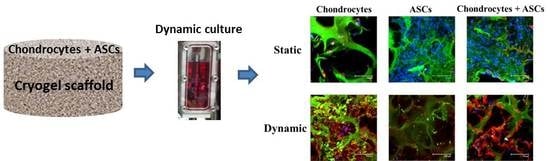
Effect of Cyclic Dynamic Compressive Loading on Chondrocytes and Adipose-Derived Stem Cells Co-Cultured in Highly Elastic Cryogel Scaffolds
Abstract
:1. Introduction
2. Results
2.1. Effects of Cyclic Compressive Loading on Gene Expression of Chondrocytes in Cryogel Scaffolds
2.2. Effects of Cyclic Compressive Loading on DNA, Glycoaminoglycans (GAGs) and Type II Collagen (Col II) Contents of Chondrocytes in Cryogel Scaffolds
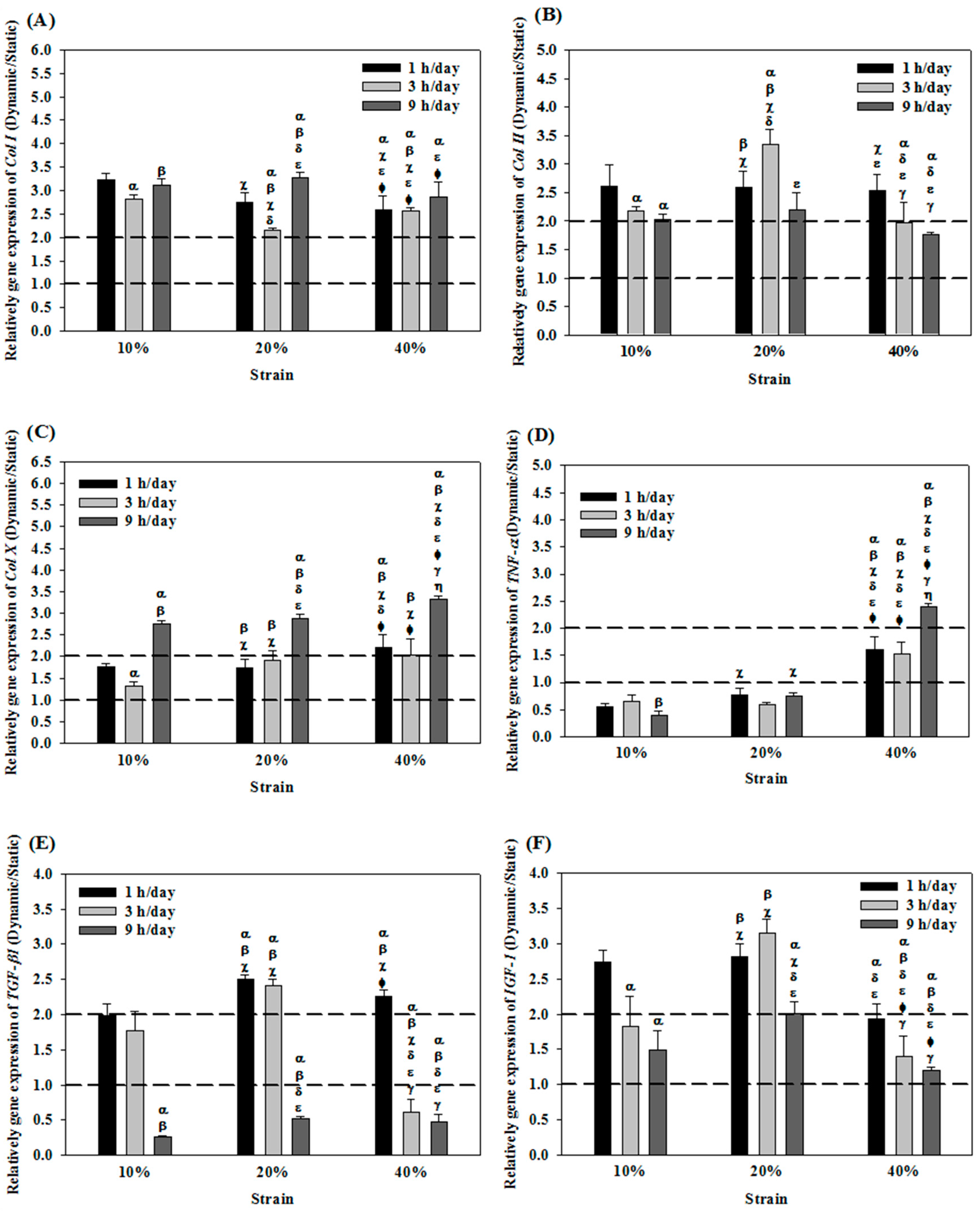
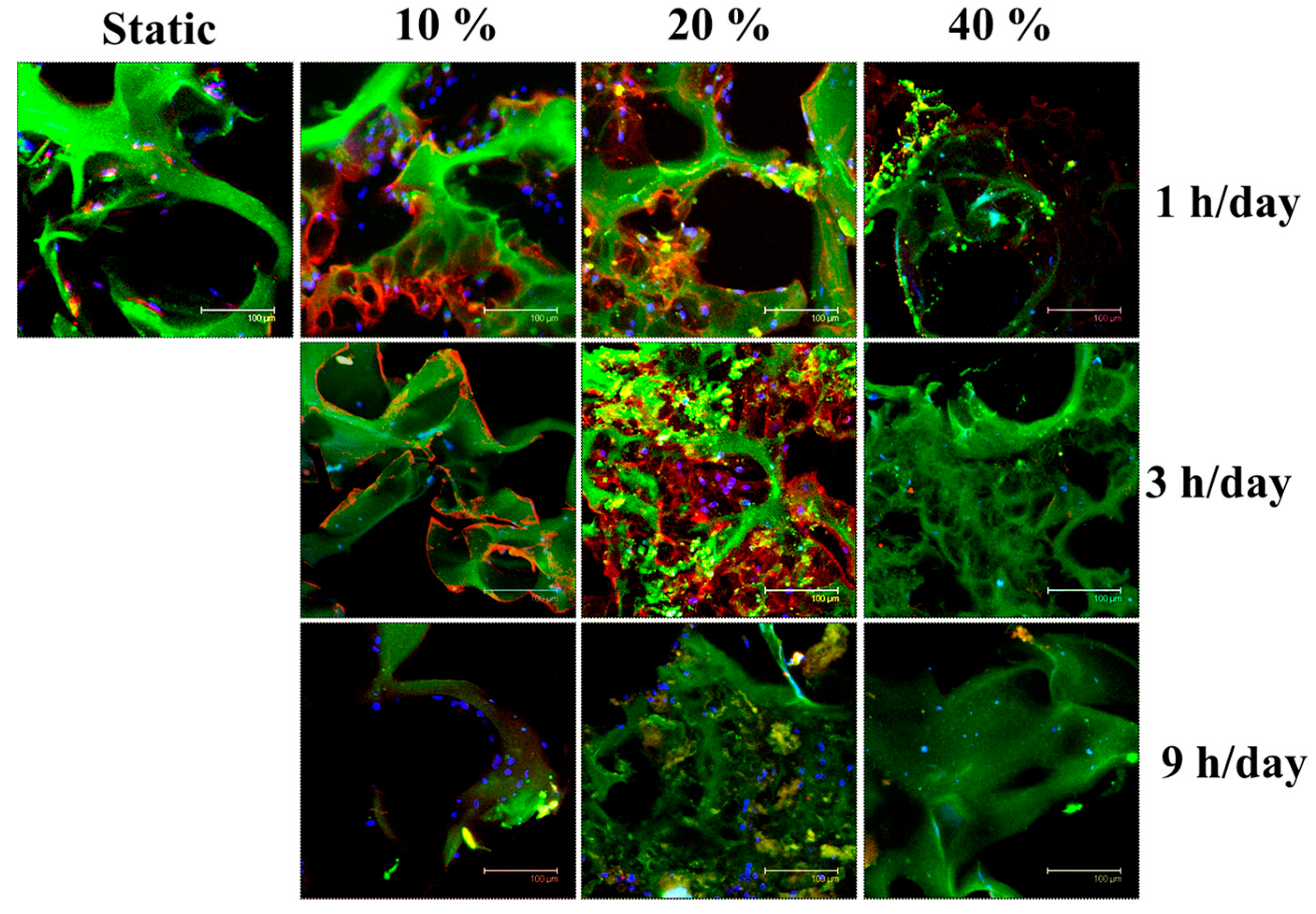
2.3. Effects of Cyclic Compressive Loading and Co-Culture on Gene Expression of Cells in Cryogel Scaffolds
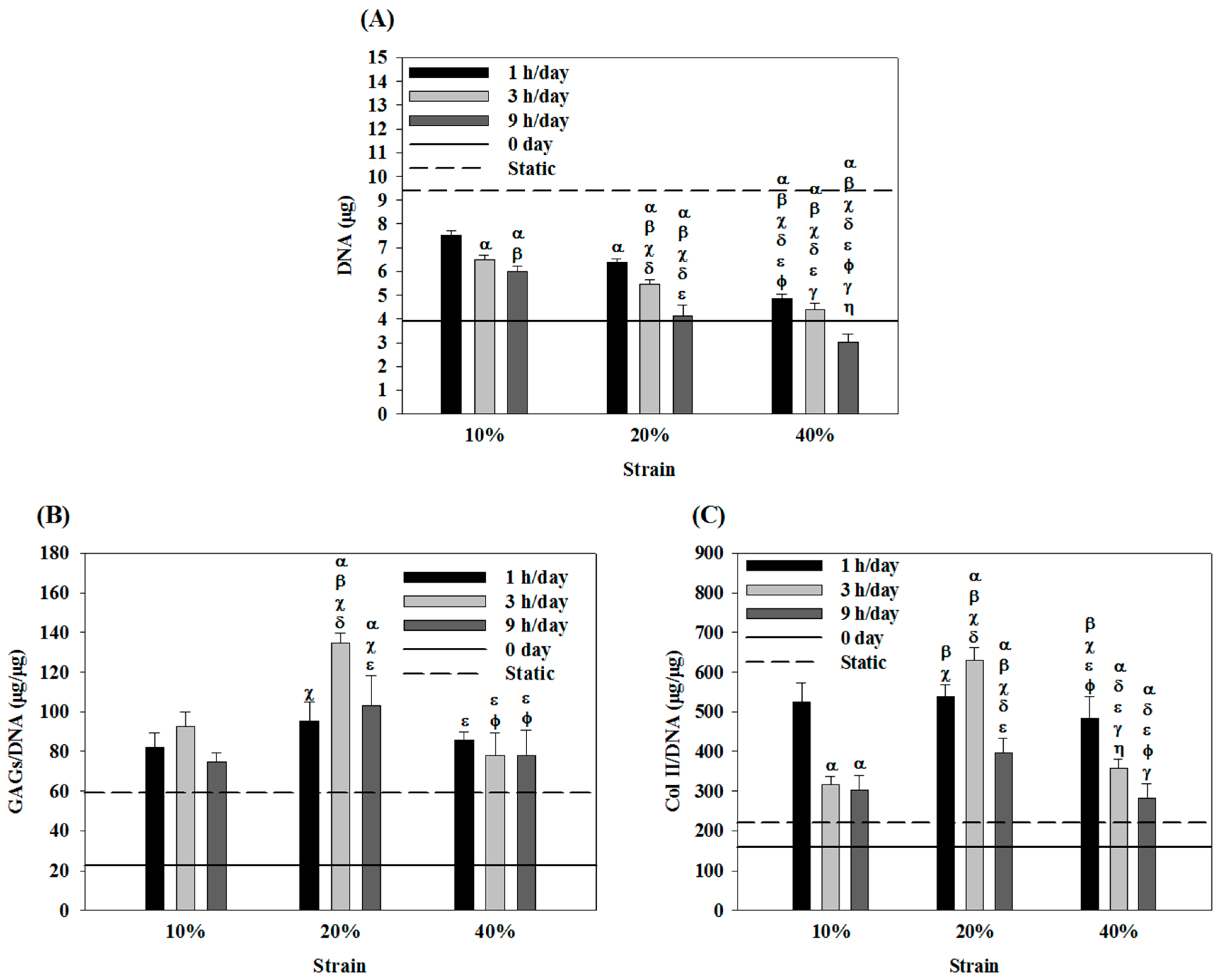
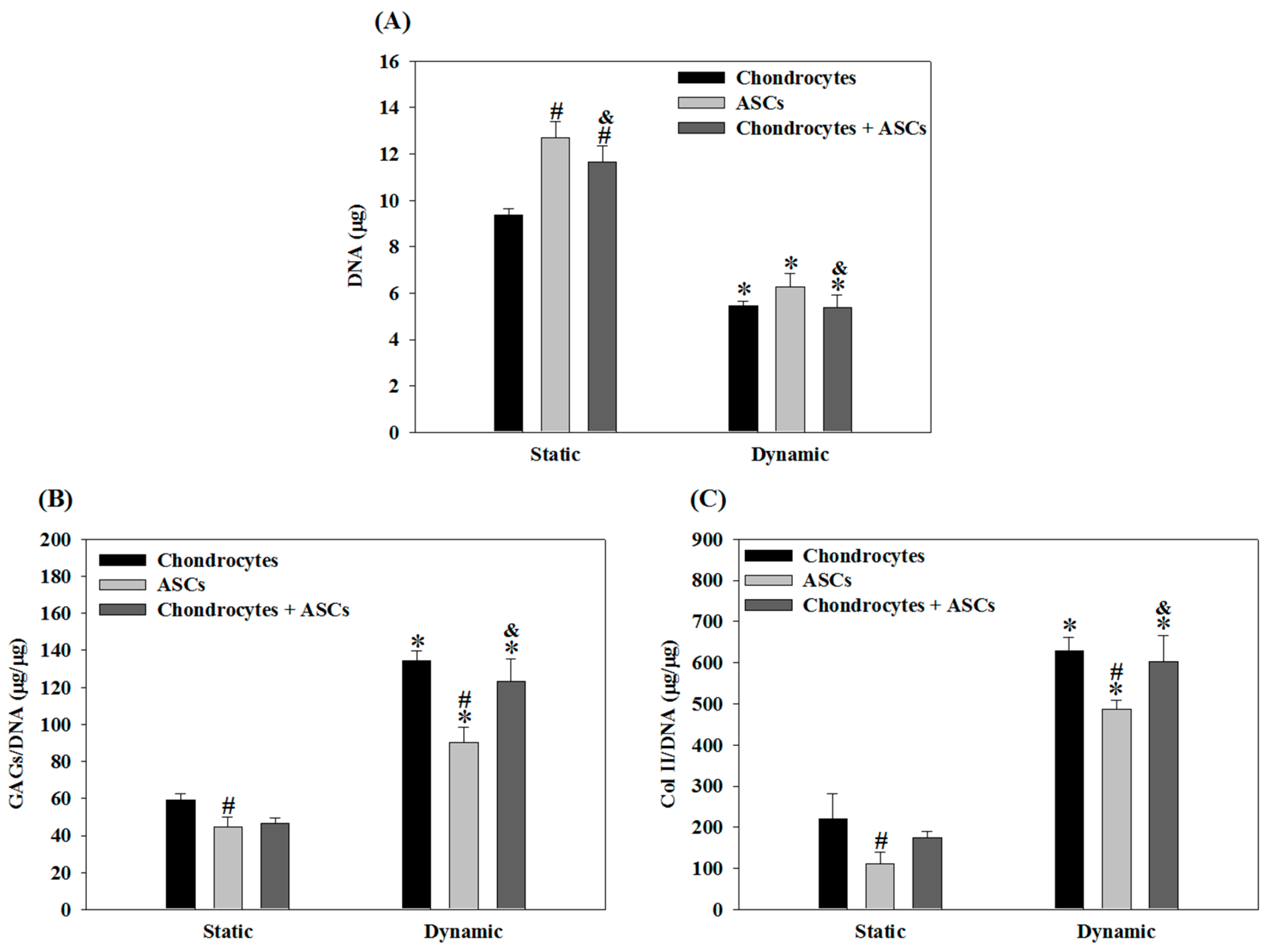
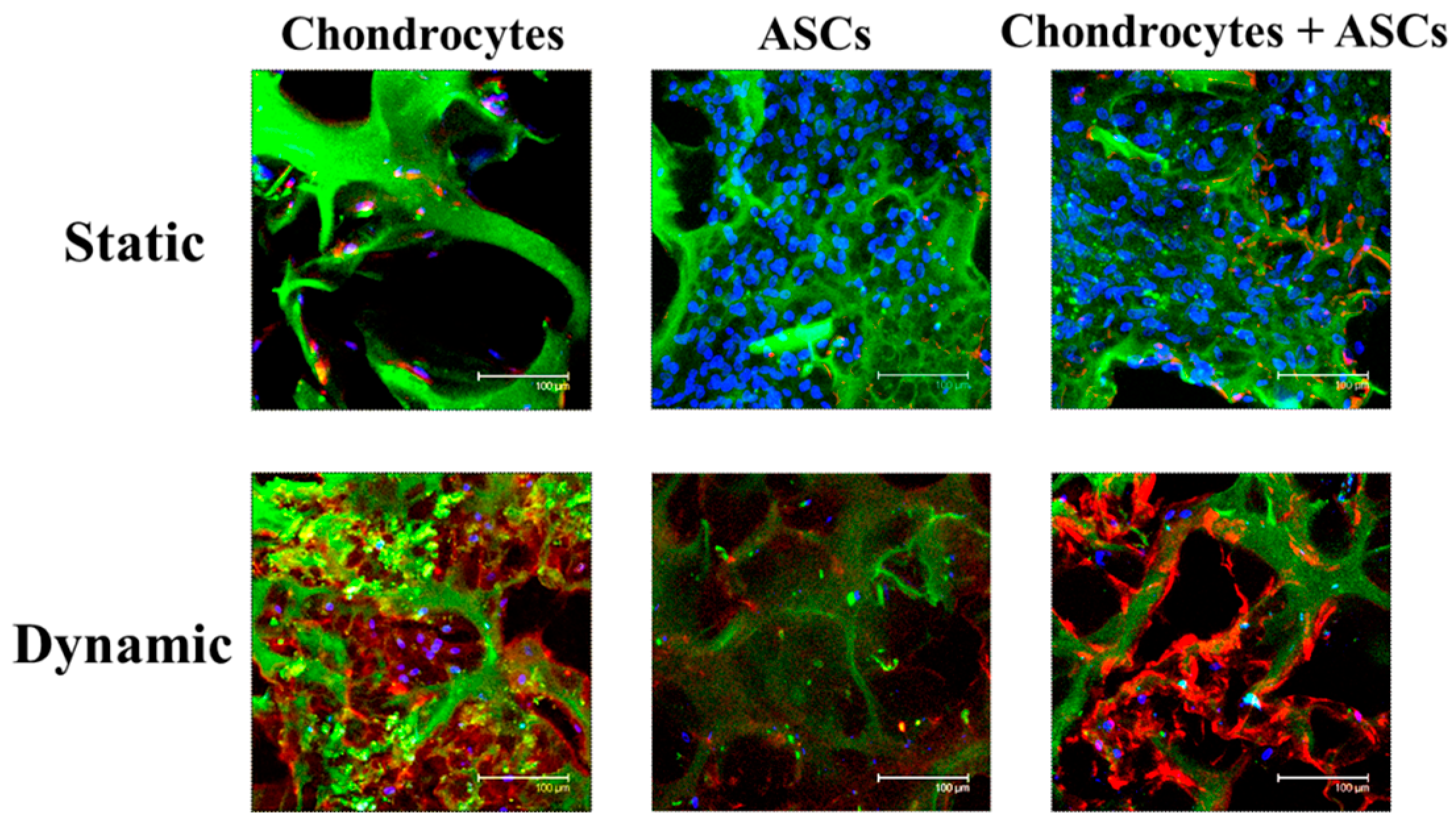
2.4. Effects of Cyclic Compressive Loading and Co-Culture on DNA, GAGs and Col II Contents of Cells in Cryogel Scaffolds
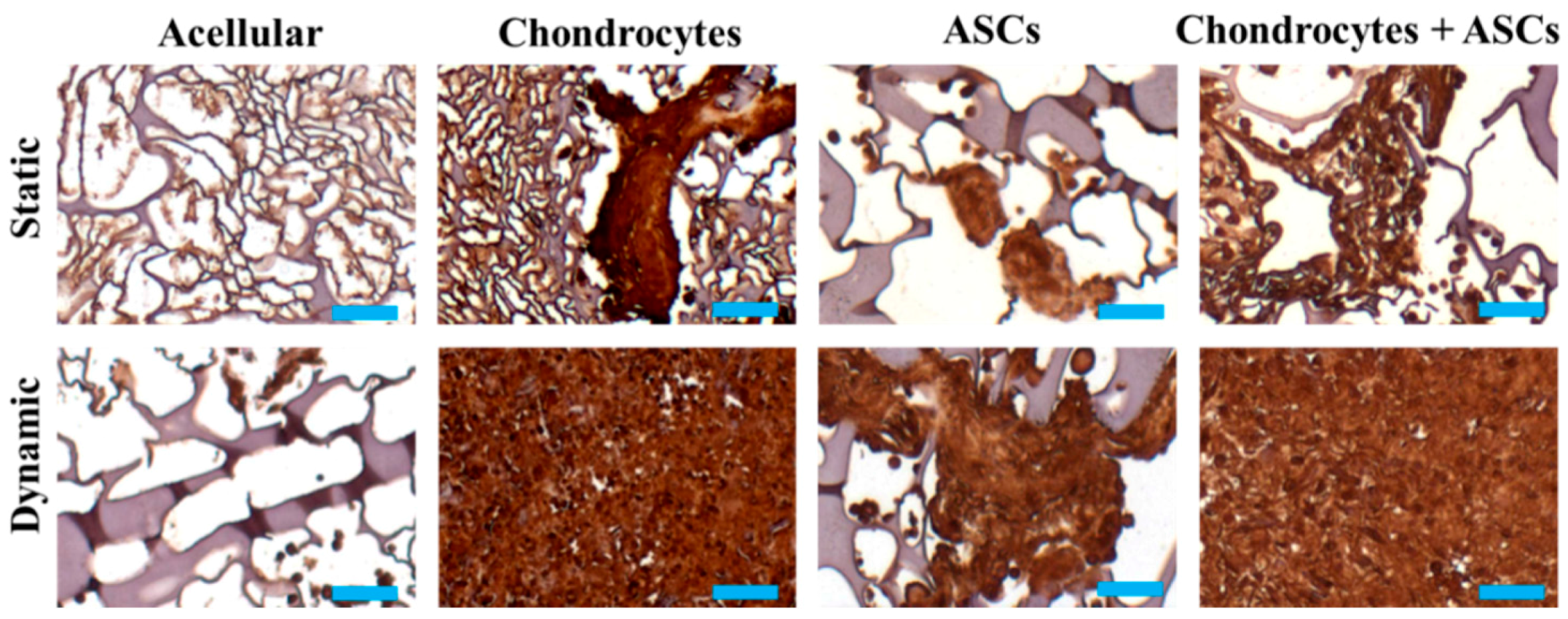
2.5. In Vivo Subcutaneous Implantation of Cells/Scaffold Implantation
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Cryogel
4.3. Isolation and Cultivation of Porcine Chondrocytes and Adipose-Derived Stem Cells (ASCs)
4.4. Dynamic Culture of Chondrocytes in Cryogel Scaffolds
4.5. Dynamic Culturing of ASCs/Chondrocytes in Cryogel Scaffolds
4.6. Gene Expression
4.7. Biochemical Analysis
4.8. Immunofluorescence Staining
4.9. In Vivo Subcutaneous Implantation
4.10. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| 3D | Three-dimensional |
| ECM | Extracellular matrix |
| Col II | Type II collagen |
| Col I | Type I collagen |
| Col X | Type X collagen |
| TNF-α | Tumor necrosis factor-α |
| IGF-1 | Insulin growth factor-1 |
| TGF-β1 | Transforming growth factor-β1 |
| BMPs | Bone morphogenetic proteins |
| GAGs | Glycoaminoglycans |
| ASCs | Adipose-derived stem cells |
| MSCs | Mesenchymal stem cells |
| DAPI | 4′,6-diamidino-2-phenylindole |
| FITC | Fluorescein isothiocyanate |
| Cy 3 | Cyanine 3 |
| RGD | Arg-Gly-Asp |
| PEG | Polyethylene glycol |
| qRT-PCR | Quantitative real-time polymerase chain reaction |
References
- Chiang, H.; Jiang, C.C. Repair of articular cartilage defects: Review and perspectives. J. Formos. Med. Assoc. 2009, 108, 87–101. [Google Scholar] [CrossRef]
- Jakob, R.P.; Franz, T.; Gautier, E.; Mainil-Varlet, P. Autologous osteochondral grafting in the knee: Indication, results, and reflections. Clin. Orthop. Relat. Res. 2002, 401, 170–184. [Google Scholar] [CrossRef]
