How Do We Study the Dynamic Structure of Unstructured Proteins: A Case Study on Nopp140 as an Example of a Large, Intrinsically Disordered Protein
Abstract
:1. Introduction
2. Molecular Mechanisms of IDPs: Regulation of the Target Protein by Specific Interaction
3. Conformational Study of IDPs Using Experimental Techniques
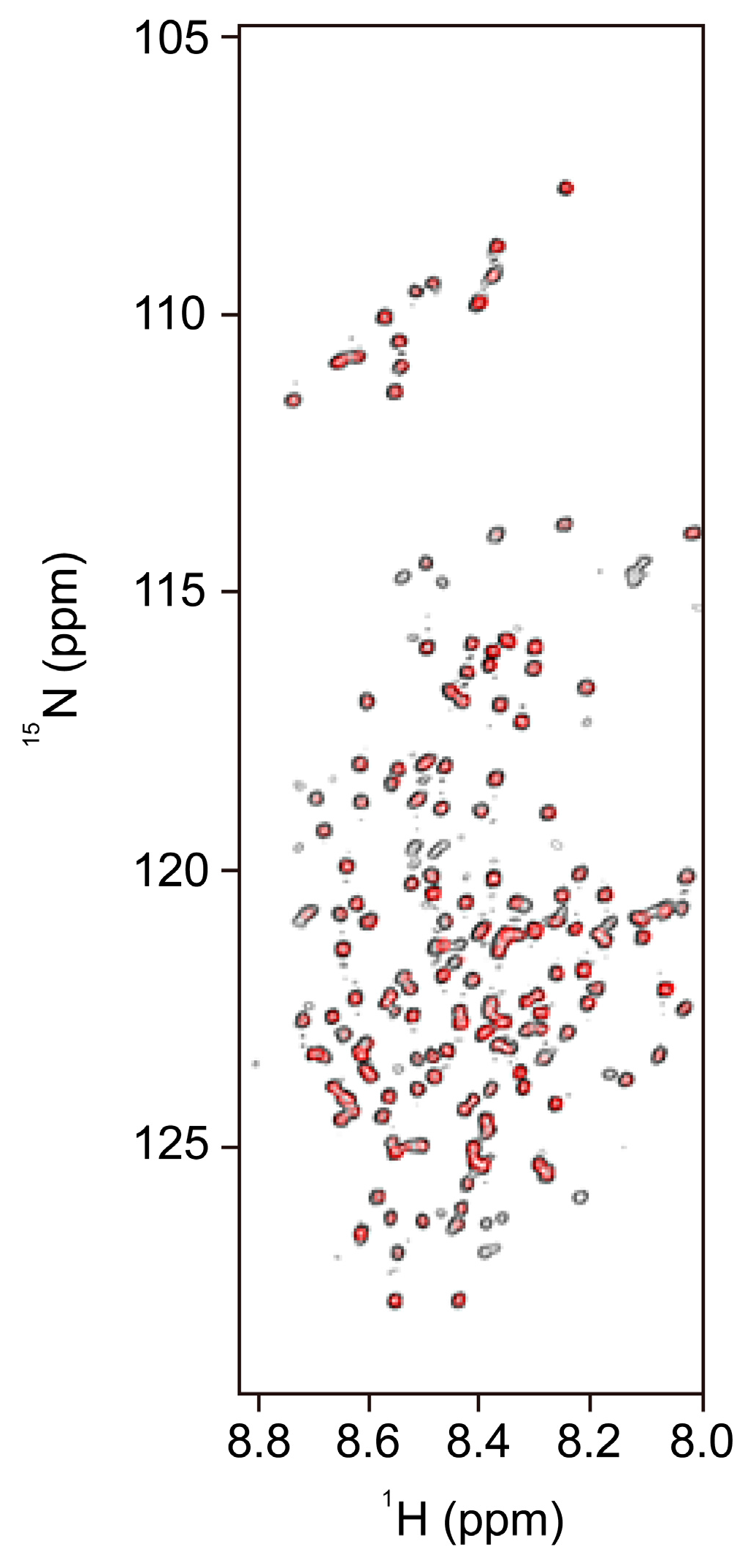
3.1. NMR Spectroscopy
3.2. EPR Spectroscopy
3.3. CD Spectroscopy
3.4. Single-Molecule Fluorescence Resonance Energy Transfer
4. Nopp140 as a Novel Class of IDP
4.1. Interaction Between Nopp140 and CK2
4.2. Biophysical Study of Nopp140
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Van der Lee, R.; Buljan, M.; Lang, B.; Weatheritt, R.J.; Daughdrill, G.W.; Dunker, A.K.; Fuxreiter, M.; Gough, J.; Gsponer, J.; Jones, D.T.; et al. Classification of Intrinsically Disordered Regions and Proteins. Chem. Rev. 2014, 114, 6589–6631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunker, A.K.; Obradovic, Z.; Romero, P.; Garner, E.C.; Brown, C.J. Intrinsic protein disorder in complete genomes. Genome inform. Workshop Genome Inform. 2000, 11, 161–171. [Google Scholar]
- Wang Jabs, E.; Li, X.; Coss, C.A.; Taylor, E.W.; Meyers, D.A.; Weber, J.L. Mapping the Treacher Collins syndrome locus to 5q31.3→q33.3. Genomics 1991, 11, 193–198. [Google Scholar] [CrossRef]
- Oldfield, C.J.; Dunker, A.K. Intrinsically disordered proteins and intrinsically disordered protein regions. Ann. Rev. Biochem. 2014, 83, 553–584. [Google Scholar] [CrossRef] [PubMed]
- Dosztanyi, Z.; Meszaros, B.; Simon, I. Bioinformatical approaches to characterize intrinsically disordered/unstructured proteins. Brief. Bioinform. 2010, 11, 225–243. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, P.H.; Zhen, W.; Poon, A.W.; Conway, K.A.; Lansbury, P.T. NACP, A Protein Implicated in Alzheimer’s Disease and Learning, Is Natively Unfolded. Biochemistry 1996, 35, 13709–13715. [Google Scholar] [CrossRef] [PubMed]
- Dyson, H.J.; Wright, P.E. Intrinsically unstructured proteins and their functions. Nat. Rev. Mol. Cell Biol. 2005, 6, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Sharma, N.; Giri, R. Therapeutic Interventions of Cancers Using Intrinsically Disordered Proteins as Drug Targets: c-Myc as Model System. Cancer Informatics 2017, 16, 1176935117699408. [Google Scholar] [CrossRef] [PubMed]
- Dosztanyi, Z.; Csizmok, V.; Tompa, P.; Simon, I. IUPred: web server for the prediction of intrinsically unstructured regions of proteins based on estimated energy content. Bioinformatics 2005, 21, 3433–3434. [Google Scholar] [CrossRef] [PubMed]
- Dunker, A.K.; Lawson, J.D.; Brown, C.J.; Williams, R.M.; Romero, P.; Oh, J.S.; Oldfield, C.J.; Campen, A.M.; Ratliff, C.M.; Hipps, K.W.; et al. Intrinsically disordered protein. J. Mol. Graph. Model. 2001, 19, 26–59. [Google Scholar] [CrossRef]
