Erinacine A-Enriched Hericium erinaceus Mycelium Produces Antidepressant-Like Effects through Modulating BDNF/PI3K/Akt/GSK-3β Signaling in Mice
Abstract
:1. Introduction
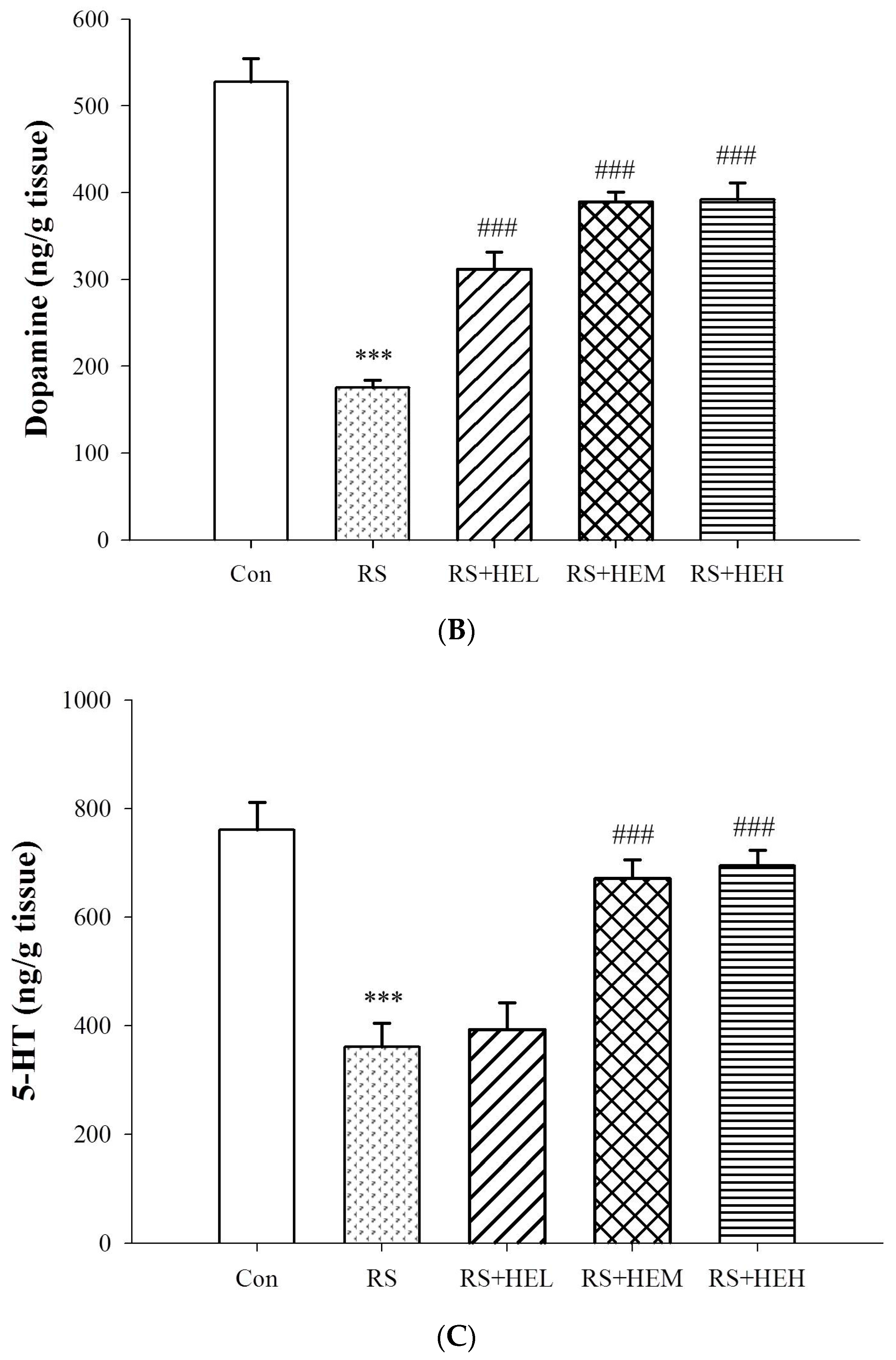
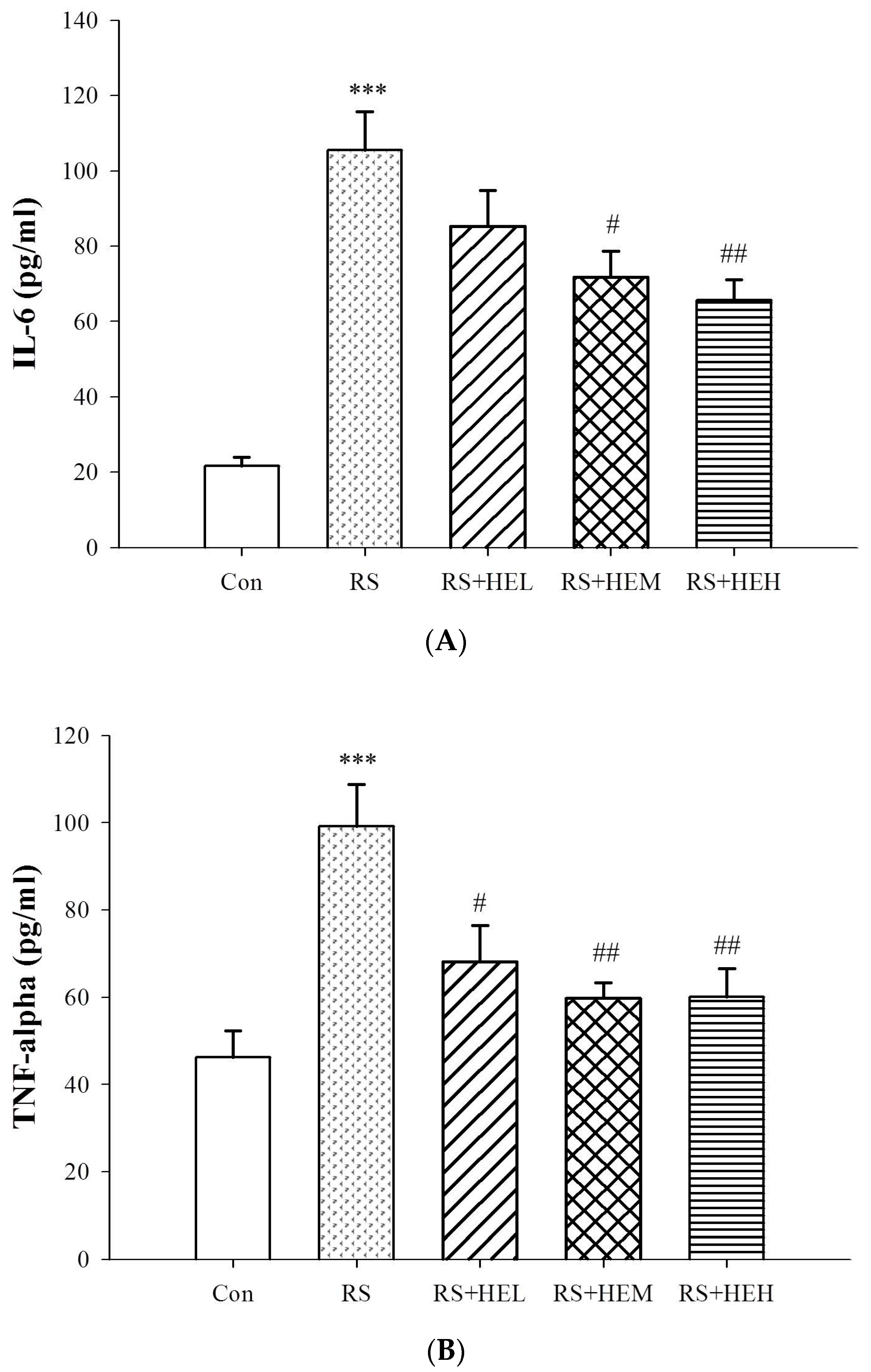
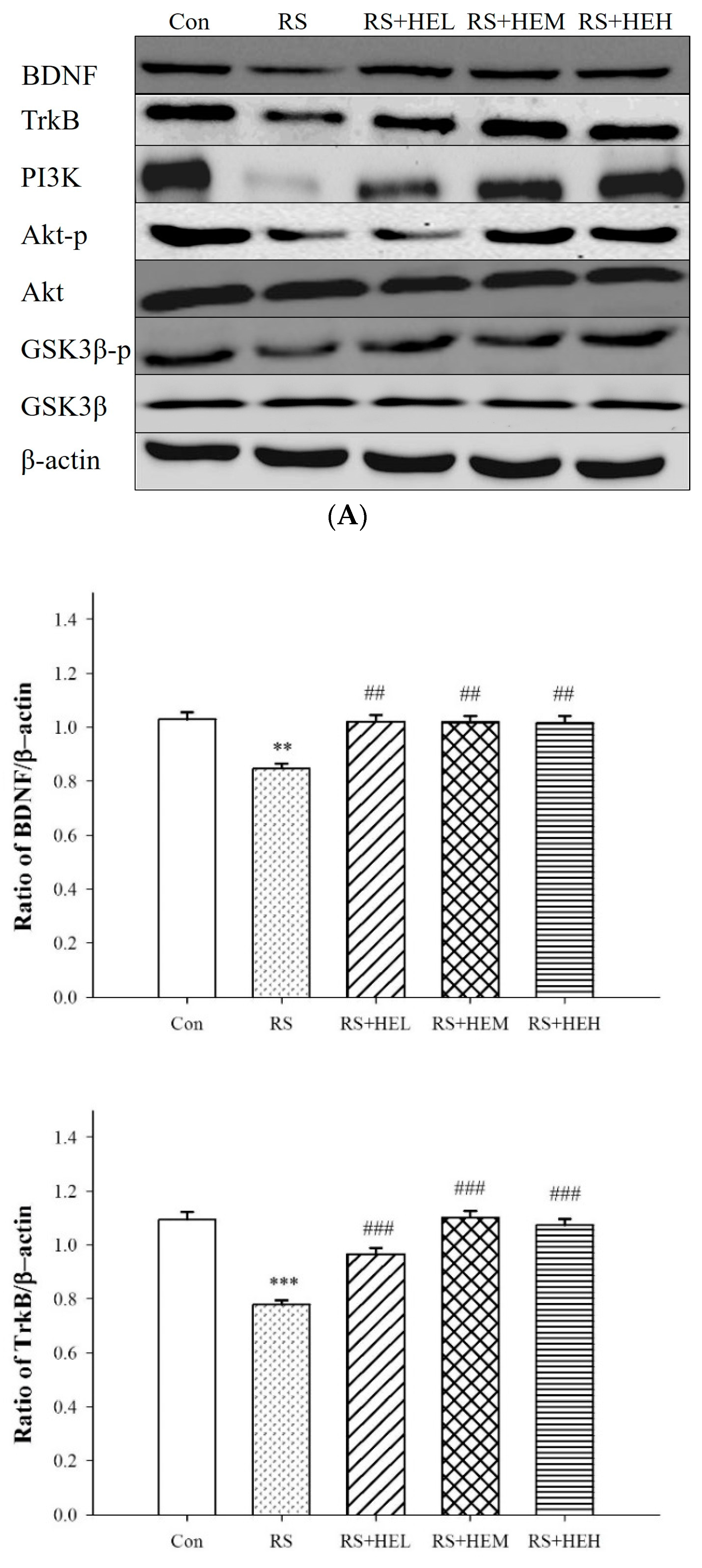
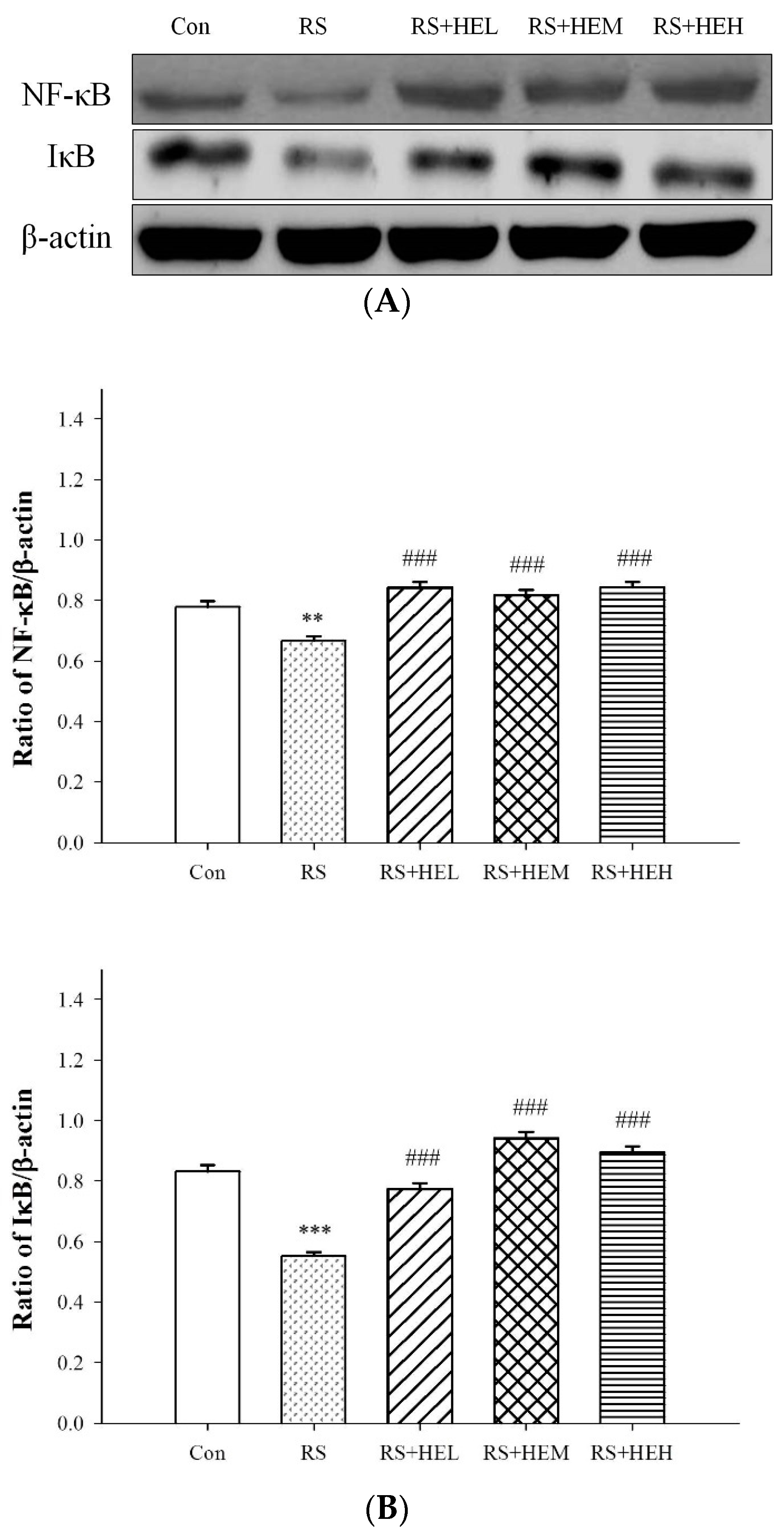
2. Results
3. Discussion
4. Materials and Methods
4.1. Cultivation of H. erinaceus
4.2. HPLC/ESI–MS Analysis of Hericium erinaceus Mycelial Ethanolic Extract
4.3. Animals and Treatments
4.4. Behavioral Tests
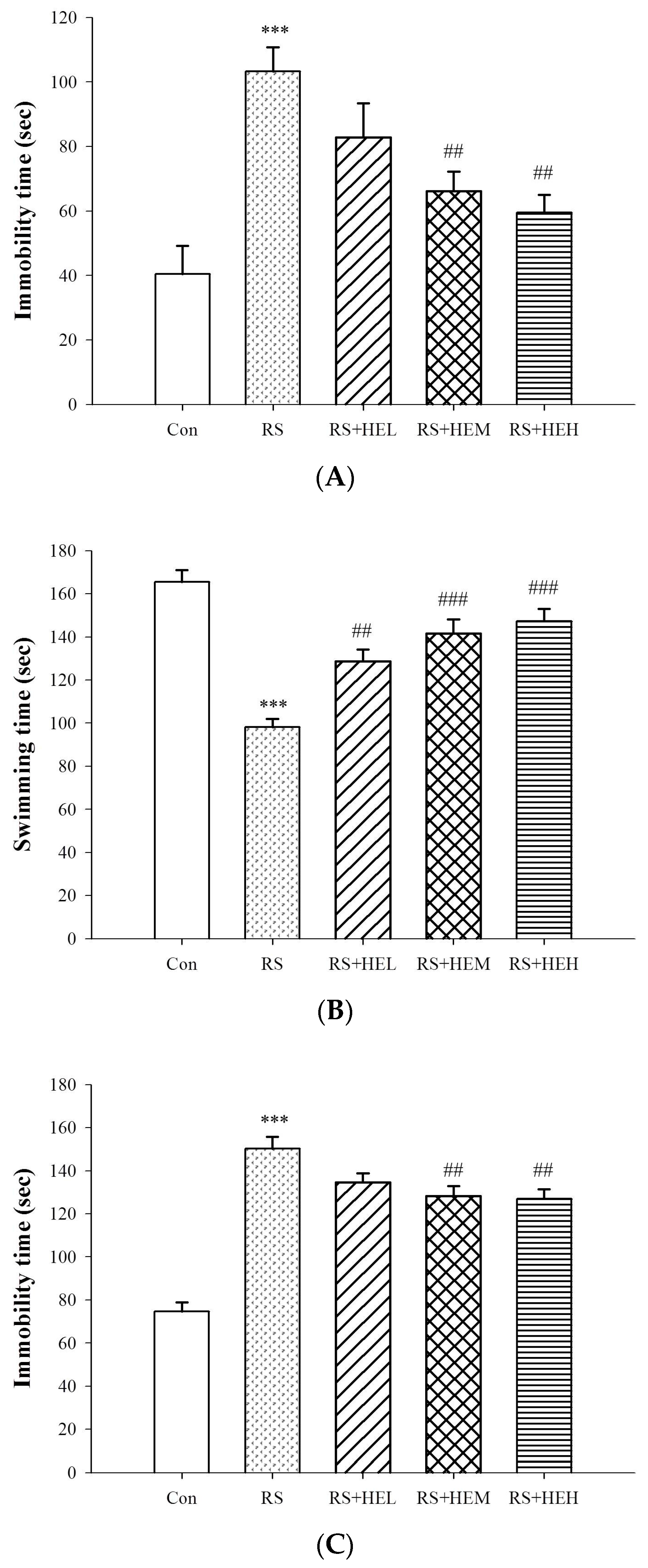
4.4.1. Tail Suspension Test (TST)
4.4.2. Forced Swimming Test (FST)
4.4.3. Elevated Plus Maze Test (EPM)
4.4.4. Open Field Test (OFT)
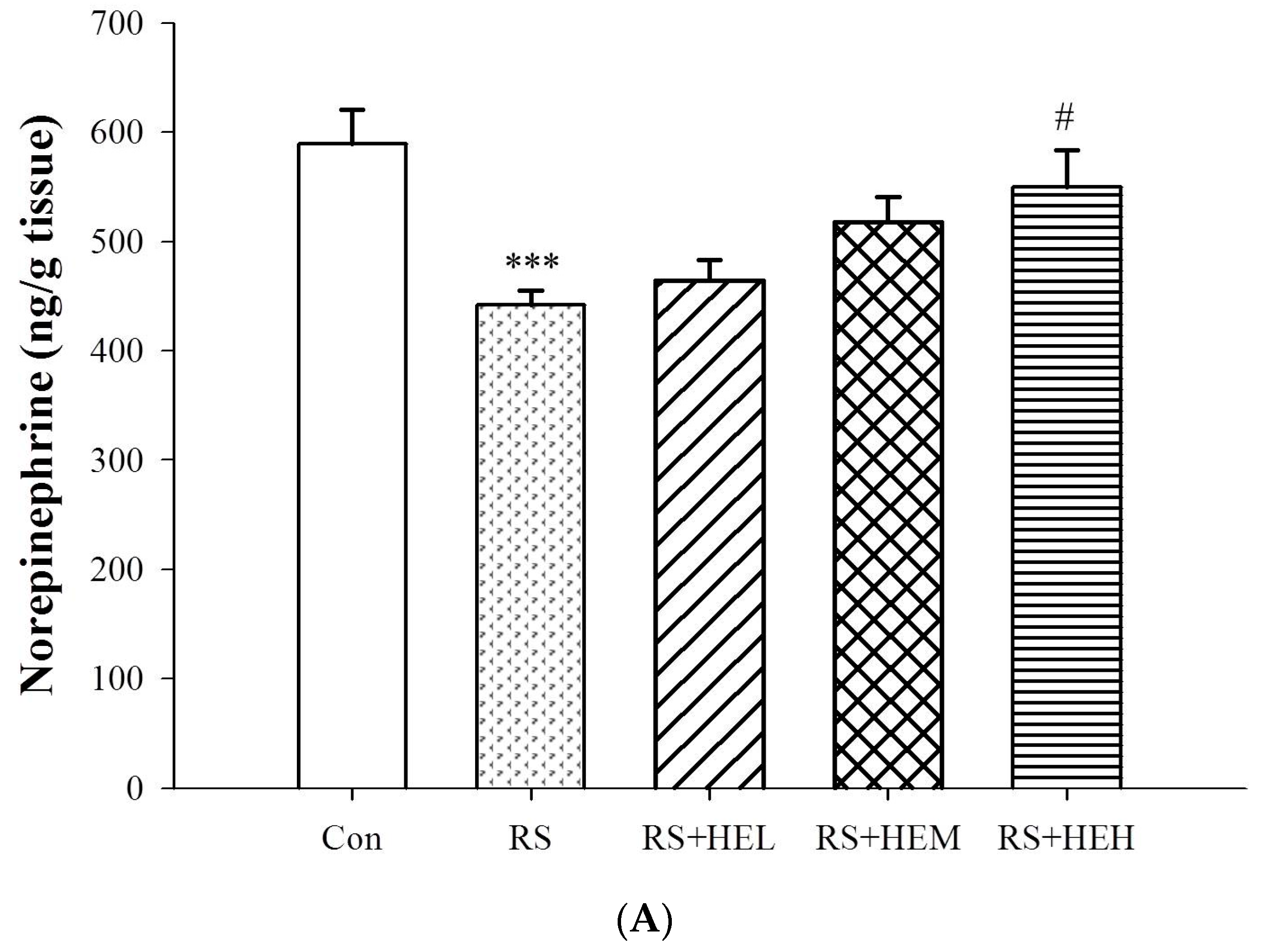
4.5. Determination of Cytokine and Monoamine Neurotransmitter Levels
4.6. Western Blot Analysis in Hippocampal Tissue
4.7. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- De Bodinat, C.; Guardiola-Lemaitre, B.; Mocaër, E.; Renard, P.; Muñoz, C.; Millan, M.J. Agomelatine, the first melatonergic antidepressant: Discovery, characterization and development. Nat. Rev. Drug Discov. 2010, 9, 628–642. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.C.; Ormel, J.; Petukhova, M.; McLaughlin, K.A.; Green, J.G.; Russo, L.J.; Stein, D.J.; Zaslavsky, A.M.; Aguilar-Gaxiola, S.; Alonso, J.; et al. Development of lifetime comorbidity in the World Health Organization world mental health surveys. Arch. Gen. Psychiatry 2011, 68, 90–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pittenger, C.; Duman, R.S. Stress, depression, and neuroplasticity: A convergence of mechanisms. Neuropsychopharmacology 2008, 33, 88–109. [Google Scholar] [CrossRef] [PubMed]
- Ulrich-Lai, Y.M.; Herman, J.P. Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 2009, 10, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Jindal, A.; Mahesh, R.; Bhatt, S. Etazolate, a phosphodiesterase 4 inhibitor reverses chronic unpredictable mild stress-induced depression-like behavior and brain oxidative damage. Pharmacol. Biochem. Behav. 2013, 105C, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Aboul-Fotouh, S. Coenzyme Q10 displays antidepressant-like activity with reduction of hippocampal oxidative/nitrosative DNA damage in chronically stressed rats. Pharmacol. Biochem. Behav. 2013, 104, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Mao, Q.Q.; Zhong, X.M.; Li, Z.Y.; Qiu, F.M.; Ip, S.P. Mechanistic Study on the Antidepressant-Like Effect of Danggui-Shaoyao-San, a Chinese Herbal Formula. Evid. Based Complement. Alternat. Med. 2012, 2012, 173565. [Google Scholar] [CrossRef] [PubMed]
