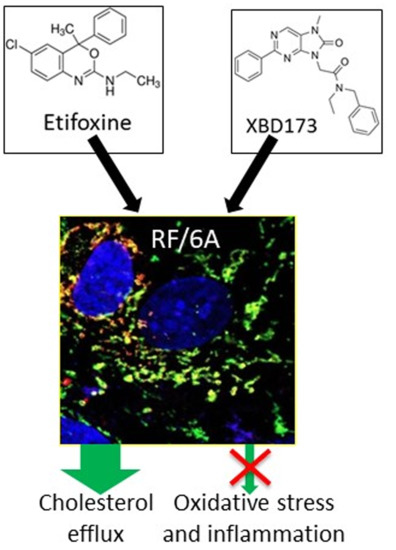
TSPO Ligands Promote Cholesterol Efflux and Suppress Oxidative Stress and Inflammation in Choroidal Endothelial Cells
Abstract
:1. Introduction
2. Results
2.1. TSPO Ligands Increase Cholesterol Efflux and Upregulate Cholesterol Metabolism and Transporter Genes
2.2. TSPO Ligands Regulate Lipogenesis in Choroidal Endothelial Cells
2.3. TSPO Ligands Supress ROS Production and Increased Antioxidant Capacity
2.4. TSPO Ligands Decrease Production of Pro-Inflammatory Cytokines
3. Discussion
4. Materials and Methods
4.1. Cell Viability Assay
4.2. Measurement of [3H]Cholesterol Efflux
4.3. Lipid Analysis
4.4. Lipid Assays
4.5. Gene Expression
4.6. Oil Red O (ORO) Staining
4.7. Immunocytochemistry
4.8. Biochemical Assays
4.9. Measurement of Cytokines
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, 106–116. [Google Scholar] [CrossRef]
- Biesemeier, A.; Taubitz, T.; Julien, S.; Yoeruek, E.; Schraermeyer, U. Choriocapillaris breakdown precedes retinal degeneration in age-related macular degeneration. Neurobiol. Aging 2014, 35, 2562–2573. [Google Scholar] [CrossRef] [PubMed]
- Whitmore, S.S.; Braun, T.A.; Skeie, J.M.; Haas, C.M.; Sohn, E.H.; Stone, E.M.; Scheetz, T.E.; Mullins, R.F. Altered gene expression in dry age-related macular degeneration suggests early loss of choroidal endothelial cells. Mol. Vis. 2013, 19, 2274–2297. [Google Scholar] [PubMed]
- Whitmore, S.S.; Sohn, E.H.; Chirco, K.R.; Drack, A.V.; Stone, E.M.; Tucker, B.A.; Mullins, R.F. Complement activation and choriocapillaris loss in early AMD: Implications for pathophysiology and therapy. Prog. Retin. Eye Res. 2015, 45, 1–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mullins, R.F.; Johnson, M.N.; Faidley, E.A.; Skeie, J.M.; Huang, J. Choriocapillaris vascular dropout related to density of drusen in human eyes with early age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1606–1612. [Google Scholar] [CrossRef] [PubMed]
- Korte, G.E.; Reppucci, V.; Henkind, P. RPE destruction causes choriocapillary atrophy. Investig. Ophthalmol. Vis. Sci. 1984, 25, 1135–1145. [Google Scholar]
- Bhutto, I.; Lutty, G. Understanding age-relted macular degeneration (AMD): Relationships between the photoreceptor/retinal pigment epithelium/Bruch’s membrane/choriocapillaaris complex. Mol. Asp. Med. 2012, 33, 295–317. [Google Scholar] [CrossRef] [PubMed]
- Braestrup, C.; Squires, R.F. Specific benzodiazepine receptors in rat brain characterized by high-affinity (3H) diazepam binding. Proc. Natl. Acad. Sci. USA 1977, 74, 3805–3809. [Google Scholar] [CrossRef] [PubMed]
- Yeliseev, A.A.; Krueger, K.E.; Kaplan, S. A mammalian mitochondrial drug receptor functions as a bacterial “oxygen” sensor. Proc. Natl. Acad. Sci. USA 1997, 94, 5101–5106. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Papadopoulos, V. Peripheral-type benzodiazepine receptor function in cholesterol transport. Identification of a putative cholesterol recognition/interaction amino acid sequence and consensus pattern. Endocrinology 1998, 139, 4991–4997. [Google Scholar] [CrossRef] [PubMed]
- Graham, A. Mitochondrial regulation of macrophage cholesterol homeostasis. Free Radic. Biol. Med. 2015, 89, 982–992. [Google Scholar] [CrossRef] [PubMed]
- Tu, L.N.; Morohaku, K.; Manna, P.R.; Pelton, S.H.; Butler, W.R.; Stocco, D.M.; Selvaraj, V. Peripheral benzodiazepine receptor/translocator protein global knockout mice are viable with no effects on steroid hormone biosynthesis. J. Biol. Chem. 2014, 289, 27444–27454. [Google Scholar] [CrossRef] [PubMed]