- Chen, C.H.; Kuo, C.Y.; Wang, Y.J.; Chen, J.P. Dual function of glucosamine in gelatin/hyaluronic acid cryogel to modulate scaffold mechanical properties and to maintain chondrogenic phenotype for cartilage tissue engineering. Int. J. Mol. Sci. 2016, 17. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, D.H.; Carelli, E.; Steffen, T.; Jarzem, P.; Haglund, L. 3D-printed ABS and PLA scaffolds for cartilage and nucleus pulposus tissue regeneration. Int. J. Mol. Sci. 2015, 16, 15118–15135. [Google Scholar] [CrossRef] [PubMed]
- Demarteau, O.; Wendt, D.; Braccini, A.; Jakob, M.; Schafer, D.; Heberer, M.; Martin, I. Dynamic compression of cartilage constructs engineered from expanded human articular chondrocytes. Biochem. Biophys. Res. Commun. 2003, 310, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Sadoshima, J.; Takahashi, T.; Jahn, L.; Izumo, S. Roles of mechano-sensitive ion channels, cytoskeleton, and contractile activity in stretch-induced immediate-early gene expression and hypertrophy of cardiac myocytes. Proc. Natl. Acad. Sci. USA 1992, 89, 9905–9909. [Google Scholar] [CrossRef] [PubMed]
- Leipzig, N.D.; Athanasiou, K.A. Static compression of single chondrocytes catabolically modifies single-cell gene expression. Biophys. J. 2008, 94, 2412–2422. [Google Scholar] [CrossRef] [PubMed]
- Herberhold, C.; Faber, S.; Stammberger, T.; Steinlechner, M.; Putz, R.; Englmeier, K.H.; Reiser, M.; Eckstein, F. In situ measurement of articular cartilage deformation in intact femoropatellar joints under static loading. J. Biomech. 1999, 32, 1287–1295. [Google Scholar] [CrossRef]
- Grad, S.; Eglin, D.; Alini, M.; Stoddart, M.J. Physical stimulation of chondrogenic cells in vitro: A review. Clin. Orthop. Relat. Res. 2011, 469, 2764–2772. [Google Scholar] [CrossRef] [PubMed]
- Sah, R.L.; Kim, Y.J.; Doong, J.Y.; Grodzinsky, A.J.; Plaas, A.H.; Sandy, J.D. Biosynthetic response of cartilage explants to dynamic compression. J. Orthop. Res. 1989, 7, 619–636. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.A.; Bader, D.L. Compressive strains at physiological frequencies influence the metabolism of chondrocytes seeded in agarose. J. Orthop. Res. 1997, 15, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Shelton, J.C.; Bader, D.L.; Lee, D.A. Mechanical conditioning influences the metabolic response of cell-seeded constructs. Cells Tissues Organs 2003, 175, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Kisiday, J.D.; Jin, M.; DiMicco, M.A.; Kurz, B.; Grodzinsky, A.J. Effects of dynamic compressive loading on chondrocyte biosynthesis in self-assembling peptide scaffolds. J. Biomech. 2004, 37, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Davisson, T.; Kunig, S.; Chen, A.; Sah, R.; Ratcliffe, A. Static and dynamic compression modulate matrix metabolism in tissue engineered cartilage. J. Orthop. Res. 2002, 20, 842–848. [Google Scholar] [CrossRef]
- Kuo, C.Y.; Chen, C.H.; Hsiao, C.Y.; Chen, J.P. Incorporation of chitosan in biomimetic gelatin/chondroitin-6-sulfate/hyaluronan cryogel for cartilage tissue engineering. Carbohydr. Polym. 2015, 117, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Dreier, R.; Gopferich, A.; Grifka, J.; Grassel, S. Soluble signalling factors derived from differentiated cartilage tissue affect chondrogenic differentiation of rat adult marrow stromal cells. Cell Physiol. Biochem. 2007, 20, 665–678. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Kandel, R.A. In vitro cartilage tissue formation by co-culture of primary and passaged chondrocytes. Tissue Eng. 2007, 13, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sun, H.; Yan, D.; Zhang, L.; Lv, X.; Liu, T.; Zhang, W.; Liu, W.; Cao, Y.; Zhou, G. In vivo ectopic chondrogenesis of bmscs directed by mature chondrocytes. Biomaterials 2010, 31, 9406–9414. [Google Scholar] [CrossRef] [PubMed]
- Grodzinsky, A.J.; Levenston, M.E.; Jin, M.; Frank, E.H. Cartilage tissue remodeling in response to mechanical forces. Ann. Rev. Biomed. Eng. 2000, 2, 691–713. [Google Scholar] [CrossRef] [PubMed]
- Wright, M.O.; Nishida, K.; Bavington, C.; Godolphin, J.L.; Dunne, E.; Walmsley, S.; Jobanputra, P.; Nuki, G.; Salter, D.M. Hyperpolarisation of cultured human chondrocytes following cyclical pressure-induced strain: Evidence of a role for α5β1 integrin as a chondrocyte mechanoreceptor. J. Orthop. Res. 1997, 15, 742–747. [Google Scholar] [CrossRef] [PubMed]