- Moll, U.M.; Petrenko, O. The MDM2-p53 interaction. Mol. Cancer Res. 2003, 1, 1001–1008. [Google Scholar] [PubMed]
- Kussie, P.H.; Gorina, S.; Marechal, V.; Elenbaas, B.; Moreau, J.; Levine, A.J.; Pavletich, N.P. Structure of the MDM2 oncoprotein bound to the p53 tumor suppressor transactivation domain. Science 1996, 274, 948–953. [Google Scholar] [CrossRef] [PubMed]
- Pomeranz Krummel, D.A.; Oubridge, C.; Leung, A.K.W.; Li, J.; Nagai, K. Crystal structure of human spliceosomal U1 snRNP at 5.5 Å resolution. Nature 2009, 458, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Eliezer, D. Biophysical characterization of intrinsically disordered proteins. Curr. Opin. Struct. Biol. 2009, 19, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Konrat, R. NMR contributions to structural dynamics studies of intrinsically disordered proteins. J. Magn. Reson. 2014, 241, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, E.B.; Cook, E.C.; Showalter, S.A. Application of NMR to studies of intrinsically disordered proteins. Arch. Biochem. Biophys. 2017, 628, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Berjanskii, M.; Wishart, D.S. NMR: prediction of protein flexibility. Nat. Protoc. 2006, 1, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.K.; Lee, S.Y.; Na, J.H.; Jang, S.; Park, C.R.; Kim, S.Y.; Lee, S.H.; Han, K.; Yu, Y.G. Mitoxantrone Binds to Nopp140, an Intrinsically Unstructured Protein, and Modulate its Interaction with Protein Kinase CK2. Bull Korean Chem. Soc. 2012, 33, 2005–2011. [Google Scholar] [CrossRef]
- Kim, D.H.; Lee, C.; Lee, S.H.; Kim, K.T.; Han, J.J.; Cha, E.J.; Lim, J.E.; Cho, Y.J.; Hong, S.H.; Han, K.H. The Mechanism of p53 Rescue by SUSP4. Angew. Chem. 2017, 56, 1278–1282. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Wright, A.; Han, K.H. An NMR study on the intrinsically disordered core transactivation domain of human glucocorticoid receptor. BMB Rep. 2017, 50, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Arai, M.; Sugase, K.; Dyson, H.J.; Wright, P.E. Conformational propensities of intrinsically disordered proteins influence the mechanism of binding and folding. Proc. Natl. Acad. Sci. USA 2015, 112, 9614–9619. [Google Scholar] [CrossRef] [PubMed]
- Bermel, W.; Bertini, I.; Chill, J.; Felli, I.C.; Haba, N.; Kumar, M.V.V.; Pierattelli, R. Exclusively heteronuclear (13)C-detected amino-acid-selective NMR experiments for the study of intrinsically disordered proteins (IDPs). ChemBioChem 2012, 13, 2425–2432. [Google Scholar] [CrossRef] [PubMed]
- Hubbell, W.L.; Altenbach, C. Investigation of structure and dynamics in membrane proteins using site-directed spin labeling. Curr. Opin. Struct. Biol. 1994, 4, 566–573. [Google Scholar] [CrossRef]