- Banati, R.B.; Middleton, R.J.; Chan, R.; Hatty, C.R.; Kam, W.W.; Quin, C.; Graeber, M.B.; Parmar, A.; Zahra, D.; Callaghan, P.; et al. Positron emission tomography and functional characterization of a complete PBR/ TSPO knockout. Nat. Commun. 2014, 5, 5452. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Campioli, E.; Midzak, A.; Culty, M.; Papadopoulos, V. Conditional steroidogenic cell-targeted deletion of TSPO unveils a crucial role in viability and hormone-dependent steroid formation. Proc. Natl. Acad. Sci. USA 2015, 112, 7261–7266. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhai, K.; Xue, Y.; Yang, J.; Yang, Q.; Fu, Y.; Hu, Y.; Liu, F.; Wang, W.; Cui, L.; et al. Global deletion of TSPO does not affect the viability and gene expression profile. PLoS ONE 2016, 11, e0167307. [Google Scholar] [CrossRef] [PubMed]
- Barron, A.M.; Ji, B.; Kito, S.; Suhara, T.; Higuchi, M. Steroidogenic abnormalities in translocator protein knockout mice and significance in the aging male. Biochem. J. 2018, 475, 75–85. [Google Scholar] [CrossRef] [PubMed]
- McEnery, M.W.; Snowman, A.M.; Trifiletti, R.R.; Snyder, S.H. Isolation of the mitochondrial benzodiazepine receptor: Association with the voltage-dependent anion channel and the adenine nucleotide carrier. Proc. Natl. Acad. Sci. USA 1992, 89, 3170–3174. [Google Scholar] [CrossRef] [PubMed]
- Gatliff, J.; Campanella, M. TSPO is a REDOX regulator of cell mitophagy. Biochem. Soc. Trans. 2015, 43, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Girard, C.; Liu, S.; Adams, D.; Lacroix, C.; Sinéus, M.; Boucher, C.; Papadopoulos, V.; Rupprecht, R.; Schumacher, M.; Groyer, G. Axonal regeneration and neuroinflammation: Roles for the translocator protein 18 kDa. J. Neuroendocrinol. 2012, 24, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, X.; Zhao, L.; Ma, W.; Rodriguez, I.R.; Fariss, R.N.; Wong, W.T. Macroglia-microglia interactions via TSPO signaling regulates microglial activation in the mouse retina. J. Neurosci. 2014, 34, 3793–3806. [Google Scholar] [CrossRef] [PubMed]
- Karlstetter, M.; Nothdurfter, C.; Aslanidis, A.; Moeller, K.; Horn, F.; Scholz, R.; Neumann, H.; Weber, B.H.; Rupprecht, R.; Langmann, T. Translocator protein (18 kDa) (TSPO) is expressed in reactive retinal microglia and modulates microglial inflammation and phagocytosis. J. Neuroinflamm. 2014, 11, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biswas, L.; Zhou, X.; Dhillon, B.; Graham, A.; Shu, X. Retinal pigment epithelium cholesterol efflux mediated by the18kDa translocator protein, TSPO, a potential target for treating age-related macular degeneration. Hum. Mol. Genet. 2017, 26, 4327–4339. [Google Scholar] [CrossRef] [PubMed]
- Falchi, A.M.; Battetta, B.; Sanna, F.; Piludu, M.; Sogos, V.; Serra, M.; Melis, M.; Putzolu, M.; Diaz, G. Intracellular cholesterol changes induced by translocator protein (18 kDa) TSPO/PBR ligands. Neuropharmacology 2007, 53, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.M.; Allen, A.M.; Graham, A. Targeting mitochondrial 18 kDa translocator protein (TSPO) regulates macrophage cholesterol efflux and lipid phenotype. Clin. Sci. 2014, 127, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Cosenza-Nashat, M.; Zhao, M.L.; Suh, H.S.; Morgan, J.; Natividad, R.; Morgello, S.; Lee, S.C. Expression of the translocator protein of 18 kDa by microglia, macrophages and astrocytes based on immunohistochemical localization in abnormal human brain. Neuropathol. Appl. Neurobiol. 