Studies on Silver Ions Releasing Processes and Mechanical Properties of Surface-Modified Titanium Alloy Implants
Abstract
1. Introduction
2. Results
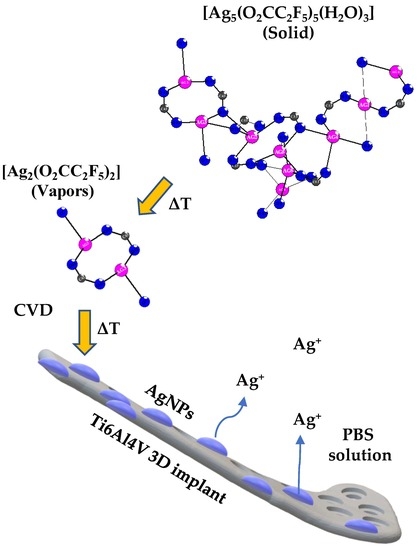
2.1. The Chemical Vapor Deposition of Silver Nanoparticles
2.1.1. Precursor—The Structure and Thermal Properties of [Ag5(O2CC2F5)5(H2O)3]
2.1.2. The Enrichment of Ti6Al4V and Ti6Al4V/TNT Substrates by Silver Nanoparticles (AgNPs)
2.2. Measurement of the Contact Angle and Surface Free Energy of Biomaterials
2.3. Mechanical Properties of Ti6Al4V/AgNPs, Ti6Al4V/TNT, and Ti6Al4V/TNT/AgNPs
2.3.1. Surface Topography
2.3.2. Hardness and Young’s Modulus
2.3.3. Adhesion Tests of Ti6Al4V/TNT and Ti6Al4V/TNT/AgNPs Composites
2.4. Evaluation of Stability and Durability of Coating Materials in the Body Fluid Environment
3. Discussion
4. Materials and Methods
4.1. Synthesis of Silver CVD Precursor and Conditions CVD Processes Carry Out
4.2. The Production of Ti6Al4V/TNT Substrates and Its Characteristics
4.3. Measurement of the Contact Angle and Surface Free Energy of Biomaterials
4.4. Topographies and Mechanical Properties of the Produced Nanocoatings on the Surface of 3D Printed Implants
4.5. Evaluation of Stability and Durability of Coating Materials in the Body Fluid Environment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Vorndran, E.; Moseke, C.; Gbureck, U. 3D printing of ceramic implants. MRS Bull. 2015, 40, 127–136. [Google Scholar] [CrossRef]
- lmquist, A.; Omar, O.M.; Esposito, M.; Lausmaa, J.; Thomsen, P. Titanium oral implants: Surface characteristics, interface biology and clinical outcome. J. Roy. Soc. Interface 2010, 7, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Streckbein, P.; Streckbein, R.G.; Wilbrand, J.F.; Malik, C.Y.; Schaaf, H.; Howaldt, H.P.; Flach, M. Non-linear 3D Evaluation of Different Oral Implant-Abutment Connections. J. Dent. Res. 2012, 91, 1184–1189. [Google Scholar] [CrossRef] [PubMed]
- Dekker, T.J.; Steele, J.R.; Federer, A.E.; Hamid, K.S.; Adams, S.B. Use of Patient-Specific 3D-Printed Titanium Implants for Complex Foot and Ankle Limb Salvage, Deformity Correction, and Arthrodesis Procedures. Foot Ankle Int. 2018, 36, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, J.; Lee, B.E.J.; Lin, A.Y.W.; Binkley, D.M.; Delaney, K.H.; Kwiecien, J.M.; Grandfield, K. Osseointegration of a 3D Printed Stemmed Titanium Dental Implant: A Pilot Study. Int. J. Dent. 2017, 2017, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Shirazi, S.F.S.; Gharehkhani, S.; Mehrali, M.; Yarmand, H.; Metselaar, H.S.C.; Kadri, N.A.; Osman, N.A.A. A review on powder-based additive manufacturing for tissue engineering: Selective laser sintering and inkjet 3D printing. J. Sci. Technol. Adv. Mater. 2015, 16, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Tran, Q.H.; Nguyen, V.Q.; Le, A.T. Silver nanoparticles: Synthesis, properties, toxicology, applications and perspectives. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4, 1–20. [Google Scholar] [CrossRef]