- Hunter, C.J.; Mouw, J.K.; Levenston, M.E. Dynamic compression of chondrocyte-seeded fibrin gels: Effects on matrix accumulation and mechanical stiffness. Osteoarthr. Cartil. 2004, 12, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Waldman, S.D.; Spiteri, C.G.; Grynpas, M.D.; Pilliar, R.M.; Kandel, R.A. Long-term intermittent compressive stimulation improves the composition and mechanical properties of tissue-engineered cartilage. Tissue Eng. 2004, 10, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.E.; Schrobback, K.; Hutmacher, D.W.; Klein, T.J. Dynamic compression improves biosynthesis of human zonal chondrocytes from osteoarthritis patients. Osteoarthr. Cartil. 2012, 20, 906–915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albro, M.B.; Nims, R.J.; Cigan, A.D.; Yeroushalmi, K.J.; Shim, J.J.; Hung, C.T.; Ateshian, G.A. Dynamic mechanical compression of devitalized articular cartilage does not activate latent TGF-β. J. Biomech. 2013, 46, 1433–1439. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, I.; Weigel, C.A.; Bryant, S.J. Cell-matrix interactions and dynamic mechanical loading influence chondrocyte gene expression and bioactivity in PEG-RGD hydrogels. Acta Biomater. 2009, 5, 2832–2846. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, T.T.; Appleby, R.N.; Salter, D.M.; Bader, D.A.; Lee, D.A. Integrin-mediated mechanotransduction in IL-1β stimulated chondrocytes. Biomech. Model. Mechanobiol. 2006, 5, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Xing, Q.; Yates, K.; Vogt, C.; Qian, Z.; Frost, M.C.; Zhao, F. Increasing mechanical strength of gelatin hydrogels by divalent metal ion removal. Sci. Rep. 2014, 4, 4706. [Google Scholar] [CrossRef] [PubMed]
- Mesallati, T.; Buckley, C.T.; Nagel, T.; Kelly, D.J. Scaffold architecture determines chondrocyte response to externally applied dynamic compression. Biomech. Model. Mechanobiol. 2013, 12, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The basic science of articular cartilage: Structure, composition, and function. Sports Health 2009, 1, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Vinatier, C.; Mrugala, D.; Jorgensen, C.; Guicheux, J.; Noel, D. Cartilage engineering: A crucial combination of cells, biomaterials and biofactors. Trends Biotechnol. 2009, 27, 307–314. [Google Scholar] [CrossRef] [PubMed]