2009, 35, 306–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, B.; Maeda, J.; Sawada, M.; Ono, M.; Okauchi, T.; Inaji, M.; Zhang, M.R.; Suzuki, K.; Ando, K.; Staufenbiel, M.; et al. Imaging of peripheral benzodiazepine receptor expression as biomarkers of detrimental versus beneficial glial responses in mouse models of Alzheimer’s and other CNS pathologies. J. Neurosci. 2008, 28, 12255–12267. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, D.T.; Schober, D.A.; Smalstig, E.B.; Mincy, R.E.; Gehlert, D.R.; Clemens, J.A. Peripheral benzodiazepine receptors are colocalized with activated microglia following transient global forebrain ischemia in the rat. J. Neurosci. 1995, 15, 5263–5274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joo, H.K.; Oh, S.C.; Cho, E.J.; Park, K.S.; Lee, J.Y.; Lee, E.J.; Lee, S.K.; Kim, H.S.; Park, J.B.; Jeon, B.H. Midazolam inhibits tumor necrosis factor-alpha-induced endothelial activation: Involvement of the peripheral benzodiazepine receptor. Anesthesiology 2009, 110, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Pol, A.; Gross, S.P.; Parton, R.G. Review: Biogenesis of the multifunctional lipid droplet: Lipids, proteins, and sites. J. Cell Biol. 2014, 204, 635–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Tassigny, A.; Assaly, R.; Schaller, S.; Pruss, R.M.; Berdeaux, A.; Morin, D. Mitochondrial translocator protein (TSPO) ligands prevent doxorubicin-induced mechanical dysfunction and cell death in isolated cardiomyocytes. Mitochondrion 2013, 13, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Joo, H.K.; Lee, Y.R.; Kang, G.; Choi, S.; Kim, C.S.; Ryoo, S.; Park, J.B.; Jeon, B.H. The 18-kDa Translocator Protein Inhibits Vascular Cell Adhesion Molecule-1 Expression via Inhibition of Mitochondrial Reactive Oxygen Species. Mol. Cells. 2015, 38, 1064–1070. [Google Scholar] [PubMed]
- Sun, X.; Guo, S.; Wang, W.; Cao, Z.; Dan, J.; Cheng, J.; Cao, W.; Tian, F.; Cao, W.; Tian, Y. Potential involvement of the 18 kDa translocator protein and reactive oxygen species in apoptosis of THP-1 macrophages induced by sonodynamic therapy. PLoS ONE 2018, 13, e0196541. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Deshmukh, A.; Sachdeva, R.; Lu, J.; Mehta, J.L. Oxidized low-density lipoprotein and atherosclerosis implications in antioxidant therapy. Am. J. Med. Sci. 2011, 342, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Miller, Y.I.; Shyy, J.Y. Context-Dependent Role of Oxidized Lipids and Lipoproteins in Inflammation. Trends Endocrinol. Metab. 2017, 28, 143–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nègre-Salvayre, A.; Augé, N.; Camaré, C.; Bacchetti, T.; Ferretti, G.; Salvayre, R. Dual signaling evoked by oxidized LDLs in vascular cells. Free Radic. Biol. Med. 2017, 106, 118–133. [Google Scholar] [CrossRef] [PubMed]