- Hubbell, W.L.; Gross, A.; Langen, R.; Lietzow, M.A. Recent advances in site-directed spin labeling of proteins. Curr. Opin. Struct. Biol. 1998, 8, 649–656. [Google Scholar] [CrossRef]
- Drescher, M. EPR in protein science : Intrinsically disordered proteins. Top. Curr. Chem. 2012, 321, 91–119. [Google Scholar] [PubMed]
- McHaourab, H.S.; Lietzow, M.A.; Hideg, K.; Hubbell, W.L. Motion of Spin-Labeled Side Chains in T4 Lysozyme. Correlation with Protein Structure and Dynamics. Biochemistry 1996, 35, 7692–7704. [Google Scholar] [CrossRef] [PubMed]
- Na, J.H.; Lee, W.K.; Kim, Y.; Jeong, C.; Song, S.S.; Cha, S.S.; Han, K.H.; Shin, Y.K.; Yu, Y.G. Biophysical characterization of the structural change of Nopp140, an intrinsically disordered protein, in the interaction with CK2α. Biochem. Biophys. Res. Commun. 2016, 477, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Morin, B.; Bourhis, J.-M.; Belle, V.; Woudstra, M.; Carrière, F.; Guigliarelli, B.; Fournel, A.; Longhi, S. Assessing induced folding of an intrinsically disordered protein by site-directed spin-labeling electron paramagnetic resonance spectroscopy. J. Phys. Chem. B 2006, 110, 20596–20608. [Google Scholar] [CrossRef] [PubMed]
- Belle, V.; Rouger, S.; Costanzo, S.; Liquiere, E.; Strancar, J.; Guigliarelli, B.; Fournel, A.; Longhi, S. Mapping alpha-helical induced folding within the intrinsically disordered C-terminal domain of the measles virus nucleoprotein by site-directed spin-labeling EPR spectroscopy. Proteins 2008, 73, 973–988. [Google Scholar] [CrossRef] [PubMed]
- Török, M.; Milton, S.; Kayed, R.; Wu, P.; McIntire, T.; Glabe, C.G.; Langen, R. Structural and Dynamic Features of Alzheimer’s Aβ Peptide in Amyloid Fibrils Studied by Site-directed Spin Labeling. J. Biol. Chem. 2002, 277, 40810–40815. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Hara, H.; Masuda, Y.; Ohigashi, H.; Irie, K. Distance measurement between Tyr10 and Met35 in amyloid beta by site-directed spin-labeling ESR spectroscopy: Implications for the stronger neurotoxicity of Abeta42 than Abeta40. Chembiochem 2007, 8, 2308–2314. [Google Scholar] [CrossRef] [PubMed]
- Ionut Iurascu, M.; Cozma, C.; Tomczyk, N.; Rontree, J.; Desor, M.; Drescher, M.; Przybylski, M. Structural characterization of beta-amyloid oligomer-aggregates by ion mobility mass spectrometry and electron spin resonance spectroscopy. Anal. Bioanal. Chem. 2009, 395, 2509–2519. [Google Scholar] [CrossRef] [PubMed]