- Van Praag, H.M. Can stress cause depression? World J. Biol. Psychiatry 2005, 6, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.P.; Schmidt, D.; Stein, C.M.; Morrow, J.D.; Salomon, R.M. Increased oxidative stress in patients with depression and its relationship to treatment. Psychiatry Res. 2013, 206, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, D.; Sur, T.K. Effect of Panax ginseng and diazepam on brain 5- hydroxytryptamine and its modification by diclofenac in rats. Indian J. Physiol. Pharmacol. 1999, 43, 505–509. [Google Scholar]
- Keeney, A.; Jessop, D.S.; Harbuz, M.S.; Marsden, C.A.; Hogg, S.; Munro, B. Differential effects of acute and chronic social defeat stress on hypothalamic–pituitary-adrenal axis function and hippocampal serotonin release in mice. J. Neuroendocrinol. 2006, 18, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Konstandi, M.; Johnson, E.; Lang, M.A.; Malamas, M.; Marselos, M. Noradrenaline, dopamine, serotonin: Different effects of psychological stress on brain biogenic amines in mice and rats. Pharmacol. Res. 2000, 41, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Khanzode, S.D.; Dakhale, G.N.; Khanzode, S.S.; Saoji, A.; Palasodkar, R. Oxidative damage and major depression: The potential antioxidant action of selective serotonin-re-uptake inhibitors. Redox. Rep. 2003, 8, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, R.S.; Gowtham, L. Microglia and regulation of inflammation-mediated neurodegeneration: Prevention and treatment by phytochemicals and metabolic nutrients. Int. J. Green Pharm. 2012, 6, 81–92. [Google Scholar] [CrossRef]
- Duman, R.S.; Monteggia, L.M. A neurotrophic model for stress-related mood disorders. Biol. Psychiatry 2006, 59, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Larsen, M.H.; Mikkelsen, J.D.; Hay-Schmidt, A.; Sandi, C. Regulation of brain derived neurotrophic factor (BDNF) in the chronic unpredictable stress rat model and the effects of chronic antidepressant treatment. J. Psychiatr. Res. 2010, 44, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, Z.; Dai, J.; Chen, L.; Huang, Y.; Zhan, Z. Beneficial effects of benzodiazepine diazepam on chronic stress-induced impairment of hippocampal structural plasticity and depression-like behavior in mice. Behav. Brain Res. 2012, 228, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Mihaljević-Peleš, A.; Sagud, M.; Janović, M.B.; Mikulić, S.K.; Jevtović, S. Do we need new therapeutic strategies for depression? Psychiatr. Danub. 2011, 23, 300–301. [Google Scholar] [PubMed]