- Pikuleva, I.A.; Curcio, C.A. Cholesterol in the retina: The best is yet to come. Prog. Retin. Eye Res. 2014, 41, 64–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curcio, C.A.; Presley, J.B.; Malek, G.; Medeiros, N.E.; Avery, D.V.; Kruth, H.S. Esterified and unesterified cholesterol in drusen and basal deposits of eyes with age-related maculopathy. Exp. Eye Res. 2005, 81, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Storti, F.; Raphael, G.; Griesser, V.; Klee, K.; Drawnel, F.; Willburger, C.; Scholz, R.; Langmann, T.; von Eckardstein, A.; Fingerle, J.; et al. Regulated efflux of photoreceptor outer segment-derived cholesterol by human RPE cells. Exp. Eye Res. 2017, 165, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Zekavat, S.M.; Lu, J.; Maugeais, C.; Mazer, N.A. An in silico model of retinal cholesterol dynamics (RCD model): Insights into the pathophysiology of dry AMD. J. Lipid Res. 2017, 58, 1325–1337. [Google Scholar] [CrossRef] [PubMed]
- Fliesler, S.J.; Bretillon, L. The ins and outs of cholesterol in the vertebrate retina. J. Lipid Res. 2010, 51, 3399–3413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prosser, H.C.; Ng, M.K.; Bursill, C.A. The role of cholesterol efflux in mechanisms of endothelial protection by HDL. Curr. Opin. Lipidol. 2012, 23, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Tu, L.N.; Zhao, A.H.; Stocco, D.M.; Selvaraj, V. PK11195 effect on steroidogenesis is not mediated through the translocator protein (TSPO). Endocrinology 2015, 156, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Wang, K.; Zirkin, B.; Papadopoulos, V. CRISPR/Cas9‒mediated Tspo gene mutations lead to reduced mitochondrial membrane potential and steroid formation in MA-10 mouse tumor Leydig cells. Endocrinology 2018, 159, 1130–1146. [Google Scholar] [CrossRef] [PubMed]
- Poisbeau, P.; Gazzo, G.; Calvel, L. Anxiolytics targeting GABAA receptors: Insights on etifoxine. World J. Biol. Psychiatry 2018, 19, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Costa, B.; Da Pozzo, E.; Cavallini, C.; Taliani, S.; Da Settimo, F.; Martini, C. Long Residence Time at the Neurosteroidogenic 18 kDa Translocator Protein Characterizes the Anxiolytic Ligand XBDACS. Chem. Neurosci. 2016, 7, 1041–1046. [Google Scholar] [CrossRef] [PubMed]
- Costa, B.; Cavallini, C.; Da Pozzo, E.; Taliani, S.; Da Settimo, F.; Martini, C. The Anxiolytic Etifoxine Binds to TSPO Ro5-4864 Binding Site with Long Residence Time Showing a High Neurosteroidogenic Activity. ACS Chem. Neurosci. 2017, 8, 1448–1454. [Google Scholar] [CrossRef] [PubMed]
- Tall, A.R.; Yvan-Charvet, L. Cholesterol, inflammation and innate immunity. Nat. Rev. Immunol. 2015, 15, 104–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonsack, F.; Sukumari-Ramesh, S. TSPO: An evolutionarily conserved protein with elusive functions. Int. J. Mol. Sci. 2018, 19, 1694. [Google Scholar] [CrossRef] [PubMed]
- Morin, D.; Musman, J.; Pons, S.; Berdeaux, A.; Ghaleh, B. Mitochondrial translocator protein (TSPO): From physiology to cardioprotection. Biochem. Pharmacol. 2016, 105, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Leducq, N.; Bono, F.; Sulpice, T.; Vin, V.; Janiak, P.; Fur, G.L.; O’Connor, S.E.; Herbert, J.M. Role of peripheral benzodiazepine receptors in mitochondrial, cellular, and cardiac damage induced by oxidative stress and ischemia-reperfusion. J. Pharmacol. Exp. Ther. 2003, 306, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Kunduzova, O.R.; Escourrou, G.; De La Farge, F.; Salvayre, R.; Séguélas, M.H.; Leducq, N.; Bono, F.; Herbert, J.M.; Parini, A. Involvement of peripheral benzodiazepine receptor in the oxidative stress, death-signaling pathways, and renal injury induced by ischemia-reperfusion. J. Am. Soc. Nephrol. 2004, 15, 2152–2160. [Google Scholar] [CrossRef] [PubMed]