- Kang, C.G.; Park, Y.B.; Choi, H.; Oh, S.; Lee, K.W.; Choi, S.H.; Shim, J.S. Osseointegration of Implants Surface-Treated with Various Diameters of TiO2 Nanotubes in Rabbit. J. Nanomater. 2015, 2015, 1–11. [Google Scholar] [CrossRef]
- Piszczek, P.; Lewandowska, Ż.; Radtke, A.; Jedrzejewski, T.; Kozak, W.; Sadowska, B.; Szubka, M.; Talik, E.; Fiori, F. Biocompatibility of Titania Nanotube Coatings Enriched with Silver Nanograins by Chemical Vapor Deposition. Nanomaterials 2017, 7, 1–19. [Google Scholar] [CrossRef]
- Radtke, A.; Topolski, A.; Jędrzejewski, T.; Kozak, W.; Sadowska, B.; Więckowska-Szakiel, M.; Szubka, M.; Talik, E.; Nielsen, L.P.; Piszczek, P. The Bioactivity and Photocatalytic Properties of Titania Nanotube Coatings Produced with the Use of the Low-Potential Anodization of Ti6Al4V Alloy Surface. Nanomaterials 2017, 7, 1–15. [Google Scholar] [CrossRef]
- Zhao, L.; Chu, P.K.; Zhang, Y.; Wu, Z. Antibacterial Coatings on Titanium Implants. J. Biomed. Mater. Res. 2009, 91, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Montanaro, L.; Arciola, C.R. Biofilm in implant infections: Its production and regulation. Int. J. Artif. Organs 2005, 28, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Fürst, M.M.; Salvi, G.E.; Lang, N.P.; Persson, G.R. Bacterial colonization immediately after installation on oral titanium implants. Clin. Oral Implants Res. 2007, 18, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Fielding, G.A.; Roy, M.; Bandyopadhyay, A.; Bose, S. Antibacterial and biological characteristics of silver containing and strontium doped plasma sprayed hydroxyapatite coatings. Acta Biomater. 2012, 8, 3144–3152. [Google Scholar] [CrossRef] [PubMed]
- Milić, M.; Leitinger, G.; Pavičić, I.; Avdičević, M.Z.; Dobrović, S.; Goessler, W.; Vrček, I.V. Cellular uptake and toxicity effects of silver nanoparticles in mammalian kidney cells. J. Appl. Toxicol. 2015, 35, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.Z.; Kim, T.N.; Kim, J.O. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef]
- Marambio-Jones, C.; Hoek, E.M.V. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J. Nanopart. Res. 2010, 12, 1531–1551. [Google Scholar] [CrossRef]
- Dastjerdi, R.; Montazer, M. A review on the application of inorganic nano-structured materials in the modification of textiles: Focus on antimicrobial properties. Colloids Surf. B Biointerfaces 2010, 79, 5–18. [Google Scholar] [CrossRef]
- Rai, M.K.; Deshmukh, S.D.; Ingle, A.P.; Gade, A.K. Silver nanoparticles: The powerful nanoweapon against multidrug-resistant bacteria. J. Appl. Microbiol. 2012, 112, 841–852. [Google Scholar] [CrossRef]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver nanoparticles as potential antibacterial agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef]
