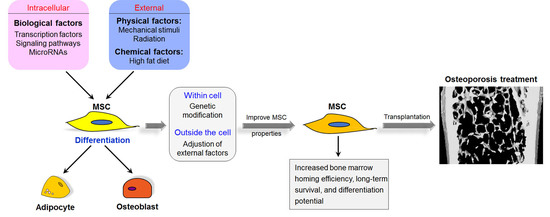
Mesenchymal Stem Cells: Cell Fate Decision to Osteoblast or Adipocyte and Application in Osteoporosis Treatment
Abstract
:1. Introduction
2. Mesenchymal Stem Cells (MSCs) and Bone Marrow MSCs (BM-MSCs) in Osteoporosis Development
3. The Molecular Mechanisms Regulating Osteoblast and Adipocyte Differentiation of MSCs
3.1. Transcription Factors
3.2. Signaling Pathways
3.3. MicroRNAs
3.4. Other Factors
4. Therapeutic Applications of MSCs for Osteoporosis
4.1. Bone Marrow MSCs (BM-MSCs)
4.2. Adipose Tissue-Derived MSCs (AD-MSCs)
5. Concerns on the Clinical Application of MSCs in Osteoporosis Treatment and Future Direction
6. Conclusions and Perspectives
Acknowledgments
Conflicts of Interest
Abbreviations
| AD-MSCs | adipose tissue-derived MSCs |
| ADSC | adipose tissue-derived stromal cell |
| ALP | alkaline phosphatase |
| bFGF | basic fibroblast growth factor |
| BMD | bone mineral density |
| BM-MSCs | bone marrow MSCs |
| BMP | bone morphogenic protein |
| BMPR | bone morphogenetic protein receptor |
| Cbfa1 | core binding factor α1 |
| Cbfβ | core binding factor β |
| C/EBPα | CCAAT/enhancer binding protein α |
| CXCR4 | C-X-C chemokine receptor type 4 |
| Dlx5 | distal-less homeobox 5 |
| ERK1/2 | extracellular signal-regulated kinase 1/2 |
| Foxa1 | forkhead transcription factor 1 |
| Foxc2 | forkhead box C2 |
| Frz | frizzled |
| GMP | good manufacturing practices |
| GSK3β | glycogen synthase kinase 3 β |
| HGF | hepatocyte growth factor |
| HLA-DR | human leukocyte antigen-antigen D related |
| HOXC8 | homeobox C8 |
| hTERT | human telomerase reverse transcriptase |
| LMHF | low magnitude high frequency |
| LRP5/6 | low-density lipoprotein receptor-related protein 5/6 |
| MAPK | mitogen-activated protein kinase |
| miRNA | microRNA |
| MSCs | mesenchymal stem cells |
| OC | osteocalcin |
| OVX | ovariectomy |
| PDGF-BB | platelet derived growth factor B |
| PI3K | phosphoinositide 3-kinase |
| PPARγ | peroxisome proliferation-activated receptor γ |
| PTH | parathyroid hormone |
| RANKL | receptor activator of nuclear factor-kappa B ligand |
| Runx2 | runt-related transcription factor 2 |
| SAMP6 | senescence accelerated mouse prone 6 |
| SDF-1 | stromal-derived factor-1 |
| TAK1 | transforming growth factor-β activated kinase 1 |
| TGF-β | transforming growth factor-β |
| Wnt | wingless and int-1 |
References
- Kanis, J.A.; Melton, L.J., III; Christiansen, C.; Johnston, C.C.; Khaltaev, N. The diagnosis of osteoporosis. J. Bone Miner. Res. 1994, 9, 1137–1141. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: Synopsis of a WHO report. WHO Study Group. Osteoporos. Int. 1994, 4, 368–381. [Google Scholar] [CrossRef] [PubMed]
- Johnell, O.; Kanis, J.A. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos. Int. 2006, 17, 1726–1733. [Google Scholar] [CrossRef] [PubMed]
- Moerman, E.J.; Teng, K.; Lipschitz, D.A.; Lecka-Czernik, B. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: The role of PPAR-γ2 transcription factor and TGF-β/BMP signaling pathways. Aging Cell 2004, 3, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, X.; Li, Y.; He, J.; Anderstam, B.; Andersson, G.; Lindgren, U. Nicotinamide phosphoribosyltransferase (Nampt) affects the lineage fate determination of mesenchymal stem cells: A possible cause for reduced osteogenesis and increased adipogenesis in older individuals. J. Bone Miner. Res. 2011, 26, 2656–2664. [Google Scholar] [CrossRef] [PubMed]
- Yeung, D.K.; Griffith, J.F.; Antonio, G.E.; Lee, F.K.; Woo, J.; Leung, P.C. Osteoporosis is associated with increased marrow fat content and decreased marrow fat unsaturation: A proton MR spectroscopy study. J. Magn. Reson. Imaging 2005, 22, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Chen, J.; Gantz, M.; Punyanitya, M.; Heymsfield, S.B.; Gallagher, D.; Albu, J.; Engelson, E.; Kotler, D.; Pi-Sunyer, X.; et al. MRI-measured pelvic bone marrow adipose tissue is inversely related to DXA-measured bone mineral in younger and older adults. Eur. J. Clin. Nutr. 2012, 66, 983–988. [Google Scholar] [CrossRef] [PubMed]
- Li, C.J.; Cheng, P.; Liang, M.K.; Chen, Y.S.; Lu, Q.; Wang, J.Y.; Xia, Z.Y.; Zhou, H.D.; Cao, X.; Xie, H.; et al. MicroRNA-188 regulates age-related switch between osteoblast and adipocyte differentiation. J. Clin. Investig. 2015, 125, 1509–1522. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.; Yang, X.; Su, X.; Hu, C.; Zhu, X.; Yang, N.; Chen, X.; Shi, S.; Shi, S.; Jin, Y. Redundant miR-3077-5p and miR-705 mediate the shift of mesenchymal stem cell lineage commitment to adipocyte in osteoporosis bone marrow. Cell Death Dis. 2013, 4, e600. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, R.S.; Roberson, P.K.; Manolagas, S.C. Giant osteoclast formation and long-term oral bisphosphonate therapy. N. Engl. J. Med. 2009, 360, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.J.; Crockett, J.C.; Coxon, F.P.; Monkkonen, J. Biochemical and molecular mechanisms of action of bisphosphonates. Bone 2011, 49, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, S.; Matsumoto, T. Recent advances in the management of osteoporosis. F1000Research 2017, 6, 625. [Google Scholar] [CrossRef] [PubMed]