- Sepkhanova, I.; Drescher, M.; Meeuwenoord, N.J.; Limpens, R.W.; Koning, R.I.; Filippov, D.V.; Huber, M. Monitoring Alzheimer Amyloid Peptide Aggregation by EPR. Appl. Magn. Reson. 2009, 36, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Drescher, M.; Godschalk, F.; Veldhuis, G.; van Rooijen, B.D.; Subramaniam, V.; Huber, M. Spin-label EPR on alpha-synuclein reveals differences in the membrane binding affinity of the two antiparallel helices. Chembiochem 2008, 9, 2411–2416. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.C. Jr. Protein secondary structure and circular dichroism: a practical guide. Proteins 1990, 7, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Maestro, B.; Galán, B.; Alfonso, C.; Rivas, G.; Prieto, M.A.; Sanz, J.M. A New Family of Intrinsically Disordered Proteins: Structural Characterization of the Major Phasin PhaF from Pseudomonas putida KT2440. PLoS ONE 2013, 8, e56904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balu, R.; Knott, R.; Cowieson, N.P.; Elvin, C.M.; Hill, A.J.; Choudhury, N.R.; Dutta, N.K. Structural ensembles reveal intrinsic disorder for the multi-stimuli responsive bio-mimetic protein Rec1-resilin. Sci. Rep. 2015, 5, 10896. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, K.; Salladini, E.; O’Brien, D.P.; Brier, S.; Chenal, A.; Yacoubi, I.; Longhi, S. Structural disorder and induced folding within two cereal, ABA stress and ripening (ASR) proteins. Sci. Rep. 2017, 7, 15544. [Google Scholar] [CrossRef] [PubMed]
- Chemes, L.B.; Alonso, L.G.; Noval, M.G.; de Prat-Gay, G. Circular dichroism techniques for the analysis of intrinsically disordered proteins and domains. In Intrinsically Disordered Protein Analysis: Volume 1, Methods and Experimental Tools; Uversky, V.N., Dunker, A.K., Eds.; Humana Press: Totowa, NJ, USA, 2012; pp. 387–404. [Google Scholar]
- Tompa, P.; Fersht, A. Structure and Function of Intrinsically Disordered Proteins; CRC Press: Boca Raton, FL, USA, 2009; pp. 126–127. [Google Scholar]
- Brucale, M.; Schuler, B.; Samorì, B. Single-Molecule Studies of Intrinsically Disordered Proteins. Chem. Rev. 2014, 114, 3281–3317. [Google Scholar] [CrossRef] [PubMed]
- Gambin, Y.; VanDelinder, V.; Ferreon, A.C.M.; Lemke, E.A.; Groisman, A.; Deniz, A.A. Visualizing a one-way protein encounter complex by ultrafast single-molecule mixing. Nat. Methods 2011, 8, 239. [Google Scholar] [CrossRef] [PubMed]
- Lamboy, J.A.; Kim, H.; Dembinski, H.; Ha, T.; Komives, E.A. Single-molecule FRET reveals the native-state dynamics of the IkappaBalpha ankyrin repeat domain. J. Mol. Biol. 2013, 425, 2578–2590. [Google Scholar] [CrossRef] [PubMed]
- Ferreon, A.C.M.; Gambin, Y.; Lemke, E.A.; Deniz, A.A. Interplay of α-synuclein binding and conformational switching probed by single-molecule fluorescence. Proc. Natl. Acad. Sci. USA 2009, 106, 5645–5650. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Krishnan, R.; Lemke, E.A.; Lindquist, S.; Deniz, A.A. A natively unfolded yeast prion monomer adopts an ensemble of collapsed and rapidly fluctuating structures. Proc. Natl. Acad. Sci. USA 2007, 104, 2649–2654. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Moran-Gutierrez, C.R.; Deniz, A.A. Probing protein disorder and complexity at single-molecule resolution. Semin. Cell Dev. Biol. 2015, 37 (Supplement C), 26–34. [Google Scholar] [CrossRef] [PubMed]