- Richelson, E. Multi-modality: A new approach for the treatment of major depressive disorder. Int. J. Neuropsychopharmacol. 2013, 16, 1433–1442. [Google Scholar] [CrossRef] [PubMed]
- Thongbai, B.; Rapior, S.; Hyde, K.D.; Wittstein, K.; Stadler, M. Hericium erinaceus, an amazing medicinal mushroom. Mycol. Progress. 2015, 14, 91. [Google Scholar] [CrossRef]
- Li, G.; Yua, K.; Li, F.; Xua, K.; Li, J.; He, S.; Caoc, S.; Tana, G. Anticancer potential of Hericium erinaceus extracts against human gastrointestinal cancers. J. Ethnopharmacol. 2014, 153, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Kawagishi, H. Anti-MRSA compounds from Hericium erinaceus (Bull.:Fr.). Pers. Int. J. Med. Mushrooms 2005, 7, 350. [Google Scholar] [CrossRef]
- Wang, J.C.; Hu, S.H.; Wang, J.T.; Chen, K.S.; Chia, Y.C. Hypoglycemic effect of extract of Hericium erinaceus. J. Sci. Food Agric. 2005, 85, 641–646. [Google Scholar] [CrossRef]
- Hiwatashi, K.; Kosaka, Y.; Suzuki, N.; Hata, K.; Mukaiyama, T.; Sakamoto, K.; Shirakawa, H.; Komai, M. Yamabushitake mushroom (Hericium erinaceus) improved lipid metabolism in mice fed a high-fat diet. Biosci. Biotechnol. Biochem. 2010, 74, 1447–1451. [Google Scholar] [CrossRef] [PubMed]
- Shimbo, M.; Kawagishi, H.; Yokogoshi, H. Erinacine A increases catecholamine and nerve growth factor content in the central nervous system of rats. Nutr. Res. 2005, 25, 617–623. [Google Scholar] [CrossRef]
- Mori, K.; Obara, Y.; Hirota, M.; Azumi, Y.; Kinugasa, S.; Inatomi, S.; Nakahata, N. Nerve growth factor-inducing activity of Hericium erinaceus in 1321N1 human astrocytoma cells. Biol. Pharm. Bull. 2008, 31, 1727–1732. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.J.; Shen, J.W.; Yu, H.Y.; Ruan, Y.; Wu, T.T.; Zhao, X. Hericenones and erinacines: Stimulators of nerve growth factor (NGF) biosynthesis in Hericium erinaceus. Mycology 2010, 1, 92–98. [Google Scholar] [CrossRef]
- Skaper, S.D. The biology of neurotrophins, signalling pathways, and functional peptide mimetics of neurotrophins and their receptors. CNS Neurol. Disord. Drug Targets 2008, 7, 46–62. [Google Scholar] [CrossRef] [PubMed]