- Cummings, S.R.; San Martin, J.; McClung, M.R.; Siris, E.S.; Eastell, R.; Reid, I.R.; Delmas, P.; Zoog, H.B.; Austin, M.; Wang, A.; et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N. Engl. J. Med. 2009, 361, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Rizzoli, R.; Yasothan, U.; Kirkpatrick, P. Denosumab. Nat. Rev. Drug Discov. 2010, 9, 591–592. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Nakamura, Y.; Kato, H. Changes of bone-related minerals during denosumab administration in post-menopausal osteoporotic patients. Nutrients 2017, 9, 871. [Google Scholar] [CrossRef] [PubMed]
- Minisola, S.; Cipriani, C.; Occhiuto, M.; Pepe, J. New anabolic therapies for osteoporosis. Intern. Emerg. Med. 2017, 12, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Cosman, F.; Crittenden, D.B.; Adachi, J.D.; Binkley, N.; Czerwinski, E.; Ferrari, S.; Hofbauer, L.C.; Lau, E.; Lewiecki, E.M.; Miyauchi, A.; et al. Romosozumab treatment in postmenopausal women with osteoporosis. N. Engl. J. Med. 2016, 375, 1532–1543. [Google Scholar] [CrossRef] [PubMed]
- Keaveny, T.M.; Crittenden, D.B.; Bolognese, M.A.; Genant, H.K.; Engelke, K.; Oliveri, B.; Brown, J.P.; Langdahl, B.L.; Yan, C.; Grauer, A.; et al. Greater gains in spine and hip strength for romosozumab compared with teriparatide in postmenopausal women with low bone mass. J. Bone Miner. Res. 2017, 32, 1956–1962. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Sandor, G.K.; Dore, E.; Morrison, A.D.; Alsahli, M.; Amin, F.; Peters, E.; Hanley, D.A.; Chaudry, S.R.; Lentle, B.; et al. Bisphosphonate associated osteonecrosis of the jaw. J. Rheumatol. 2009, 36, 478–490. [Google Scholar] [CrossRef] [PubMed]
- Fernandez Ayora, A.; Herion, F.; Rompen, E.; Reginster, J.Y.; Magremanne, M.; Lambert, F. Dramatic osteonecrosis of the jaw associated with oral bisphosphonates, periodontitis, and dental implant removal. J. Clin. Periodontol. 2015, 42, 190–195. [Google Scholar] [CrossRef] [PubMed]
- da Silva Meirelles, L.; Chagastelles, P.C.; Nardi, N.B. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J. Cell Sci. 2006, 119, 2204–2213. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.; Unger, M.; van Griensven, M.; Balmayor, E.R. Adipose-derived mesenchymal stem cells from liposuction and resected fat are feasible sources for regenerative medicine. Eur. J. Med. Res. 2017, 22, 17. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.J.; Chailakhjan, R.K.; Lalykina, K.S. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Prolif. 1970, 3, 393–403. [Google Scholar] [CrossRef]
- Friedenstein, A.J.; Chailakhyan, R.K.; Latsinik, N.V.; Panasyuk, A.F.; Keiliss-Borok, I.V. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation 1974, 17, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.M.; Elabd, C.; Amri, E.Z.; Ailhaud, G.; Dani, C. The human adipose tissue is a source of multipotent stem cells. Biochimie 2005, 87, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Sarugaser, R.; Lickorish, D.; Baksh, D.; Hosseini, M.M.; Davies, J.E. Human umbilical cord perivascular (HUCPV) cells: A source of mesenchymal progenitors. Stem Cells 2005, 23, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Kumagai, G.; Wada, K.; Tanaka, T.; Asari, T.; Oishi, K.; Fujita, T.; Mizukami, H.; Furukawa, K.I.; Ishibashi, Y. High osteogenic potential of adipose- and muscle-derived mesenchymal stem cells in spinal-ossification model mice. Spine 2017, 42, E1342–E1349. [Google Scholar] [CrossRef] [PubMed]
- Batouli, S.; Miura, M.; Brahim, J.; Tsutsui, T.W.; Fisher, L.W.; Gronthos, S.; Robey, P.G.; Shi, S. Comparison of stem-cell-mediated osteogenesis and dentinogenesis. J. Dent. Res. 2003, 82, 976–981. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Peister, A.; Mellad, J.A.; Larson, B.L.; Hall, B.M.; Gibson, L.F.; Prockop, D.J. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood 2004, 103, 1662–1668. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Lv, F.J.; Tuan, R.S.; Cheung, K.M.; Leung, V.Y. Concise review: The surface markers and identity of human mesenchymal stem cells. Stem Cells 2014, 32, 1408–1419. [Google Scholar] [CrossRef] [PubMed]