- Santillána, M.J.; Quarantab, N.E.; Boccaccinic, A.R. Titania and titania–silver nanocomposite coatings grown by electrophoretic deposition from aqueous suspensions. Surf. Coat. Technol. 2010, 205, 2562–2571. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Self-accumulated Ag nanoparticles on mesoporous TiO2 thin film with high bactericidal activities. Surf. Coat. Technol. 2010, 204, 3676–3683. [Google Scholar] [CrossRef]
- Yates, H.M.; Brook, L.A.; Sheel, D.W. Photoactive Thin Silver Films by Atmospheric Pressure CVD. Int. J. Photoenergy 2008, 1–8. [Google Scholar] [CrossRef]
- Golrokhi, Z.; Chalker, S.; Sutcliffe, C.J.; Potter, R.J. Self-limiting atomic layer deposition of conformal nanostructured silver films. Appl. Surf. Sci. 2016, 364, 789–797. [Google Scholar] [CrossRef]
- Grodzicki, A.; Łakomska, I.; Piszczek, P.; Szymańska, I.; Szłyk, E. Copper(I), silver(I) and gold(I) carboxylate complexes as precursors in chemical vapour deposition of thin metallic films. Coord. Chem. Rev. 2005, 249, 2232–2258. [Google Scholar] [CrossRef]
- Dryden, N.H.; Vittal, J.J.; Puddephatt, R.J. New precursors for chemical vapor deposition of silver. Chem. Mater. 1993, 5, 765–766. [Google Scholar] [CrossRef]
- Piszczek, P.; Szłyk, E.; Chaberski, M.; Taeschner, C.; Leonhardt, A.; Bała, W.; Bartkiewicz, K. Characterization of Silver Trimethylacetate Complexes with Tertiary Phosphines as CVD Precursors of Thin Silver Films. Chem. Vap. Depos. 2005, 11, 53–59. [Google Scholar] [CrossRef]
- Szłyk, E.; Piszczek, P.; Chaberski, M.; Goliński, A. Studies of thermal decomposition process of Ag(I) perfluorinated carboxylates with temperature variable IR and MS. Polyhedron 2001, 20, 2853–2861. [Google Scholar] [CrossRef]
- Szłyk, E.; Piszczek, P.; Grodzicki, A.; Chaberski, M.; Goliński, A.; Szatkowski, J.; Błaszczyk, T. CVD of AgI Complexes with tertiary Phosphines and Perfluorinated Carboxylates—A New Class of Silver Precursors. Chem. Vap. Dep. 2001, 7, 1–6. [Google Scholar]
- Lutz, H.D. Bonding and structure of water molecules in solid hydrates. Correlation of spectroscopic and structural data. In Solid Materials; Structure and Bonding; Springer: Berlin/Heidelberg, Germany, 1988; Volume 69, pp. 97–125. [Google Scholar]
- Lewandowska, Ż.; Piszczek, P.; Radtke, A.; Jędrzejewski, T.; Kozak, W.; Sadowska, B. The evaluation of the impact of titania nanotube covers morphology and crystal phase on their biological properties. J. Mater. Sci. 2015, 26, 163. [Google Scholar] [CrossRef]
- Brammer, K.S.; Oh, S.; Cobb, C.J.; Bjursten, L.M.; van der Heyde, H.; Jin, S. Improved bone forming functionality on diameter-controlled TiO2 nanotube surface. Acta Biomater. 2009, 5, 215–3223. [Google Scholar] [CrossRef] [PubMed]
- Caihong, L.; Jiang, W.; Xiaoming, L. A visible-light-controlled platform for prolonged drug release based on Ag-doped TiO2 nanotubes with a hydrophobic layer. Beilstein J. Nanotechnol. 2018, 9, 1793–1801. [Google Scholar] [CrossRef]