- Madduri, S.; Papaloizos, M.; Gander, B. Synergistic effect of GDNF and NGF onaxonal branching and elongation in vitro. Neurosci. Res. 2009, 65, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.C.; Lu, C.C.; Shen, C.H.; Tung, S.Y.; Hsieh, M.C.; Lee, K.C.; Lee, L.Y.; Chen, C.C.; Teng, C.C.; Huang, W.S.; et al. Hericium erinaceus mycelium and its isolated erinacine A protection from MPTP-induced neurotoxicity through the ER stress, triggering an apoptosis cascade. J. Transl. Med. 2016, 4, 78. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.F.; Chen, J.H.; Teng, C.C.; Shen, C.H.; Hsieh, M.C.; Lu, C.C.; Lee, K.C.; Lee, L.Y.; Chen, W.P.; Chen, C.C.; et al. Protective effects of Hericium erinaceus mycelium and its isolated erinacine A against ischemia-injury-induced neuronal cell death via the inhibition of iNOS/p38 MAPK and nitrotyrosine. Int. J. Mol. Sci. 2014, 15, 15073–15089. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, T.T.; Chen, C.C.; Lee, L.Y.; Chen, W.P.; Lu, C.K.; Shen, C.C.; Huang, F.C.Y.; Chen, C.C.; Shiao, Y.J. Erinacine A-enriched Hericium erinaceus mycelium ameliorates Alzheimer’s disease-related pathologies in APPswe/PS1dE9 transgenic mice. J. Biomed. Sci. 2016, 23, 49. [Google Scholar] [CrossRef]
- Freitas, A.E.; Bettio, L.E.; Neis, V.B.; Santos, D.B.; Ribeiro, C.M.; Rosa, P.B.; Farina, M.; Rodrigues, A.L. Agmatine abolishes restraint stress-induced depressive-like behavior and hippocampal antioxidant imbalance in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 50, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Kenmoku, H.; Shimai, T.; Toyomasu, T.; Kato, N.; Sassa, T. Erinacine Q, a new erinacine from Hericium erinaceum, and its biosynthetic route to erinacine C in the basidiomycete. Biosci. Biotechnol. Biochem. 2002, 66, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Tzeng, T.T.; Chen, C.C.; Ni, C.L.; Lee, L.Y.; Chen, W.P.; Shiao, Y.J.; Shen, C.C. Erinacine S, a rare sesterterpene from the mycelia of Hericium erinaceus. J. Nat. Prod. 2016, 79, 438–441. [Google Scholar] [CrossRef] [PubMed]