- Boxall, S.A.; Jones, E. Markers for characterization of bone marrow multipotential stromal cells. Stem Cells Int. 2012, 2012, 975871. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Greenberger, J.S.; Epperly, M.W.; Goff, J.P.; Adler, C.; Leboff, M.S.; Glowacki, J. Age-related intrinsic changes in human bone-marrow-derived mesenchymal stem cells and their differentiation to osteoblasts. Aging Cell 2008, 7, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Astudillo, P.; Rios, S.; Pastenes, L.; Pino, A.M.; Rodriguez, J.P. Increased adipogenesis of osteoporotic human-mesenchymal stem cells (MSCs) characterizes by impaired leptin action. J. Cell Biochem. 2008, 103, 1054–1065. [Google Scholar] [CrossRef] [PubMed]
- Augello, A.; De Bari, C. The regulation of differentiation in mesenchymal stem cells. Hum. Gene Ther. 2010, 21, 1226–1238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komori, T.; Yagi, H.; Nomura, S.; Yamaguchi, A.; Sasaki, K.; Deguchi, K.; Shimizu, Y.; Bronson, R.T.; Gao, Y.H.; Inada, M.; et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 1997, 89, 755–764. [Google Scholar] [CrossRef]
- Komori, T. Regulation of osteoblast differentiation by transcription factors. J. Cell Biochem. 2006, 99, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Gao, Y.; Ueta, C.; Yamaguchi, A.; Komori, T. Multilineage differentiation of Cbfa1-deficient calvarial cells in vitro. Biochem. Biophys. Res. Commun. 2000, 273, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, C.A.; Furuichi, T.; Fujita, T.; Fukuyama, R.; Kanatani, N.; Kobayashi, S.; Satake, M.; Takada, K.; Komori, T. Core-binding factor β interacts with Runx2 and is required for skeletal development. Nat. Genet. 2002, 32, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, R.T.; Xiao, G.; Jiang, D.; Gopalakrishnan, R.; Yang, S.; Reith, E. Multiple signaling pathways converge on the Cbfa1/Runx2 transcription factor to regulate osteoblast differentiation. Connect. Tissue Res. 2003, 44, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; Zhou, X.; Kunkel, G.; Zhang, Z.; Deng, J.M.; Behringer, R.R.; de Crombrugghe, B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 2002, 108, 17–29. [Google Scholar] [CrossRef]
- Nishio, Y.; Dong, Y.; Paris, M.; O’Keefe, R.J.; Schwarz, E.M.; Drissi, H. Runx2-mediated regulation of the zinc finger Osterix/Sp7 gene. Gene 2006, 372, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.M.; Liu, Z.B.; Zhao, Y.; Gong, Z.W.; Li, D.; Wang, X.Y.; Zeng, X.L.; Liu, W.G. Runx2 is involved in regulating osterix promoter activity and gene expression. Prog. Biochem. Biophys. 2006, 33, 957–964. [Google Scholar]
- Hong, J.H.; Hwang, E.S.; McManus, M.T.; Amsterdam, A.; Tian, Y.; Kalmukova, R.; Mueller, E.; Benjamin, T.; Spiegelman, B.M.; Sharp, P.A.; et al. TAZ, a transcriptional modulator of mesenchymal stem cell differentiation. Science 2005, 309, 1074–1078. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Cho, K.W.; Choi, H.S.; Park, S.J.; Rhee, Y.; Jung, H.S.; Lim, S.K. The forkhead transcription factor Foxc2 stimulates osteoblast differentiation. Biochem. Biophys. Res. Commun. 2009, 386, 532–536. [Google Scholar] [CrossRef] [PubMed]
- Bialek, P.; Kern, B.; Yang, X.; Schrock, M.; Sosic, D.; Hong, N.; Wu, H.; Yu, K.; Ornitz, D.M.; Olson, E.N.; et al. A twist code determines the onset of osteoblast differentiation. Dev. Cell 2004, 6, 423–435. [Google Scholar] [CrossRef]
- Zhuang, H.; Zhang, X.; Zhu, C.; Tang, X.; Yu, F.; Shang, G.W.; Cai, X. Molecular mechanisms of PPAR-γ governing MSC osteogenic and adipogenic differentiation. Curr. Stem Cell Res. Ther. 2016, 11, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.H.; Li, F.G.; Chen, X.Y.; Li, J.T.; Wu, Y.H.; Huang, L.H.; Wang, Z.; Li, P.; Wang, T.; Lahn, B.T.; et al. PPARγ suppression inhibits adipogenesis but does not promote osteogenesis of human mesenchymal stem cells. Int. J. Biochem. Cell Biol. 2012, 44, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.T.; Lane, M.D. CCAAT/enhancer binding protein α is sufficient to initiate the 3T3-L1 adipocyte differentiation program. Proc. Natl. Acad. Sci. USA 1994, 91, 8757–8761. [Google Scholar] [CrossRef] [PubMed]
- Okitsu, Y.; Takahashi, S.; Minegishi, N.; Kameoka, J.; Kaku, M.; Yamamoto, M.; Sasaki, T.; Harigae, H. Regulation of adipocyte differentiation of bone marrow stromal cells by transcription factor GATA-2. Biochem. Biophys. Res. Commun. 2007, 364, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Fujimori, K.; Amano, F. Forkhead transcription factor Foxa1 is a novel target gene of C/EBPβ and suppresses the early phase of adipogenesis. Gene 2011, 473, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Deng, C.; Li, Y.P. TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int. J. Biol. Sci. 2012, 8, 272–288. [Google Scholar] [CrossRef] [PubMed]
- Kang, Q.; Song, W.X.; Luo, Q.; Tang, N.; Luo, J.; Luo, X.; Chen, J.; Bi, Y.; He, B.C.; Park, J.K.; et al. A comprehensive analysis of the dual roles of BMPs in regulating adipogenic and osteogenic differentiation of mesenchymal progenitor cells. Stem Cells Dev. 2009, 18, 545–559. [Google Scholar] [CrossRef] [PubMed]