- Piszczek, P.; Radtke, A. Silver Nanoparticles Fabricated Using Chemical Vapor Deposition and Atomic Layer Deposition Techniques: Properties. Applications and Perspectives: Review. In Noble and Precious Metals; Seehra, M.S., Bristow, A.D., Eds.; IntechOpen: London, UK, 2018; pp. 187–213. ISBN 978-1-78923-292-9. [Google Scholar]
- Antoci, V., Jr.; Adams, C.S.; Parvizi, J.; Davidson, H.M.; Composto, R.J.; Freeman, T.A.; Wickstrom, E.; Ducheyne, P.; Jungkind, D.; Shapiro, I.M.; et al. The inhibition of Staphylococcus epidermidis biofilm formation by vancomycin-modified titanium alloy and implications for the treatment of periprosthetic infection. Biomaterials 2008, 29, 4684–4690. [Google Scholar] [CrossRef] [PubMed]
- Zaho, C.; Feng, B.; Li, Y.; Tan, J.; Lu, X.; Weng, J. Preparation and antibacterial activity of titanium nanotubes loaded with Ag Nanoparticles in the dark and under the UV light. Appl. Surf. Sci. 2013, 280, 8–14. [Google Scholar] [CrossRef]
- Von Wilmowsky, C.; Bauer, S.; Lutz, R.; Meisel, M.; Neukam, F.W.; Toyoshima, T.; Schmuki, P.; Nkenke, E.; Schlegel, K.A. In vivo evaluation of anodic TiO2 nanotubes: An experimental study in the pig. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 89B, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Lee, K.; Roy, P.; Birajdar, B.I.; Spiecker, E.; Schmuki, P. Formation of a Non-Thickness-Limited Titanium Dioxide Mesosponge and its Use in Dye-Sensitized Solar Cells. Angew. Chem. 2009, 121, 9490–9493. [Google Scholar] [CrossRef]
- Lee, K.; Kim, D.; Roy, P.; Paramasivam, I.; Birajdar, B.I.; Spiecker, E.; Schmuki, P. Anodic Formation of Thick Anatase TiO2 Mesosponge Layers for High-Efficiency Photocatalysis. J. Am. Chem. Soc. 2010, 132, 1478–1479. [Google Scholar] [CrossRef] [PubMed]
- Regonini, D.; Satka, A.; Jaroenworaluck, A.; Allsopp, D.W.E.; Bowen, C.R.; Stevens, R. Factors influencing surface morphology of anodized TiO2 nanotubes. Electrochim. Acta 2012, 74, 244–253. [Google Scholar] [CrossRef]
- Macak, J.M.; Hildebrand, H.; Marten-Jahns, U.; Schmuki, P. Mechanistic aspects and growth of large diameter self-organized TiO2 nanotubes. J. Electroanal. Chem. 2008, 621, 254–266. [Google Scholar] [CrossRef]
- Bauer, S.; Kleber, S.; Schmuki, P. TiO2 nanotubes: Tailoring the geometry in H3PO4/HF electrolytes. Electrochem. Commun. 2006, 8, 1321–1325. [Google Scholar] [CrossRef]
- Kar, A.; Raja, K.S.; Misra, M. Electrodeposition of hydroxyapatite onto nanotubular TiO2 for implant applications. Surf. Coat. Technol. 2006, 201, 3723–3731. [Google Scholar] [CrossRef]
- Xiong, J.; Wang, Y.; Li, Y.; Hodgson, P. Phase transformation and thermal structure stability of titania nanotube films with different morphologies. Thin Solid Films 2012, 526, 116–119. [Google Scholar] [CrossRef]
- Yang, B.; Ng, C.K.; Fung, M.K.; Ling, C.C.; Djurišić, A.B.; Fung, S. Annealing study of titanium oxide nanotube arrays. Mater. Chem. Phys. 2011, 130, 1227–1231. [Google Scholar] [CrossRef]