- Milles, S.; Koehler, C.; Gambin, Y.; Deniz, A.A.; Lemke, E.A. Intramolecular three-colour single pair FRET of intrinsically disordered proteins with increased dynamic range. Mol. Biosyst. 2012, 8, 2531–2534. [Google Scholar] [CrossRef] [PubMed]
- Mujumdar, R.B.; Ernst, L.A.; Mujumdar, S.R.; Lewis, C.J.; Waggoner, A.S. Cyanine dye labeling reagents: sulfoindocyanine succinimidyl esters. Bioconjug. Chem. 1993, 4, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Panchuk-Voloshina, N.; Haugland, R.P.; Bishop-Stewart, J.; Bhalgat, M.K.; Millard, P.J.; Mao, F.; Leung, W.Y.; Haugland, R.P. Alexa dyes, a series of new fluorescent dyes that yield exceptionally bright, photostable conjugates. J. Histochem. Cytochem. 1999, 47, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Isaac, C.; Yang, Y.; Thomas Meier, U. Nopp140 Functions as a Molecular Link Between the Nucleolus and the Coiled Bodies. J. Cell Biol. 1998, 142, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Meier, U.T.; Blobel, G. A nuclear localization signal binding protein in the nucleolus. J. Cell Biol. 1990, 111, 2235–2245. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Isaac, C.; Wang, C.; Dragon, F.; Pogac̆ić, V.; Meier, U.T. Conserved composition of mammalian box H/ACA and box C/D small nucleolar ribonucleoprotein particles and their interaction with the common factor Nopp140. Mol. Biol. Cell 2000, 11, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Meier, U.T.; Blobel, G. NAP57, a mammalian nucleolar protein with a putative homolog in yeast and bacteria. J. Cell Biol. 1994, 127, 1505–1514. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Meier, U.T.; Dobrowolska, G.; Krebs, E.G. Specific Interaction between Casein Kinase 2 and the Nucleolar Protein Nopp140. J. Biol. Chem. 1997, 272, 3773–3779. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Lee, W.K.; Jin, Y.; Lee, K.J.; Jeon, H.; Yu, Y.G. Doxorubicin binds to un-phosphorylated form of hNopp140 and reduces protein kinase CK2-dependent phosphorylation of hNopp140. J. Biochem. Mol. Biol. 2006, 39, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Henriksson, M.; Lüscher, B. Proteins of the Myc network: essential regulators of cell growth and differentiation. Adv. Cancer Res. 1996, 68, 109–182. [Google Scholar]
- Keller, D.M.; Zeng, X.; Wang, Y.; Zhang, Q.H.; Kapoor, M.; Shu, H.; Goodman, R.; Lozano, G.; Zhao, Y.; Lu, H. A DNA damage–induced p53 serine 392 kinase complex contains CK2, hSpt16, and SSRP1. Mol. Cell 2001, 7, 283–292. [Google Scholar] [CrossRef]