- Gori, F.; Thomas, T.; Hicok, K.C.; Spelsberg, T.C.; Riggs, B.L. Differentiation of human marrow stromal precursor cells: Bone morphogenetic protein-2 increases OSF2/CBFA1, enhances osteoblast commitment, and inhibits late adipocyte maturation. J. Bone Miner. Res. 1999, 14, 1522–1535. [Google Scholar] [CrossRef] [PubMed]
- Javed, A.; Bae, J.S.; Afzal, F.; Gutierrez, S.; Pratap, J.; Zaidi, S.K.; Lou, Y.; van Wijnen, A.J.; Stein, J.L.; Stein, G.S.; et al. Structural coupling of Smad and Runx2 for execution of the BMP2 osteogenic signal. J. Biol. Chem. 2008, 283, 8412–8422. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.A.; Israel, D.I.; Kelly, S.; Luxenberg, D.P. Bone morphogenetic protein-2 causes commitment and differentiation in C3H10T1/2 and 3T3 cells. Growth Factors 1993, 9, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.Q.; Otto, T.C.; Lane, M.D. Commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc. Natl. Acad. Sci. USA 2004, 101, 9607–9611. [Google Scholar] [CrossRef] [PubMed]
- Hata, K.; Nishimura, R.; Ikeda, F.; Yamashita, K.; Matsubara, T.; Nokubi, T.; Yoneda, T. Differential roles of Smad1 and p38 kinase in regulation of peroxisome proliferator-activating receptor γ during bone morphogenetic protein 2-induced adipogenesis. Mol. Biol. Cell 2003, 14, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Kim, M.; Jho, E.H. Wnt/β-catenin signalling: From plasma membrane to nucleus. Biochem. J. 2013, 450, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Etheridge, S.L.; Spencer, G.J.; Heath, D.J.; Genever, P.G. Expression profiling and functional analysis of wnt signaling mechanisms in mesenchymal stem cells. Stem Cells 2004, 22, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Glowacki, J.; Zhou, S. Inhibition of adipocytogenesis by canonical WNT signaling in human mesenchymal stem cells. Exp. Cell Res. 2011, 317, 1796–1803. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Li, Q.; Luo, S.; Liu, Z.; Luo, D.; Zhang, B.; Zhang, D.; Rao, P.; Xiao, J. PPARγ and Wnt signaling in adipogenic and osteogenic differentiation of mesenchymal stem cells. Curr. Stem Cell Res. Ther. 2016, 11, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, C. A Wnt canon orchestrating osteoblastogenesis. Trends Cell Biol. 2006, 16, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Piters, E.; Boudin, E.; Van Hul, W. Wnt signaling: A win for bone. Arch. Biochem. Biophys. 2008, 473, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Byun, M.R.; Hwang, J.H.; Kim, A.R.; Kim, K.M.; Hwang, E.S.; Yaffe, M.B.; Hong, J.H. Canonical Wnt signalling activates TAZ through PP1A during osteogenic differentiation. Cell Death Differ. 2014, 21, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Bennett, C.N.; Longo, K.A.; Wright, W.S.; Suva, L.J.; Lane, T.F.; Hankenson, K.D.; MacDougald, O.A. Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc. Natl. Acad. Sci. USA 2005, 102, 3324–3329. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Bennett, C.N.; Gerin, I.; Rapp, L.A.; Hankenson, K.D.; Macdougald, O.A. Wnt signaling stimulates osteoblastogenesis of mesenchymal precursors by suppressing CCAAT/enhancer-binding protein α and peroxisome proliferator-activated receptor γ. J. Biol. Chem. 2007, 282, 14515–14524. [Google Scholar] [CrossRef] [PubMed]
- Cawthorn, W.P.; Bree, A.J.; Yao, Y.; Du, B.; Hemati, N.; Martinez-Santibanez, G.; MacDougald, O.A. Wnt6, Wnt10a and Wnt10b inhibit adipogenesis and stimulate osteoblastogenesis through a β-catenin-dependent mechanism. Bone 2012, 50, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, B.; Eliasson, B.; Smith, U. Thiazolidinediones increase the wingless-type MMTV integration site family (WNT) inhibitor Dickkopf-1 in adipocytes: A link with osteogenesis. Diabetologia 2010, 53, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Horvitz, H.R.; Sulston, J.E. Isolation and genetic characterization of cell-lineage mutants of the nematode Caenorhabditis elegans. Genetics 1980, 96, 435–454. [Google Scholar] [PubMed]
- Arfat, Y.; Xiao, W.Z.; Ahmad, M.; Zhao, F.; Li, D.J.; Sun, Y.L.; Hu, L.; Zhihao, C.; Zhang, G.; Iftikhar, S.; et al. Role of microRNAs in osteoblasts differentiation and bone disorders. Curr. Med. Chem. 2015, 22, 748–758. [Google Scholar] [CrossRef] [PubMed]