- Kaczmarek, D.; Domaradzki, J.; Wojcieszak, D.; Prociow, E.; Mazur, M.; Placido, F.; Lapp, S. Hardness of Nanocrystalline TiO2 Thin Films. J. Nano Res. 2012, 18–19, 195–200. [Google Scholar] [CrossRef]
- Wojcieszak, D.; Mazur, M.; Indyka, J.; Jurkowska, A.; Kalisz, M.; Domanowski, P.; Kaczmarek, D.; Domaradzki, J. Mechanical and structural properties of titanium dioxide deposited by innovative magnetron sputtering process. Mater. Sci.-Poland 2015, 33, 660–668. [Google Scholar] [CrossRef]
- Oh, K.; Lee, K.; Choi, J. Influence of geometry and crystal structures of TiO2 nanotubes on micro Vickers hardness. Mater. Lett. 2017, 192, 137–141. [Google Scholar] [CrossRef]
- Munirathinam, B.; Neelakantan, L. Role of crystallinity on the nanomechanical and electrochemical properties of TiO2 nanotubes. J. Electroanal. Chem. 2016, 770, 73–83. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Brandenburg, K. DIAMOND, Release 2.1e.; Crystal Impact GbR: Bonn, Germany, 2001. [Google Scholar]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]








| Bond Length | |||||
| Ag1-O12 | 2.138(5) | Ag3-O42 | 2.205(4) | Ag5-O21 | 2.234(5) |
| Ag1-O12 i | 2.138(5) | Ag3-O41 | 2.218(4) | Ag5-O32 | 2.249(4) |
| Ag1-O8 | 2.825(6) | Ag3-O7 | 2.547(4) | Ag5-O52 | 2.379(5) |
| Ag1-Ag4 i | 3.0058(5) | Ag3-O51 | 2.588(4) | Ag5-O9 | 2.641(6) |
| Ag1-Ag4 | 3.0058(5) | Ag3-Ag3 iii | 2.8932(9) | Ag5-Ag2 ii | 2.8951(7) |
| Ag2-O31 ii | 2.219(5) | Ag4-O11 | 2.324(4) | Ag5-Ag6 | 3.239(2) |
| Ag2-O22 | 2.237(5) | Ag4-O7 | 2.426(4) | Ag6-O9 | 2.318(7) |
| Ag2-O51 | 2.553(4) | Ag4-O51 | 2.511(4) | Ag6-O9 iv | 2.412(8) |
| Ag2-O41 | 2.610(4) | Ag4-O32 | 2.540(5) | Ag6-O52 iv | 2.472(6) |
| Ag2-Ag5 ii | 2.8950(7) | Ag4-O8 | 2.577(6) | Ag6-O52 | 2.579(6) |
| Ag2-Ag5 | 3.3236(8) | Ag6-O21 | 2.593(5) | ||
| Angles | |||||
| O12-Ag1-O12 i | 180.0 | O42-Ag3-O41 | 162.69(16) | O21-Ag5-O32 | 155.4(2) |
| O12-Ag1-Ag4 i | 95.09(12) | O42-Ag3-O7 | 91.16(14) | O21-Ag5-O52 | 93.4(2) |
| O12 i -Ag1-Ag4 i | 84.91(12) | O41-Ag3-O7 | 104.90(15) | O32-Ag5-O52 | 108.9(2) |
| O12-Ag1-Ag4 | 84.91(12) | O42-Ag3-O51 | 106.76(16) | O21-Ag5-Ag2 ii | 82.41(13) |
| O12 i -Ag1-Ag4 | 95.09(12) | O41-Ag3-O51 | 84.01(16) | O32-Ag5-Ag2 ii | 81.11(12) |
| Ag4 i -Ag1-Ag4 | 180.0 | O7-Ag3-O51 | 75.23(13) | O52-Ag5-Ag2 ii | 156.0(2) |
| O31 i -Ag2-O22 | 159.63(18) | O42-Ag3-Ag3 iii | 82.99(11) | O21-Ag5-Ag6 | 52.75(13) |
| O31 i -Ag2-O51 | 91.49(16) | O41-Ag3-Ag3 iii | 79.70(11) | O32-Ag5-Ag6 | 136.00(13) |
| O22-Ag2-O51 | 108.03(16) | O7-Ag3-Ag3 iii | 158.57(10) | O52-Ag5-Ag6 | 51.94(14) |
| O31 i -Ag2-Ag5 ii | 81.38(12) | O51-Ag3-Ag3 iii | 126.20(10) | Ag2 ii-Ag5-Ag6 | 133.99(5) |
| O22-Ag2-Ag5 ii | 78.26(13) | O11-Ag4-O7 | 153.99(15) | O21-Ag5-Ag2 | 134.02(15) |
| O51-Ag2-Ag5 ii | 163.39(10) | O11-Ag4-O51 | 126.55(15) | O32-Ag5-Ag2 | 61.71(13) |
| O31 ii -Ag2-Ag5 | 61.33(14) | O7-Ag4-O51 | 78.78(14) | O52-Ag5-Ag2 | 82.47(13) |