- Socała, K.; Nieoczym, D.; Pieróg, M.; Szuster-Ciesielska, A.; Wyska, E.; Wlaź, P. Antidepressant-like activity of sildenafil following acute and subchronic treatment in the forced swim test in mice: Effects of restraint stress and monoamine depletion. Metab. Brain Dis. 2016, 31, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Shim, I.; Lee, H.; Hahm, D.H. Fucoidan prevents depression-like behavior in rats exposed to repeated restraint stress. J. Nat. Med. 2013, 67, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Sunanda; Rao, B.S.; Raju, T.R. Restraint stress-induced alterations in the levels of biogenic amines, amino acids, and AChE activity in the hippocampus. Neurochem. Res. 2000, 25, 1547–1552. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, D.; Sharma, A. Antidepressant-like activity of Glycyrrhiza glabra L. in mouse models of immobility tests. Prog. Neuropsychopharmacol. Biol. Psychiatry 2006, 30, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.L.; da Silva, G.L.; Mateussi, A.S.; Fernandes, E.S.; Miguel, O.G.; Yunes, R.A.; Calixto, J.B.; Santos, A.R. Involvement of monoaminergic system in the antidepressant-like effect of the hydroalcoholic extract of Siphocampylus verticillatus. Life Sci. 2002, 70, 1347–1358. [Google Scholar] [CrossRef]
- Song, C.; Wang, H. Cytokines mediated inflammation and decreased neurogenesis in animal models of depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.K.; Kavelaars, A.; Heijnen, C.J.; Dantzer, R. Neuroinflammation and comorbidity of pain and depression. Pharmacol. Rev. 2014, 66, 80–101. [Google Scholar] [CrossRef] [PubMed]
- Edwards, K.M.; Burns, V.E.; Ring, C.; Carroll, D. Sex differences in the interleukin-6 response to acute psychological stress. Biol. Psychol. 2006, 71, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Alexander, G.M.; van Rijn, M.A.; van Hilten, J.J.; Perreault, M.J.; Schwartzman, R.J. Changes in cerebrospinal fluid levels of pro-inflammatory cytokines in CRPS. Pain 2005, 116, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, L.L.; Gawuga, C.E.; Tyrka, A.R.; Lee, J.K.; Anderson, G.M.; Price, L.H. Association between plasma IL-6 response to acute stress and early-life adversity in healthy adults. Neuropsychopharmacology 2010, 35, 2617–2623. [Google Scholar] [CrossRef] [PubMed]
- Savignac, H.M.; Hyland, N.P.; Dinan, T.G.; Cryan, J.F. The effects of repeated social interaction stress on behavioural and physiological parameters in a stress-sensitive mouse strain. Behav. Brain Res. 2011, 216, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Noh, H.J.; Yoon, J.Y.; Kim, G.S.; Lee, S.E.; Lee, D.Y.; Choi, J.H.; Kim, S.Y.; Kang, K.S.; Cho, J.Y.; Kim, K.H. Benzyl alcohol derivatives from the mushroom Hericium erinaceum attenuate LPS-stimulated inflammatory response through the regulation of NF-κB and AP-1 activity. Immunopharmacol. Immunotoxicol. 