- Sekar, D.; Saravanan, S.; Karikalan, K.; Thirugnanasambantham, K.; Lalitha, P.; Islam, V.I. Role of microRNA 21 in mesenchymal stem cell (MSC) differentiation: A powerful biomarker in MSCs derived cells. Curr. Pharm. Biotechnol. 2015, 16, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Hamam, D.; Ali, D.; Kassem, M.; Aldahmash, A.; Alajez, N.M. microRNAs as regulators of adipogenic differentiation of mesenchymal stem cells. Stem Cells Dev. 2015, 24, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Gennari, L.; Bianciardi, S.; Merlotti, D. MicroRNAs in bone diseases. Osteoporos. Int. 2017, 28, 1191–1213. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.B.; Li, X.; Li, Z.Y.; Zhao, J.; Yuan, X.B.; Ren, Y.; Cui, Z.D.; Liu, Y.D.; Yang, X.J. microRNA-21 promotes osteogenic differentiation of mesenchymal stem cells by the PI3K/β-catenin pathway. J. Orthop. Res. 2015, 33, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Hwang, S.J.; Bae, Y.C.; Jung, J.S. MiR-21 regulates adipogenic differentiation through the modulation of TGF-β signaling in mesenchymal stem cells derived from human adipose tissue. Stem Cells 2009, 27, 3093–3102. [Google Scholar] [CrossRef] [PubMed]
- Eskildsen, T.; Taipaleenmaki, H.; Stenvang, J.; Abdallah, B.M.; Ditzel, N.; Nossent, A.Y.; Bak, M.; Kauppinen, S.; Kassem, M. MicroRNA-138 regulates osteogenic differentiation of human stromal (mesenchymal) stem cells in vivo. Proc. Natl. Acad. Sci. USA 2011, 108, 6139–6144. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhao, L.; Xing, L.; Chen, D. MicroRNA-204 regulates Runx2 protein expression and mesenchymal progenitor cell differentiation. Stem Cells 2010, 28, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.F.; Fu, W.M.; He, M.L.; Wang, H.; Wang, W.M.; Yu, S.C.; Bian, X.W.; Zhou, J.; Lin, M.C.; Lu, G.; et al. MiR-637 maintains the balance between adipocytes and osteoblasts by directly targeting Osterix. Mol. Biol. Cell 2011, 22, 3955–3961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baglio, S.R.; Devescovi, V.; Granchi, D.; Baldini, N. MicroRNA expression profiling of human bone marrow mesenchymal stem cells during osteogenic differentiation reveals Osterix regulation by miR-31. Gene 2013, 527, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Delaine-Smith, R.M.; Reilly, G.C. Mesenchymal stem cell responses to mechanical stimuli. Muscles Ligaments Tendons J. 2012, 2, 169–180. [Google Scholar] [PubMed]
- Luu, Y.K.; Pessin, J.E.; Judex, S.; Rubin, J.; Rubin, C.T. Mechanical signals as a non-invasive means to influence mesenchymal stem cell fate, promoting bone and suppressing the fat phenotype. Bonekey Osteovis. 2009, 6, 132–149. [Google Scholar] [CrossRef] [PubMed]
- Menuki, K.; Mori, T.; Sakai, A.; Sakuma, M.; Okimoto, N.; Shimizu, Y.; Kunugita, N.; Nakamura, T. Climbing exercise enhances osteoblast differentiation and inhibits adipogenic differentiation with high expression of PTH/PTHrP receptor in bone marrow cells. Bone 2008, 43, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Maredziak, M.; Smieszek, A.; Chrzastek, K.; Basinska, K.; Marycz, K. Physical activity increases the total number of none-marrow-derived mesenchymal stem cells, enhances their osteogenic potential, and inhibits their adipogenic properties. Stem. Cells Int. 2015, 2015, 379093. [Google Scholar] [CrossRef] [PubMed]
- Luu, Y.K.; Capilla, E.; Rosen, C.J.; Gilsanz, V.; Pessin, J.E.; Judex, S.; Rubin, C.T. Mechanical stimulation of mesenchymal stem cell proliferation and differentiation promotes osteogenesis while preventing dietary-induced obesity. J. Bone Miner. Res. 2009, 24, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Rubin, C.T.; Capilla, E.; Luu, Y.K.; Busa, B.; Crawford, H.; Nolan, D.J.; Mittal, V.; Rosen, C.J.; Pessin, J.E.; Judex, S. Adipogenesis is inhibited by brief, daily exposure to high-frequency, extremely low-magnitude mechanical signals. Proc. Natl. Acad. Sci. USA 2007, 104, 17879–17884. [Google Scholar] [CrossRef] [PubMed]
- Demiray, L.; Ozcivici, E. Bone marrow stem cells adapt to low-magnitude vibrations by altering their cytoskeleton during quiescence and osteogenesis. Turk. J. Biochem. 2015, 39, 88–97. [Google Scholar]
- Baskan, O.; Mese, G.; Ozcivici, E. Low-intensity vibrations normalize adipogenesis-induced morphological and molecular changes of adult mesenchymal stem cells. Proc. Inst. Mech. Eng. H. 2017, 231, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; He, F.; Zhong, D.Y.; Luo, Z.P. Acoustic-frequency vibratory stimulation regulates the balance between osteogenesis and adipogenesis of human bone marrow-derived mesenchymal stem cells. BioMed Res. Int. 2015, 2015, 540731. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Guan, X.; Zhu, Z.; Gao, S.; Zhang, C.; Li, C.; Zhou, K.; Hou, W.; Yu, H. Osteogenic differentiation of bone marrow-derived mesenchymal stromal cells on bone-derived scaffolds: Effect of microvibration and role of ERK1/2 activation. Eur. Cell Mater. 2011, 22, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Papachristou, D.J.; Papachroni, K.K.; Basdra, E.K.; Papavassiliou, A.G. Signaling networks and transcription factors regulating mechanotransduction in bone. Bioessays 2009, 31, 794–804. [Google Scholar] [CrossRef] [PubMed]
- Ge, C.; Xiao, G.; Jiang, D.; Yang, Q.; Hatch, N.E.; Roca, H.; Franceschi, R.T. Identification and functional characterization of ERK/MAPK phosphorylation sites in the Runx2 transcription factor. J. Biol. Chem. 2009, 284, 32533–32543. [Google Scholar] [CrossRef] [PubMed]