- Kulartz, M.; Kreitz, S.; Hiller, E.; Damoc, E.-C.; Przybylski, M.; Knippers, R. Expression and phosphorylation of the replication regulator protein geminin. Biochem. Biophys. Res. Commun. 2003, 305, 412–420. [Google Scholar] [CrossRef]
- Lin, A.; Frost, J.; Deng, T.; Smeal, T.; Al-Alawi, N.; Kikkawa, U.; Hunter, T.; Brenner, D.; Karin, M. Casein kinase II is a negative regulator of c-Jun DNA binding and AP-1 activity. Cell 1992, 70, 777–789. [Google Scholar] [CrossRef]
- Tawfic, S.; Yu, S.; Wang, H.; Faust, R.; Davis, A.; Ahmed, K. Protein kinase CK2 signal in neoplasia. Histol. Histopathol. 2001, 16, 573–582. [Google Scholar] [PubMed]
- Pai, C.Y.; Chen, H.K.; Sheu, H.L.; Yeh, N.H. Cell-cycle-dependent alterations of a highly phosphorylated nucleolar protein p130 are associated with nucleologenesis. J. Cell Sci. 1995, 108, 1911–1920. [Google Scholar] [PubMed]
- Kim, Y.-K.; Lee, K.J.; Jeon, H.; Yu, Y.G. Protein kinase CK2 is inhibited by human nucleolar phosphoprotein p140 in an inositol hexakisphosphate-dependent manner. J. Biol. Chem. 2006, 281, 36752–36757. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.K.; Lee, S.Y.; Kim, W.I.; Rho, Y.H.; Bae, Y.S.; Lee, C.; Kim, I.Y.; Yu, Y.G. Characterization of the InsP6-dependent interaction between CK2 and Nopp140. Biochem. Biophys. Res. Commun. 2008, 376, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.K.; Son, S.H.; Jin, B.S.; Na, J.H.; Kim, S.Y.; Kim, K.H.; Kim, E.E.; Yu, Y.G.; Lee, H.H. Structural and functional insights into the regulation mechanism of CK2 by IP6 and the intrinsically disordered protein Nopp140. Proc. Natl. Acad. Sci. USA 2013, 110, 19360–19365. [Google Scholar] [CrossRef] [PubMed]





| Disordered protein | Nopp140 | Treacle |
|---|---|---|
| % of disordered-promoting residues | 54.4 | 49.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Na, J.-H.; Lee, W.-K.; Yu, Y.G. How Do We Study the Dynamic Structure of Unstructured Proteins: A Case Study on Nopp140 as an Example of a Large, Intrinsically Disordered Protein. Int. J. Mol. Sci. 2018, 19, 381. https://doi.org/10.3390/ijms19020381
Na J-H, Lee W-K, Yu YG. How Do We Study the Dynamic Structure of Unstructured Proteins: A Case Study on Nopp140 as an Example of a Large, Intrinsically Disordered Protein. International Journal of Molecular Sciences. 2018; 19(2):381. https://doi.org/10.3390/ijms19020381
Chicago/Turabian StyleNa, Jung-Hyun, Won-Kyu Lee, and Yeon Gyu Yu. 2018. "How Do We Study the Dynamic Structure of Unstructured Proteins: A Case Study on Nopp140 as an Example of a Large, Intrinsically Disordered Protein" International Journal of Molecular Sciences 19, no. 2: 381. https://doi.org/10.3390/ijms19020381






