
| Wy-14,643/Control | p-Value | Wy-14,643/Control | p-Value | |||
| L-pbe | 20.07 | <0.001 | ** | 378.08 | <0.001 | ** |
| Pde1C | 1.05 | 0.9 | NS | 9.51 | <0.001 | ** |
| WT HEART | Pparα−/−HEART | TmcsMed1−/−HEART | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Wy-14,643/Control | p-Value | Wy-14,643/Control | p-Value | Wy-14,643/Control | p-Value | ||||
| L-pbe | 378.08 | <0.001 | ** | 1.2 | 0.41 | NS | 0.95 | 0.06 | NS |
| Pde1C | 9.51 | <0.001 | ** | 0.91 | 0.38 | NS | 0.94 | 0.7 | NS |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shete, V.; Liu, N.; Jia, Y.; Viswakarma, N.; Reddy, J.K.; Thimmapaya, B. Mouse Cardiac Pde1C Is a Direct Transcriptional Target of Pparα. Int. J. Mol. Sci. 2018, 19, 3704. https://doi.org/10.3390/ijms19123704
Shete V, Liu N, Jia Y, Viswakarma N, Reddy JK, Thimmapaya B. Mouse Cardiac Pde1C Is a Direct Transcriptional Target of Pparα. International Journal of Molecular Sciences. 2018; 19(12):3704. https://doi.org/10.3390/ijms19123704
Chicago/Turabian StyleShete, Varsha, Ning Liu, Yuzhi Jia, Navin Viswakarma, Janardan K. Reddy, and Bayar Thimmapaya. 2018. "Mouse Cardiac Pde1C Is a Direct Transcriptional Target of Pparα" International Journal of Molecular Sciences 19, no. 12: 3704. https://doi.org/10.3390/ijms19123704