- Ge, C.; Xiao, G.; Jiang, D.; Franceschi, R.T. Critical role of the extracellular signal-regulated kinase-MAPK pathway in osteoblast differentiation and skeletal development. J. Cell Biol 2007, 176, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Uzer, G.; Pongkitwitoon, S.; Ete Chan, M.; Judex, S. Vibration induced osteogenic commitment of mesenchymal stem cells is enhanced by cytoskeletal remodeling but not fluid shear. J. Biomech. 2013, 46, 2296–2302. [Google Scholar] [CrossRef] [PubMed]
- Sen, B.; Xie, Z.; Case, N.; Styner, M.; Rubin, C.T.; Rubin, J. Mechanical signal influence on mesenchymal stem cell fate is enhanced by incorporation of refractory periods into the loading regimen. J. Biomech. 2011, 44, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Lau, E.; Lee, W.D.; Li, J.; Xiao, A.; Davies, J.E.; Wu, Q.; Wang, L.; You, L. Effect of low-magnitude, high-frequency vibration on osteogenic differentiation of rat mesenchymal stromal cells. J. Orthop. Res. 2011, 29, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Lu, Y.; Gan, X.; Yu, H. Low magnitude high frequency vibration promotes adipogenic differentiation of bone marrow stem cells via P38 MAPK signal. PLoS ONE 2017, 12, e0172954. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Liang, L.; Dou, Y.; Huang, Z.; Mo, H.; Wang, Y.; Yu, B. Mechanical strain regulates osteogenic and adipogenic differentiation of bone marrow mesenchymal stem cells. BioMed Res. Int. 2015, 2015, 873251. [Google Scholar] [CrossRef] [PubMed]
- Simmons, C.A.; Matlis, S.; Thornton, A.J.; Chen, S.; Wang, C.Y.; Mooney, D.J. Cyclic strain enhances matrix mineralization by adult human mesenchymal stem cells via the extracellular signal-regulated kinase (ERK1/2) signaling pathway. J. Biomech. 2003, 36, 1087–1096. [Google Scholar] [CrossRef]
- Carroll, S.F.; Buckley, C.T.; Kelly, D.J. Cyclic tensile strain can play a role in directing both intramembranous and endochondral ossification of mesenchymal stem cells. Front. Bioeng. Biotechnol. 2017, 5, 73. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, Y.; Koga, M.; Saito, M.; Matsunaga, Y.; Nakayama, K. Inhibition of adipocyte differentiation by mechanical stretching through ERK-mediated downregulation of PPARγ2. J. Cell Sci. 2004, 117, 3605–3614. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhao, Z.; Li, J.; Zou, L.; Shuler, C.; Zou, Y.; Huang, X.; Li, M.; Wang, J. Hydrostatic pressures promote initial osteodifferentiation with ERK1/2 not p38 MAPK signaling involved. J. Cell Biochem. 2009, 107, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Sittichokechaiwut, A.; Edwards, J.H.; Scutt, A.M.; Reilly, G.C. Short bouts of mechanical loading are as effective as dexamethasone at inducing matrix production by human bone marrow mesenchymal stem cell. Eur. Cell Mater. 2010, 20, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Holtorf, H.L.; Jansen, J.A.; Mikos, A.G. Flow perfusion culture induces the osteoblastic differentiation of marrow stroma cell-scaffold constructs in the absence of dexamethasone. J. Biomed. Mater. Res. A. 2005, 72, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Stavenschi, E.; Labour, M.N.; Hoey, D.A. Oscillatory fluid flow induces the osteogenic lineage commitment of mesenchymal stem cells: The effect of shear stress magnitude, frequency, and duration. J. Biomech. 2017, 55, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.F.; Li, R.Z.; Su, P.H.; Arfat, Y.; Zhang, G.; Shang, P.; Qian, A.R. Response and adaptation of bone cells to simulated microgravity. Acta Astronaut. 2014, 104, 396–408. [Google Scholar] [CrossRef]
- Arfat, Y.; Xiao, W.Z.; Iftikhar, S.; Zhao, F.; Li, D.J.; Sun, Y.L.; Zhang, G.; Shang, P.; Qian, A.R. Physiological effects of microgravity on bone cells. Calcified Tissue Int. 2014, 94, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Yang, J.; Guo, C.; Shi, D.; Shen, D.; Zheng, Q.; Chen, R.; Xu, Y.; Xi, Y.; Wang, J. Effects of hindlimb unloading on ex vivo growth and osteogenic/adipogenic potentials of bone marrow-derived mesenchymal stem cells in rats. Stem Cells Dev. 2008, 17, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Visigalli, D.; Strangio, A.; Palmieri, D.; Manduca, P. Hind limb unloading of mice modulates gene expression at the protein and mRNA level in mesenchymal bone cells. BMC Musculoskelet. Disord. 2010, 11, 147. [Google Scholar] [CrossRef] [PubMed]