- De Croos, J.N.; Dhaliwal, S.S.; Grynpas, M.D.; Pilliar, R.M.; Kandel, R.A. Cyclic compressive mechanical stimulation induces sequential catabolic and anabolic gene changes in chondrocytes resulting in increased extracellular matrix accumulation. Matrix Biol. 2006, 25, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.Q.; Chen, Q. Mechanoregulation of chondrocyte proliferation, maturation, and hypertrophy: Ion-channel dependent transduction of matrix deformation signals. Exp. Cell Res. 2000, 256, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Vezeridis, P.S.; Nicholas, B.; Crisco, J.J.; Moore, D.C.; Chen, Q. Differential expression of type X collagen in a mechanically active 3-D chondrocyte culture system: A quantitative study. J. Orthop. Surg. Res. 2006, 1, 15. [Google Scholar] [CrossRef] [PubMed]
- Walker, G.D.; Fischer, M.; Gannon, J.; Thompson, R.C., Jr.; Oegema, T.R., Jr. Expression of type-X collagen in osteoarthritis. J. Orthop. Res. 1995, 13, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, S.; Joosten, L.A.B.; Bendele, A.M.; Edwards Iii, C.K.; Arntz, O.J.; Helsen, M.M.A.; van de Loo, F.A.J.; van den Berg, W.B. Different roles of tumour necrosis factor α and interleukin 1 in murine streptococcal cell wall arthritis. Cytokine 1998, 10, 690–702. [Google Scholar] [CrossRef] [PubMed]
- Lotz, M.; Hashimoto, S.; Kühn, K. Mechanisms of chondrocyte apoptosis. Osteoarthr. Cartil. 1999, 7, 389–391. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, T.; Kon, T.; Einhorn, T.A.; Gerstenfeld, L.C. Induction of apoptosis in chondrocytes by tumor necrosis factor-α. J. Orthop. Res. 2001, 19, 785–796. [Google Scholar] [CrossRef]
- Long, P.; Gassner, R.; Agarwal, S. Tumor necrosis factor α-dependent proinflammatory gene induction is inhibited by cyclic tensile strain in articular chondrocytes in vitro. Arthritis Rheum. 2001, 44, 2311–2319. [Google Scholar] [CrossRef]
- Lian, J.B.; Stein, G.S. Concepts of osteoblast growth and differentiation: Basis for modulation of bone cell development and tissue formation. Crit. Rev. Oral. Biol. Med. 1992, 3, 269–305. [Google Scholar] [CrossRef] [PubMed]
- Madry, H.; Kaul, G.; Cucchiarini, M.; Stein, U.; Zurakowski, D.; Remberger, K.; Menger, M.D.; Kohn, D.; Trippel, S.B. Enhanced repair of articular cartilage defects in vivo by transplanted chondrocytes overexpressing insulin-like growth factor I (IGF-I). Gene Ther. 2005, 12, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- Yaeger, P.C.; Masi, T.L.; de Ortiz, J.L.; Binette, F.; Tubo, R.; McPherson, J.M. Synergistic action of transforming growth factor-β and insulin-like growth factor-i induces expression of type ii collagen and aggrecan genes in adult human articular chondrocytes. Exp. Cell Res. 1997, 237, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Maeda, S.; Nishida, J.; Sato, T.; Inomata, Y.; Shimamura, T.; Horiuchi, S. Changes in microstructure and gene expression of articular chondrocytes cultured in a tube under mechanical stress. Osteoarthr. Cartil. 2005, 13, 154–161. [Google Scholar] [CrossRef] [PubMed]