2014, 36, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Martinowich, K.; Manji, H.; Lu, B. New insights into BDNF function in depression and anxiety. Nat. Neurosci. 2007, 10, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Lu, B. BDNF and activity-dependent synaptic modulation. Learn. Mem. 2003, 10, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Itami, C.; Kimura, F.; Kohno, T.; Matsuoka, M.; Ichikawa, M.; Tsumoto, T.; Nakamura, S. Brain-derived neurotrophic factor-dependent unmasking of ‘‘silent’’ synapses in the developing mouse barrel cortex. Proc. Natl. Acad. Sci. USA 2003, 100, 13069–13074. [Google Scholar] [CrossRef] [PubMed]
- Fukuhara, K.; Ishikawa, K.; Yasuda, S.; Kishishita, Y.; Kim, H.K.; Kakeda, T.; Yamamoto, M.; Norii, T.; Ishikawa, T. Intracerebroventricular 4-methylcatechol (4-MC) ameliorates chronic pain associated with depression-like behavior via induction of brain-derived neurotrophic factor (BDNF). Cell Mol. Neurobiol. 2012, 32, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Wittstein, K.; Rascher, M.; Rupcic, Z.; Löwen, E.; Winter, B.; Köster, R.W.; Stadler, M. Corallocins A-C, nerve growth and brain-derived neurotrophic factor inducing metabolites from the mushroom Hericium coralloides. J. Nat. Prod. 2016, 79, 2264–2269. [Google Scholar] [CrossRef] [PubMed]
- Mahar, I.; Bambico, F.R.; Mechawar, N.; Nobrega, J.N. Stress, serotonin, and hippocampal neurogenesis in relation to depression and antidepressant effects. Neurosci. Biobehav. Rev. 2014, 38, 173–192. [Google Scholar] [CrossRef] [PubMed]
- Rosa, A.O.; Kaster, M.P.; Binfare, R.W.; Morales, S.; Martin-Aparicio, E.; Navarro-Rico, M.L.; Martinez, A.; Medina, M.; Garcia, A.G.; Lopez, M.G.; et al. Antidepressant-like effect of the novel thiadiazolidinone NP031115 in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Weichhart, T.; Säemann, M.D. The PI3K/Akt/mTOR pathway in innate immune cells: Emerging therapeutic applications. Ann. Rheum. Dis. 2008, 67, iii70–iii74. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, J.M.; Gainetdinov, R.R.; Caron, M.G. Akt/GSK3 signaling in the action of psychotropic drugs. Annu. Rev. Pharmacol. Toxicol. 2009, 49, 327–347. [Google Scholar] [CrossRef] [PubMed]