- Zayzafoon, M.; Gathings, W.E.; McDonald, J.M. Modeled microgravity inhibits osteogenic differentiation of human mesenchymal stem cells and increases adipogenesis. Endocrinology 2004, 145, 2421–2432. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Dai, Z.Q.; Ling, S.K.; Zhang, H.Y.; Wan, Y.M.; Li, Y.H. Gravity, a regulation factor in the differentiation of rat bone marrow mesenchymal stem cells. J. Biomed. Sci. 2009, 16, 87. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Meng, R.; Deng, W.; Ding, W.; Zheng, Q.; Yuan, W.; Liu, L.; Zong, C.; Shang, P.; Wang, J. Effects of microgravity modeled by large gradient high magnetic field on the osteogenic initiation of human mesenchymal stem cells. Stem Cell Rev. 2010, 6, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Meyers, V.E.; Zayzafoon, M.; Gonda, S.R.; Gathings, W.E.; McDonald, J.M. Modeled microgravity disrupts collagen I/integrin signaling during osteoblastic differentiation of human mesenchymal stem cells. J. Cell Biochem. 2004, 93, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Meyers, V.E.; Zayzafoon, M.; Douglas, J.T.; McDonald, J.M. RhoA and cytoskeletal disruption mediate reduced osteoblastogenesis and enhanced adipogenesis of human mesenchymal stem cells in modeled microgravity. J. Bone Miner. Res. 2005, 20, 1858–1866. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Huang, G.; Yang, J.; Xu, Y.; Guo, C.; Xi, Y.; Pan, Z.; Wang, J. Could the effect of modeled microgravity on osteogenic differentiation of human mesenchymal stem cells be reversed by regulation of signaling pathways? Biol. Chem. 2007, 388, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Stemig, M.E.; Takahashi, Y.; Hui, S.K. Radiation response of mesenchymal stem cells derived from bone marrow and human pluripotent stem cells. J. Radiat. Res. 2015, 56, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Parhami, F.; Tintut, Y.; Beamer, W.G.; Gharavi, N.; Goodman, W.; Demer, L.L. Atherogenic high-fat diet reduces bone mineralization in mice. J. Bone Miner. Res. 2001, 16, 182–188. [Google Scholar] [CrossRef] [PubMed]
- da Silva, S.V.; Renovato-Martins, M.; Ribeiro-Pereira, C.; Citelli, M.; Barja-Fidalgo, C. Obesity modifies bone marrow microenvironment and directs bone marrow mesenchymal cells to adipogenesis. Obesity 2016, 24, 2522–2532. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Yu, X. Lipid metabolism disorders and bone dysfunction--interrelated and mutually regulated. Mol. Med. Rep. 2015, 12, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Barry, F.P.; Murphy, J.M. Mesenchymal stem cells: Clinical applications and biological characterization. Int. J. Biochem. Cell Biol. 2004, 36, 568–584. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Qu, X.; Zhao, R.C. Clinical applications of mesenchymal stem cells. J. Hematol. Oncol. 2012, 5, 19. [Google Scholar] [CrossRef] [PubMed]
- Antebi, B.; Pelled, G.; Gazit, D. Stem cell therapy for osteoporosis. Curr. Osteoporos. Rep. 2014, 12, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Goh, J.; Das De, S.; Ge, Z.; Ouyang, H.; Chong, J.S.; Low, S.L.; Lee, E.H. Efficacy of bone marrow-derived stem cells in strengthening osteoporotic bone in a rabbit model. Tissue Eng. 2006, 12, 1753–1761. [Google Scholar] [CrossRef] [PubMed]
- Ichioka, N.; Inaba, M.; Kushida, T.; Esumi, T.; Takahara, K.; Inaba, K.; Ogawa, R.; Iida, H.; Ikehara, S. Prevention of senile osteoporosis in SAMP6 mice by intrabone marrow injection of allogeneic bone marrow cells. Stem Cells 2002, 20, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Ocarino Nde, M.; Boeloni, J.N.; Jorgetti, V.; Gomes, D.A.; Goes, A.M.; Serakides, R. Intra-bone marrow injection of mesenchymal stem cells improves the femur bone mass of osteoporotic female rats. Connect. Tissue Res. 2010, 51, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Sui, B.; Hu, C.; Zhang, X.; Zhao, P.; He, T.; Zhou, C.; Qiu, X.; Chen, N.; Zhao, X.; Jin, Y. Allogeneic mesenchymal stem cell therapy promotes osteoblastogenesis and prevents glucocorticoid-induced osteoporosis. Stem Cells Transl Med. 2016, 5, 1238–1246. [Google Scholar] [CrossRef] [PubMed]
- Kiernan, J.; Hu, S.; Grynpas, M.D.; Davies, J.E.; Stanford, W.L. Systemic mesenchymal stromal cell transplantation prevents functional bone loss in a mouse model of age-related osteoporosis. Stem Cells Transl Med. 2016, 5, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Kern, S.; Eichler, H.; Stoeve, J.; Kluter, H.; Bieback, K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 2006, 24, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.T.; Lee, M.J.; Chen, C.H.; Chuang, S.C.; Chang, L.F.; Ho, M.L.; Hung, S.H.; Fu, Y.C.; Wang, Y.H.; Wang, H.I.; et al. Proliferation and differentiation potential of human adipose-derived mesenchymal stem cells isolated from elderly patients with osteoporotic fractures. J. Cell Mol. Med. 2012, 16, 582–593. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.W.; Sun, H.J.; Yang, J.Y.; Jung, J.Y.; Choi, H.J.; An, J.H.; Kim, S.W.; Kim, S.Y.; Park, K.J.; Shin, C.S. Human adipose tissue-derived stromal cell therapy prevents bone loss in ovariectomized nude mouse. Tissue Eng. Part A 2012, 18, 1067–1078. [Google Scholar] [CrossRef] [PubMed]