- McQuillan, D.J.; Handley, C.J.; Campbell, M.A.; Bolis, S.; Milway, V.E.; Herington, A.C. Stimulation of proteoglycan biosynthesis by serum and insulin-like growth factor-i in cultured bovine articular cartilage. Biochem. J. 1986, 240, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Bohme, K.; Conscience-Egli, M.; Tschan, T.; Winterhalter, K.H.; Bruckner, P. Induction of proliferation or hypertrophy of chondrocytes in serum-free culture: The role of insulin-like growth factor-I, insulin, or thyroxine. J. Cell. Biol. 1992, 116, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Fox, D.B.; Cook, J.L.; Kuroki, K.; Cockrell, M. Effects of dynamic compressive load on collagen-based scaffolds seeded with fibroblast-like synoviocytes. Tissue Eng. 2006, 12, 1527–1537. [Google Scholar] [CrossRef] [PubMed]
- Bian, L.; Fong, J.V.; Lima, E.G.; Stoker, A.M.; Ateshian, G.A.; Cook, J.L.; Hung, C.T. Dynamic mechanical loading enhances functional properties of tissue-engineered cartilage using mature canine chondrocytes. Tissue Eng. Part A 2010, 16, 1781–1790. [Google Scholar] [CrossRef] [PubMed]
- Hubka, K.M.; Dahlin, R.L.; Meretoja, V.V.; Kasper, F.K.; Mikos, A.G. Enhancing chondrogenic phenotype for cartilage tissue engineering: Monoculture and coculture of articular chondrocytes and mesenchymal stem cells. Tissue Eng. Part B 2014, 20, 641–654. [Google Scholar] [CrossRef] [PubMed]
- Worster, A.A.; Brower-Toland, B.D.; Fortier, L.A.; Bent, S.J.; Williams, J.; Nixon, A.J. Chondrocytic differentiation of mesenchymal stem cells sequentially exposed to transforming growth factor-β1 in monolayer and insulin-like growth factor-I in a three-dimensional matrix. J. Orthop. Res. 2001, 19, 738–749. [Google Scholar] [CrossRef]