- Li, I.C.; Chen, Y.L.; Lee, L.Y.; Chen, W.P.; Tsai, Y.T.; Chen, C.C.; Chen, C.S. Evaluation of the toxicological safety of erinacine A-enriched Hericium erinaceus in a 28-day oral feeding study in Sprague–Dawley rats. Food Chem. Toxicol. 2014, 70, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.T.; Chyau, C.C.; Hsu, C.C.; Kuo, S.M.; Chuang, C.W.; Lin, H.H.; Chen, J.H. Pepino polyphenolic extract improved oxidative, inflammatory and glycative stress in the sciatic nerves of diabetic mice. Food Funct. 2016, 7, 1111–1121. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Kwon, H.J.; Baek, I.S.; Han, P.L. Repeated short-term (2 h × 14 d) emotional stress induces lasting depression-like behavior in mice. Exp. Neurobiol. 2012, 21, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Steru, L.; Chermat, R.; Thierry, B.; Simon, P. The tail suspension test: A new method for screening antidepressants in mice. Psychopharmacology 1985, 85, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Porsolt, R.D.; Bertin, A.; Jalfre, M. Behavioral despair in mice: A primary screening test for antidepressants. Arch. Int. Pharmacodyn. Ther. 1977, 229, 327–336. [Google Scholar] [PubMed]
- Braida, D.; Capurro, V.; Zani, A.; Rubino, T.; Viganò, D.; Parolaro, D.; Sala, M. Potential anxiolytic-and antidepressant-like effects of salvinorin a, the main active ingredient of Salvia divinorum, in rodents. Br. J. Pharmacol. 2009, 157, 844–853. [Google Scholar] [CrossRef] [PubMed]
- Mahesh, R.; Bhatt, S.; Pandey, D.K.; Gautam, B. Antidepressant like Effect of pimozide in acute and chronic animal models of depression. Indian J. Pharm. Edu. Res. 2011, 45, 46–53. [Google Scholar]
- Chiu, C.H.; Chyau, C.C.; Chen, C.C.; Lin, C.H.; Cheng, C.H.; Mong, M.C. Polysaccharide extract of Cordyceps sobolifera attenuates renal injury in endotoxemic rats. Food Chem. Toxicol. 2014, 69, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Huang, R.; Lin, X.; Huang, J.; Huang, Z.; Liu, H. Effects of Yulangsan polysaccharide on monoamine neurotransmitters, adenylate cyclase activity and brain-derived neurotrophic factor expression in a mouse model of depression induced by unpredictable chronic mild stress. Neural. Regen. Res. 2012, 7, 191–196. [Google Scholar] [PubMed]
- Ai, Z.; Cheng, A.F.; Yu, Y.T.; Yu, L.J.; Jin, W. Antidepressant-like behavioral, anatomical, and biochemical effects of petroleum ether extract from maca (Lepidium meyenii) in mice exposed to chronic unpredictable mild stress. J. Med. Food 2014, 17, 535–542. [Google Scholar] [CrossRef] [PubMed]