- Mirsaidi, A.; Genelin, K.; Vetsch, J.R.; Stanger, S.; Theiss, F.; Lindtner, R.A.; von Rechenberg, B.; Blauth, M.; Muller, R.; Kuhn, G.A.; et al. Therapeutic potential of adipose-derived stromal cells in age-related osteoporosis. Biomaterials 2014, 35, 7326–7335. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Zhang, P.; Xue, S.; Xu, Y.; Tan, J.; Liu, G. Adipose-derived stem cells alleviate osteoporosis by enhancing osteogenesis and inhibiting adipogenesis in a rabbit model. Cytotherapy 2014, 16, 1643–1655. [Google Scholar] [CrossRef] [PubMed]
- Lien, C.Y.; Chih-Yuan Ho, K.; Lee, O.K.; Blunn, G.W.; Su, Y. Restoration of bone mass and strength in glucocorticoid-treated mice by systemic transplantation of CXCR4 and cbfa-1 co-expressing mesenchymal stem cells. J. Bone Miner. Res. 2009, 24, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Ponnazhagan, S. Bone homing of mesenchymal stem cells by ectopic α4 integrin expression. FASEB J. 2007, 21, 3917–3927. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.M.; Ng, W.M.; Tan, H.S.; Vinitha, D.; Yang, Z.; Fan, J.B.; Zou, Y.; Hui, J.H.; Lee, E.H.; Lim, B. Molecular basis of immortalization of human mesenchymal stem cells by combination of p53 knockdown and human telomerase reverse transcriptase overexpression. Stem Cells Dev. 2013, 22, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Fierro, F.A.; Kalomoiris, S.; Sondergaard, C.S.; Nolta, J.A. Effects on proliferation and differentiation of multipotent bone marrow stromal cells engineered to express growth factors for combined cell and gene therapy. Stem Cells 2011, 29, 1727–1737. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Shi, X.; Xiao, F.; Yang, Y.; Zhang, X.; Wang, L.S.; Wu, C.T.; Wang, H. Transplantation of hepatocyte growth factor-modified dental pulp stem cells prevents bone loss in the early phase of ovariectomy-induced osteoporosis. Hum. Gene Ther. 2017. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.C.; Yew, T.L.; Yang, D.C.; Huang, W.H.; Hung, S.C. Benefits of hypoxic culture on bone marrow multipotent stromal cells. Am. J. Blood Res. 2012, 2, 148–159. [Google Scholar] [PubMed]


| Cell Type | Treatment Method | Therapeutic Outcomes | References |
|---|---|---|---|
| BM-MSCs | Local transplantation of autologous BM-MSCs | Increased trabecular thickness, improved microstructures with newly formed osteoids, and enhanced trabecular thickness and stiffness of bone. | [125] |
| BM-MSCs | Local injection of the normal allogeneic BM-MSCs | Increased trabecular bone, attenuated the loss of BMD, improved the femur bone mass and prevented osteoporosis. | [126,127] |
| BM-MSCs | Systemic injection of allogeneic BM-MSCs | Promoted osteoblastogenesis, maintained bone formation, and prevented the reduction of bone mass and strength in osteoporotic mouse model. Increased bone formation and sustained microarchitectural competence in a mouse model of age-related osteoporosis. | [128,129] |
| AD-MSCs | Systemic injection of allogeneic AD-MSCs | Prevented OVX-induced bone loss. | [132] |
| AD-MSCs | Local injection of autologous AD-MSCs | Improved trabecular bone quality and induced a significant increase in several molecular markers of bone turnover. Promoted osteogenesis, inhibited adipogenesis, and increased bone mineral density and new bone formation. | [133,134] |
| Cell Type | Treatment Method | Disease Treated | No. of Patients | Dose (No. of Treatment) | Phase | Therapeutic Outcomes | Clinical Trial No. |
|---|---|---|---|---|---|---|---|
| BM-MSCs | Intravenous injection of autologous BM-MSCs that were fucosylated | Osteoporosis, Spinal fractures | 10 | First 4 patients receive 2 million cells/kg; last 6 receive 5 million cells/kg (Single) | Phase I | Still in progress, no results | NCT02566655 |
| AD-MSCs | AD-MSCs were seeded within a composite graft and transplanted back into the fracture site | Osteoporotic fractures | 8 | Unknown | Phase II | Terminated, no results | NCT01532076 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, L.; Yin, C.; Zhao, F.; Ali, A.; Ma, J.; Qian, A. Mesenchymal Stem Cells: Cell Fate Decision to Osteoblast or Adipocyte and Application in Osteoporosis Treatment. Int. J. Mol. Sci. 2018, 19, 360. https://doi.org/10.3390/ijms19020360
Hu L, Yin C, Zhao F, Ali A, Ma J, Qian A. Mesenchymal Stem Cells: Cell Fate Decision to Osteoblast or Adipocyte and Application in Osteoporosis Treatment. International Journal of Molecular Sciences. 2018; 19(2):360. https://doi.org/10.3390/ijms19020360
Chicago/Turabian StyleHu, Lifang, Chong Yin, Fan Zhao, Arshad Ali, Jianhua Ma, and Airong Qian. 2018. "Mesenchymal Stem Cells: Cell Fate Decision to Osteoblast or Adipocyte and Application in Osteoporosis Treatment" International Journal of Molecular Sciences 19, no. 2: 360. https://doi.org/10.3390/ijms19020360