- Longobardi, L.; O’Rear, L.; Aakula, S.; Johnstone, B.; Shimer, K.; Chytil, A.; Horton, W.A.; Moses, H.L.; Spagnoli, A. Effect of IGF-I in the chondrogenesis of bone marrow mesenchymal stem cells in the presence or absence of TGF-β signaling. J. Bone Min. Res. 2006, 21, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.H.; Kajiyama, G.; Smith, R.L.; Maloney, W.; Yang, F. Stem cells catalyze cartilage formation by neonatal articular chondrocytes in 3D biomimetic hydrogels. Sci. Rep. 2013, 3, 3553. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Hagar, K.L.; Frost, L.E.; Sun, Y.; Cheung, H.S. Effects of cyclic compressive loading on chondrogenesis of rabbit bone-marrow derived mesenchymal stem cells. Stem Cells 2004, 22, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Mouw, J.K.; Connelly, J.T.; Wilson, C.G.; Michael, K.E.; Levenston, M.E. Dynamic compression regulates the expression and synthesis of chondrocyte-specific matrix molecules in bone marrow stromal cells. Stem Cells 2007, 25, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ke, J.; Long, X.; Meng, Q.; Deng, M.; Fang, W.; Li, J.; Cai, H.; Chen, S. Insulin-like growth factor-1 boosts the developing process of condylar hyperplasia by stimulating chondrocytes proliferation. Osteoarthr. Cartil. 2012, 20, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Griffon, D.J.; Sedighi, M.R.; Schaeffer, D.V.; Eurell, J.A.; Johnson, A.L. Chitosan scaffolds: Interconnective pore size and cartilage engineering. Acta Biomater. 2006, 2, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Shyu, V.B.; Chen, J.P.; Lee, M.Y. Selective laser sintered poly-epsilon-caprolactone scaffold hybridized with collagen hydrogel for cartilage tissue engineering. Biofabrication 2014, 6. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.A.; Han, J.; Kim, B.S. Stimulation of chondrogenic differentiation of mesenchymal stem cells. Int. J. Stem Cells 2012, 5, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Somoza, R.A.; Welter, J.F.; Correa, D.; Caplan, A.I. Chondrogenic differentiation of mesenchymal stem cells: Challenges and unfulfilled expectations. Tissue Eng. Part B Rev. 2014, 20, 596–608. [Google Scholar] [CrossRef] [PubMed]
- O’Conor, C.J.; Case, N.; Guilak, F. Mechanical regulation of chondrogenesis. Stem Cell Res. Ther. 2013, 4, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, C.C.; Chiang, H.; Liao, C.J.; Lin, Y.J.; Kuo, T.F.; Shieh, C.S.; Huang, Y.Y.; Tuan, R.S. Repair of porcine articular cartilage defect with a biphasic osteochondral composite. J. Orthop. Res. 2007, 25, 1277–1290. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.H.; Liao, H.T.; Chen, J.P. Preparation and characterization of gelatin/hyaluronic acid cryogels for adipose tissue engineering: In vitro and in vivo studies. Acta Biomater. 2013, 9, 9012–9026. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Sah, R.L.Y.; Doong, J.-Y.H.; Grodzinsky, A.J. Fluorometric assay of DNA in cartilage explants using hoechst 33258. Anal. Biochem. 1988, 174, 168–176. [Google Scholar] [CrossRef]
- Enobakhare, B.O.; Bader, D.L.; Lee, D.A. Quantification of sulfated glycosaminoglycans in chondrocyte/alginate cultures, by use of 1,9-dimethylmethylene blue. Anal Biochem. 1996, 243, 189–191. [Google Scholar] [CrossRef] [PubMed]


| Gene | Primer Sequence | |
|---|---|---|
| β-actin | forward | AAGCCAACCGTGAGAAGATG |
| reverse | GTACATGGCTGGGGTGTTG | |
| Col I | forward | CCAACAAGGCCAAGAAGAAG |
| reverse | ATGGTACCTGAGGCCGTTCT | |
| Col II | forward | GCACGGATGGTCCCAAAG |
| reverse | CAGCAGCTCCCCTCTCAC | |
| Col X | forward | CACCAAGGCACAGTTCTTCA |
| reverse | ACCGGAATACCTTGCTCTC | |
| TNF-α | forward | CCCTTCCACCAACGTTTTCCT |
| reverse | TGATGGCAGAGAGGAGGTTG | |
| TGF-β1 | forward | TTTCGCCTCAGTGCCCA |
| reverse | GCCAGAATTGAACCCGTTAA | |
| IGF-1 | forward | TTCGCATCTCTTCTACTTGGCCCT |
| reverse | CGTACCCTGTGGGCTTGTTGAAAT | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-H.; Kuo, C.-Y.; Chen, J.-P. Effect of Cyclic Dynamic Compressive Loading on Chondrocytes and Adipose-Derived Stem Cells Co-Cultured in Highly Elastic Cryogel Scaffolds. Int. J. Mol. Sci. 2018, 19, 370. https://doi.org/10.3390/ijms19020370
Chen C-H, Kuo C-Y, Chen J-P. Effect of Cyclic Dynamic Compressive Loading on Chondrocytes and Adipose-Derived Stem Cells Co-Cultured in Highly Elastic Cryogel Scaffolds. International Journal of Molecular Sciences. 2018; 19(2):370. https://doi.org/10.3390/ijms19020370
Chicago/Turabian StyleChen, Chih-Hao, Chang-Yi Kuo, and Jyh-Ping Chen. 2018. "Effect of Cyclic Dynamic Compressive Loading on Chondrocytes and Adipose-Derived Stem Cells Co-Cultured in Highly Elastic Cryogel Scaffolds" International Journal of Molecular Sciences 19, no. 2: 370. https://doi.org/10.3390/ijms19020370
APA StyleChen, C.-H., Kuo, C.-Y., & Chen, J.-P. (2018). Effect of Cyclic Dynamic Compressive Loading on Chondrocytes and Adipose-Derived Stem Cells Co-Cultured in Highly Elastic Cryogel Scaffolds. International Journal of Molecular Sciences, 19(2), 370. https://doi.org/10.3390/ijms19020370