- Schaller, S.; Paradis, S.; Ngoh, G.A.; Assaly, R.; Buisson, B.; Drouot, C.; Ostuni, M.A.; Lacapere, J.J.; Bassissi, F.; Bordet, T.; et al. TRO40303, a new cardioprotective compound, inhibits mitochondrial permeability transition. J. Pharmacol. Exp. Ther. 2010, 333, 696–706. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Liang, D.; Zhang, H.; Liu, Y.; Li, F.; Chen, Y.H. 4′-Chlorodiazepam, a translocator protein (18 kDa) antagonist, improves cardiac functional recovery during postischemia reperfusion in rats. Exp. Biol. Med. 2010, 235, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Paradis, S.; Leoni, V.; Caccia, C.; Berdeaux, A.; Morin, D. Cardioprotection by the TSPO ligand 4′-chlorodiazepam is associated with inhibition of mitochondrial accumulation of cholesterol at reperfusion. Cardiovasc. Res. 2013, 98, 420–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baez, E.; Guio-Vega, G.P.; Echeverria, V.; Sandoval-Rueda, D.A.; Barreto, G.E. 4′-Chlorodiazepam Protects Mitochondria in T98G Astrocyte Cell Line from Glucose Deprivation. Neurotox. Res. 2017, 32, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Mattace Raso, G.; Taliani, S.; Da Pozzo, E.; Simorini, F.; Costa, B.; Martini, C.; Laneri, S.; Sacchi, A.; Cosimelli, B.; et al. TSPO-ligands prevent oxidative damage and inflammatory response in C6 glioma cells by neurosteroid synthesis. Eur. J. Pharm. Sci. 2016, 88, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, B.; Crawford, D.; Dellovade, T.; Savinainen, A.; Graham, D.; Liere, P.; Oudinet, J.P.; Webb, M.; Hering, H. Differential efficacy of the TSPO ligands Etifoxine and XBD-173 in two rodent models of Multiple Sclerosis. Neuropharmacology 2016, 108, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.B.; Khoo, C.; Ryu, J.K.; van Breemen, E.; Kim, S.U.; McLarnon, J.G. Inhibition of lipopolysaccharide-induced cyclooxygenase-2, tumor necrosis factor-alpha and [Ca2+]i responses in human microglia by the peripheral benzodiazepine receptor ligand PKJ 11195. J. Neurochem. 2002, 83, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Wilms, H.; Claasen, J.; Röhl, C.; Sievers, J.; Deuschl, G.; Lucius, R. Involvement of benzodiazepine receptors in neuroinflammatory and neurodegenerative diseases: Evidence from activated microglial cells in vitro. Neurobiol. Dis. 2003, 14, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.K. Linking endothelial dysfunction with endothelial cell activation. J. Clin. Investig. 2013, 123, 540–541. [Google Scholar] [CrossRef] [PubMed]
- Arbo, B.D.; Benetti, F.; Garcia-Segura, L.M.; Ribeiro, M.F. Therapeutic actions of translocator protein (18 kDa) ligands in experimental models of psychiatric disorders and neurodegenerative diseases. J. Steroid Biochem. Mol. Biol. 2015, 154, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Barron, A.M.; Garcia-Segura, L.M.; Caruso, D.; Jayaraman, A.; Lee, J.W.; Melcangi, R.C.; Pike, C.J. Ligand for translocator protein reverses pathology in a mouse model of Alzheimer’s disease. J. Neurosci. 2013, 33, 8891–8897. [Google Scholar] [CrossRef] [PubMed]