| O22-Ag2-Ag5 | 120.51(13) | O11-Ag4-O32 | 88.59(16) | Ag2 ii-Ag5-Ag2 | 83.578(19) |
| O51-Ag2-Ag5 | 67.06(10) | O7-Ag4-O32 | 100.33(14) | Ag6-Ag5-Ag2 | 133.31(6) |
| Ag5 ii -Ag2-Ag5 | 96.42(2) | O51-Ag4-O32 | 85.85(15) | O9-Ag6-O9 iv | 154.41(13) |
| O11-Ag4-O8 | 93.10(17) | O9-Ag6-O52 iv | 98.8(3) | ||
| O7-Ag4-O8 | 78.17(17) | O9 iv-Ag6-O52 iv | 78.7(2) | ||
| O51-Ag4-O8 | 93.07(17) | O9-Ag6-O52 | 78.3(2) | ||
| O32-Ag4-O8 | 178.31(16) | O9 iv-Ag6-O52 | 93.6(2) | ||
| O11-Ag4-Ag1 | 74.81(10) | O52 iv-Ag6-O52 | 156.09(11) | ||
| O7-Ag4-Ag1 | 79.64(9) | O9-Ag6-O21 | 79.1(2) | ||
| O51-Ag4-Ag1 | 148.61(10) | O9 iv-Ag6-O21 | 124.0(2) | ||
| O32-Ag4-Ag1 | 120.40(11) | O52 iv-Ag6-O21 | 122.1(2) | ||
| O8-Ag4-Ag1 | 60.22(15) | O52-Ag6-O21 | 81.03(19) | ||
| O9-Ag6-Ag5 | 53.70(16) | ||||
| O9 iv-Ag6-Ag5 | 134.01(17) | ||||
| O52 iv-Ag6-Ag5 | 147.11(18) | ||||
| O52-Ag6-Ag5 | 46.58(12) | ||||
| O21-Ag6-Ag5 | 43.30(11) |
| Fragmentation Ions | m/z | 403 K | 503 K | 523 K |
|---|---|---|---|---|
| [Ag(CO)]+ | 136 | 8 | - | - |
| [Ag(O2C)]+ | 147 | 21 | 11 | 4 |
| [Ag(O2CF)]+ | 171 | 23 | >2 | - |
| [Ag(C2F5)]+ | 209 | 10 | 31 | 12 |
| [Ag(O2CC2F5)(H2O)]+ | 289 | 100 | - | - |
| [Ag2(C2F5)]+ | 335 | 58 | 100 | 38 |
| [Ag2(O2CC2F5)]+ | 379 | - | 68 | 6 |
| [Ag(O2CC2F5)2(H2O)]+ | 452 | 10 | - | - |
| [Ag2(O2CC2F5)(C2F5)]+ | 498 | 30 | 5 | >2 |
| [Ag2(O2CC2F5)2(CO)]+ | 586 | >2 | >1 | - |
| [Ag2(O2CC2F5)2(CO)2]+ | 598 | >1 | >1 | - |
| [Ag3(O2CC2F5)(C2F5)(CO)]+ | 635 | >2 | - | - |
| [Ag3(O2CC2F5)2(CO)]+ | 679 | >1 | - | - |
| [Ag2(O2CC2F5)3(OC)(H2O)]+ | 752 | >1 | - | - |
| [Ag2(O2CC2F5)3(OOC)(H2O)2]+ | 784 | >1 | - | - |
| [Ag5(O2CC2F5)5(H2O)3] | Ag(O2CC2F5) [29] | |
|---|---|---|
| Total reactor pressure (p) [hPa] | 5 × 10−1 | 4 |
| Substrate temperature (TD) [K] | 553 | 563 |
| Vaporization temperature (TV) [K] | 508 | 513 |
| Deposition rate (rD) [mg·min−1] | 2.25–2.57 | 2.56 |
| Carrier gas | Ar | Ar |
| Deposition time [min] | 30 | 30 |
| Precursor mass [mg] | 100 | 100 |
| Biomaterial Sample | Average Contact Angle [°] ± Standard Deviation | SFE [mJ/m2] | |
|---|---|---|---|
| Measuring Liquid | |||
| Water | Diiodomethane | ||
| Ti6Al4V | 108.3 ± 0.09 | 37 ± 0.16 | 45.37 ± 0.05 |
| Ti6Al4V/AgNPs | 131.9 ± 0.12 | 89.6 ± 0.50 | 15.09 ± 0.09 |
| Ti6Al4V/TNT5 | ˂10 | 36 ± 6.82 | ˃72.06 |
| Ti6Al4V/TNT15 | ˂10 | 32.3 ± 2.75 | ˃72.30 |
| Ti6Al4V/TNT20 | ˂10 | 30.7 ± 2.18 | >72.42 |
| Ti6Al4V/TNT5/AgNPs | 124.2 ± 0.06 | 41.9 ± 0.47 | 51.97 ± 0.15 |
| Ti6Al4V/TNT15/AgNPs | 120.5 ± 0.1 | 67.3 ± 0.96 | 28.46 ± 0.23 |
| Ti6Al4V/TNT20/AgNPs | 110.2 ± 0.5 | 72.3 ± 0.73 | 21.7 ± 0.05 |
| Biomaterial Sample | Hardness [GPa] | Young’s Modulus [GPa] | Maximum Depth of Indentation [nm] |
|---|---|---|---|
| Ti6Al4V | 6.18 ± 2.88 | 230.12 ± 21.68 | 162.18 ± 12.18 |
| Ti6Al4V/AgNPs | 6.81 ± 2.55 | 187.54 ± 54.33 | 253.09 ± 51.55 |