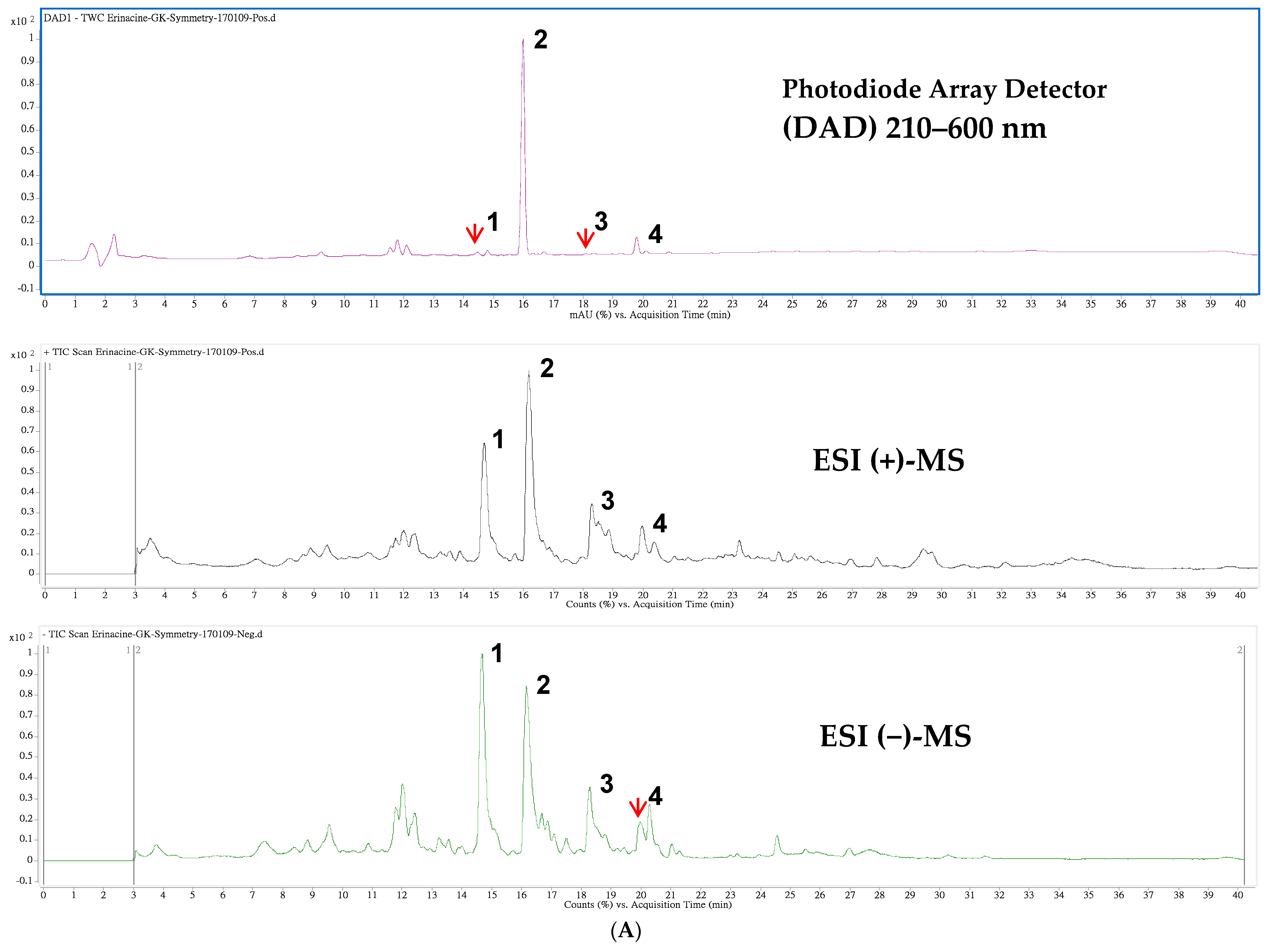
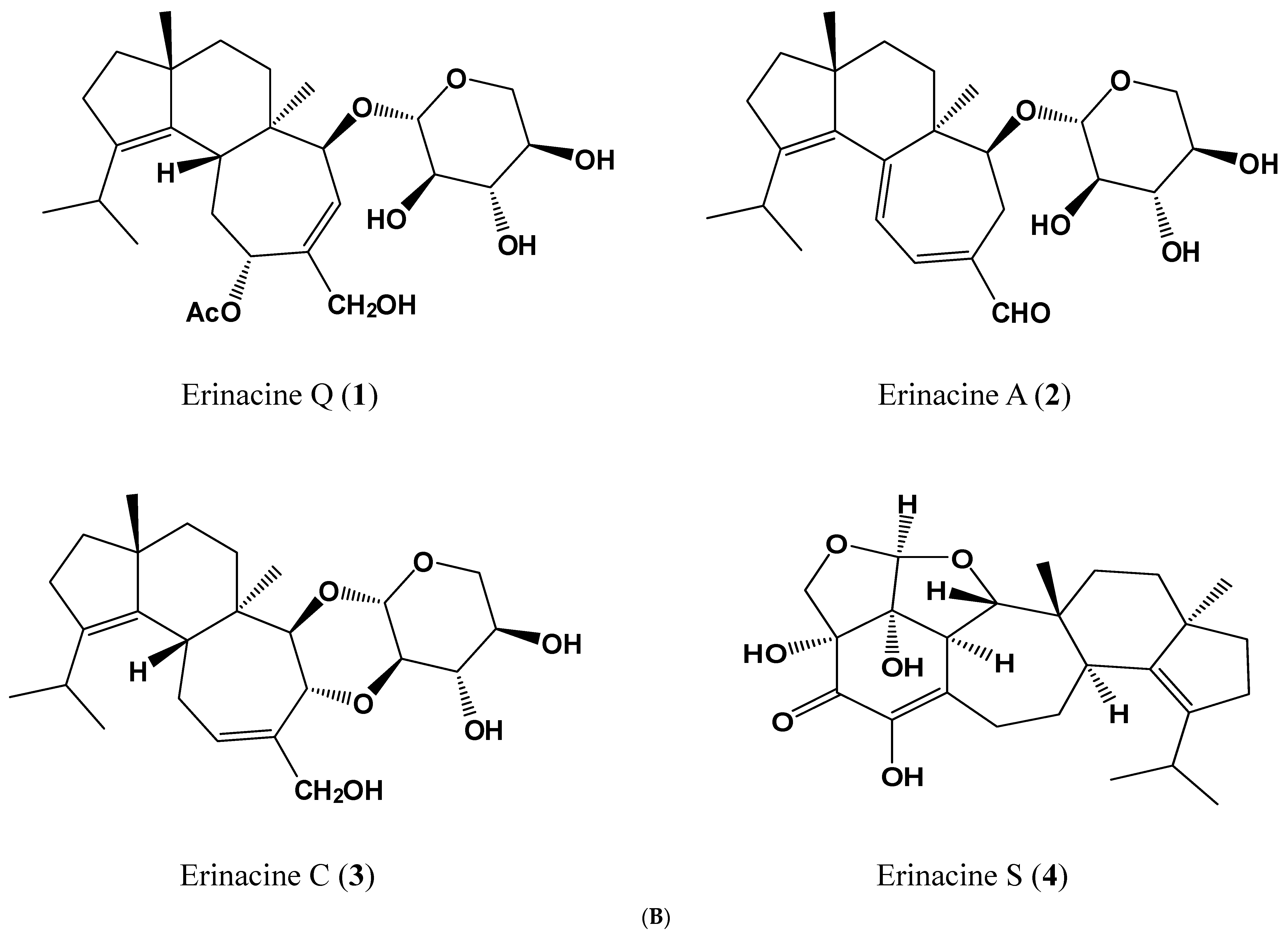

| Peak 1 | tR (min) | λmax (nm) | [M + H]+ | [M + Na]+ | m/z | [M − H]− | [M + HCOO]− | m/z | MW | Content 2 | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 14.47 | 210 | 517 | 285,267 | 539 | 529,410 | 494 | 0.094 | [34] | ||
| 2 | 15.98 | 344 | 433 | 455 | 301,283 | 477 | 467 | 432 | 5.010 | [32] | |
| 3 | 18.10 | 222 | 457 | 399,417 | 479 | 469 | 434 | 0.019 | [34] | ||
| 4 | 19.79 | 284,222 | 453 | 431,355 | 429 | 409 | 430 | 0.374 | [35] |
| Con | RS | RS + HEL | RS + HEM | RS + HEH | |
|---|---|---|---|---|---|
| POAE | 52.3 ± 2.71 | 39.4 ± 2.35 ** | 44.8 ± 2.62 | 47.7 ± 2.78 # | 48.9 ± 2.80 # |
| PTOA | 42.6 ± 2.49 | 31.7 ± 2.33 ** | 37.6 ± 2.09 | 38.8 ± 1.78 # | 38.7 ± 2.12 # |
| CAE | 11.1 ± 1.10 | 13.3 ± 0.53 | 12.3 ± 0.83 | 11.0 ± 1.17 | 11.5 ± 0.73 |
| Con | RS | RS + HEL | RS + HEM | RS + HEH | |
|---|---|---|---|---|---|
| Crossing | 35.6 ± 2.68 | 40.8 ± 2.74 | 33.6 ± 2.76 | 35.8 ± 4.82 | 40.2 ± 3.71 |
| Rearing | 24.2 ± 4.02 | 18.9 ± 1.76 | 17.9 ± 1.47 | 19.8 ± 1.88 | 16.5 ± 2.15 |
| Defecation | 6.1 ± 0.93 | 11.1 ± 0.97 ** | 10.6 ± 1.04 | 8.1 ± 0.76 # | 8.2 ± 0.70 # |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiu, C.-H.; Chyau, C.-C.; Chen, C.-C.; Lee, L.-Y.; Chen, W.-P.; Liu, J.-L.; Lin, W.-H.; Mong, M.-C. Erinacine A-Enriched Hericium erinaceus Mycelium Produces Antidepressant-Like Effects through Modulating BDNF/PI3K/Akt/GSK-3β Signaling in Mice. Int. J. Mol. Sci. 2018, 19, 341. https://doi.org/10.3390/ijms19020341
Chiu C-H, Chyau C-C, Chen C-C, Lee L-Y, Chen W-P, Liu J-L, Lin W-H, Mong M-C. Erinacine A-Enriched Hericium erinaceus Mycelium Produces Antidepressant-Like Effects through Modulating BDNF/PI3K/Akt/GSK-3β Signaling in Mice. International Journal of Molecular Sciences. 2018; 19(2):341. https://doi.org/10.3390/ijms19020341
Chicago/Turabian StyleChiu, Chun-Hung, Charng-Cherng Chyau, Chin-Chu Chen, Li-Ya Lee, Wan-Ping Chen, Jia-Ling Liu, Wen-Hsin Lin, and Mei-Chin Mong. 2018. "Erinacine A-Enriched Hericium erinaceus Mycelium Produces Antidepressant-Like Effects through Modulating BDNF/PI3K/Akt/GSK-3β Signaling in Mice" International Journal of Molecular Sciences 19, no. 2: 341. https://doi.org/10.3390/ijms19020341