- Daugherty, D.J.; Selvaraj, V.; Chechneva, O.V.; Liu, X.B.; Pleasure, D.E.; Deng, W. A TSPO ligand is protective in a mouse model of multiple sclerosis. EMBO Mol. Med. 2013, 5, 891–903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leva, G.; Klein, C.; Benyounes, J.; Hallé, F.; Bihel, F.; Collongues, N.; De Seze, J.; Mensah-Nyagan, A.G.; Patte-Mensah, C. The translocator protein ligand XBD173 improves clinical symptoms and neuropathological markers in the SJL/J mouse model of multiple sclerosis. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 3016–3027. [Google Scholar] [CrossRef] [PubMed]
- Scholz, R.; Caramoy, A.; Bhuckory, M.B.; Rashid, K.; Chen, M.; Xu, H.; Grimm, C.; Langmann, T. Targeting translocator protein (18 kDa) (TSPO) dampens pro-inflammatory microglia reactivity in the retina and protects from degeneration. J. Neuroinflammation 2015, 12, 201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rupprecht, R.; Rammes, G.; Eser, D.; Baghai, T.C.; Schüle, C.; Nothdurfter, C.; Troxler, T.; Gentsch, C.; Kalkman, H.O.; Chaperon, F.; et al. Translocator protein (18 kD) as target for anxiolytics without benzodiazepine-like side effects. Science 2009, 325, 490–493. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.M.; Kim, K.H. Etifoxine for pain patients with anxiety. Korean J. Pain 2015, 28, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Yasin, N.; Veenman, L.; Singh, S.; Azrad, M.; Bode, J.; Vainshtein, A.; Caballero, B.; Marek, I.; Gavish, M. Classical and Novel TSPO Ligands for the Mitochondrial TSPO Can Modulate Nuclear Gene Expression: Implications for Mitochondrial Retrograde Signaling. Int. J. Mol. Sci. 2017, 18, 786. [Google Scholar] [CrossRef] [PubMed]






| Gene | Gene Bank Accession Number | Primer Sequences (5′–3′) Forward | Primer Sequences (5′–3′) Reverse |
|---|---|---|---|
| β-ACTIN | NM_001033084.1 | TCCACGAAACTACCTTCAACTC | GTCATACTCCTGCTTGCTGAT |
| LXRα | XM_015114352.1 | CCCCATGACCGACTGATGTT | CGGAGGCTCACCAGTTTCATT |
| ABCG1 | XM_015132831.1 | AAGGTGTCCTGCTACATCAT | CAGTATCTCCTTGACCATCTC |
| ABCA1 | XM_015117158.1 | GAAGTACATCAGAACATGGGC | GATCAAAGCCATGGCTGTAG |
| CYP46A1 | XM_015144470.1 | CCTTCTTCATTGCTGGTCACG | TCCATCACTGTGAACGCCAAG |
| CYP27A1 | NM_001194021.1 | GGCAAGTACCCAGTACGG | AGCAAATAGCTTCCAAGG |
| IL-1 β | NM_001042756.1 | ACCTGAGCTCGCCAGTGAAA | GCCGGAAGCCCTCGTTGTAG |
| TNF-α | NM_001047149.1 | TCTCCTTCCTGCTCGTGGCA | GGGTTTGCTACAACATGGGCTAC |
| IL6 | XM_015133872.1 | CCTTCCAAAGATGGCTGAAA | CAGGGGTGGTTATTGCATCT |
| VEGFA | NM_001278410.1 | AAGGAGGAGGGCAGAATCAT | ATCTGCATGGTGATGTTGGA |
| GPX1 | NM_001159298.2 | CTCTTCGAGAAGTGCGAGGT | TCGATGTCAATGGTCTGGAA |
| SOD1 | NM_001032804.1 | AGGGCACCATCAATTTCGAG | ACATTGCCCAGGTCTCCAAC |
| Catalase | XM_001115625.3 | CGCCTATGCAGCGAAGCTTA | TTTGCGCATCTAGCACCGGA |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biswas, L.; Farhan, F.; Reilly, J.; Bartholomew, C.; Shu, X. TSPO Ligands Promote Cholesterol Efflux and Suppress Oxidative Stress and Inflammation in Choroidal Endothelial Cells. Int. J. Mol. Sci. 2018, 19, 3740. https://doi.org/10.3390/ijms19123740
Biswas L, Farhan F, Reilly J, Bartholomew C, Shu X. TSPO Ligands Promote Cholesterol Efflux and Suppress Oxidative Stress and Inflammation in Choroidal Endothelial Cells. International Journal of Molecular Sciences. 2018; 19(12):3740. https://doi.org/10.3390/ijms19123740
Chicago/Turabian StyleBiswas, Lincoln, Fahad Farhan, James Reilly, Chris Bartholomew, and Xinhua Shu. 2018. "TSPO Ligands Promote Cholesterol Efflux and Suppress Oxidative Stress and Inflammation in Choroidal Endothelial Cells" International Journal of Molecular Sciences 19, no. 12: 3740. https://doi.org/10.3390/ijms19123740