| Ti6Al4V/TNT5 | 7.42 ± 3.30 | 229.71 ± 88.07 | 302.40 ± 61.85 |
| Ti6Al4V/TNT15 | 16.23 ± 8.81 | 350.64 ± 157.57 | 168.11 ± 46.04 |
| Ti6Al4V/TNT5/AgNPs | 9.86 ± 4.61 | 253.93 ± 87.14 | 211.53 ± 56.38 |
| Ti6Al4V/TNT15/AgNPs | 13.60 ± 7.24 | 287.03 ± 92.92 | 184.46 ± 40.60 |
| Nano Scratch—Test Properties | ||
|---|---|---|
| Biomaterial Sample | Critical Friction [mN] | Critical Load [mN] |
| Ti6Al4V/TNT5 | 155.76 ± 69.02 | 197.713 ± 78.62 |
| Ti6Al4V/TNT15 | 234.68 ± 21.05 | 275.03 ± 28.91 |
| Ti6Al4V/TNT5/AgNPs | 213.57 ± 49.50 | 275.11 ± 58.15 |
| Ti6Al4V/TNT15/AgNPs | 238.27 ± 53.54 | 267.74 ± 75.73 |
| Formula sum | C15 H6 Ag5 F25 O13 |
| Formula weight | 1408.55 |
| Crystal system | triclinic |
| Space group | P-1 |
| Unit cell dimensions | a = 11.3277(5) Å b = 13.0765(5) Å c = 13.7547(5) Å α = 116.746(4)° β = 100.869(3)° γ = 99.819(3)° |
| Cell volume [Å3] | 1709.36(13) |
| Density (calculated) [Mg/m3] | 2.737 |
| Z | 2 |
| Absorption coefficient [mm−1] | 3.005 |
| F(000) | 1320 |
| Crystal size [mm] | 0.57 × 0.51 × 0.38 |
| Theta range for data collection [dego] | 2.16 to 26.37 |
| Index ranges | −14 ≤ h ≤ 14 −16 ≤ k ≤ 16 −17 ≤ l ≤ 17 |
| Reflections collected | 18624 |
| Reflections unique/Rint | 6960/0.0424 |
| Completeness to theta = 26.37 | 99.5% |
| Transmission Max/Min | 0.3947/0.2792 |
| Refinement method | Full-matrix least-squares on F^2 |
| Data/restraints/parameters | 6960/20/592 |
| Goodness-of-fit on F^2 | 1.043 |
| Final R indices [I > 2sigma(I)] | R1 = 0.0474 wR2 = 0.1339 |
| R indices (all data) | R1 = 0.0627 wR2 = 0.1439 |
| Largest diff. peak and hole [e.Å−3] | 0.937 and −0.846 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radtke, A.; Grodzicka, M.; Ehlert, M.; Muzioł, T.M.; Szkodo, M.; Bartmański, M.; Piszczek, P. Studies on Silver Ions Releasing Processes and Mechanical Properties of Surface-Modified Titanium Alloy Implants. Int. J. Mol. Sci. 2018, 19, 3962. https://doi.org/10.3390/ijms19123962
Radtke A, Grodzicka M, Ehlert M, Muzioł TM, Szkodo M, Bartmański M, Piszczek P. Studies on Silver Ions Releasing Processes and Mechanical Properties of Surface-Modified Titanium Alloy Implants. International Journal of Molecular Sciences. 2018; 19(12):3962. https://doi.org/10.3390/ijms19123962
Chicago/Turabian StyleRadtke, Aleksandra, Marlena Grodzicka, Michalina Ehlert, Tadeusz M. Muzioł, Marek Szkodo, Michał Bartmański, and Piotr Piszczek. 2018. "Studies on Silver Ions Releasing Processes and Mechanical Properties of Surface-Modified Titanium Alloy Implants" International Journal of Molecular Sciences 19, no. 12: 3962. https://doi.org/10.3390/ijms19123962
APA StyleRadtke, A., Grodzicka, M., Ehlert, M., Muzioł, T. M., Szkodo, M., Bartmański, M., & Piszczek, P. (2018). Studies on Silver Ions Releasing Processes and Mechanical Properties of Surface-Modified Titanium Alloy Implants. International Journal of Molecular Sciences, 19(12), 3962. https://doi.org/10.3390/ijms19123962