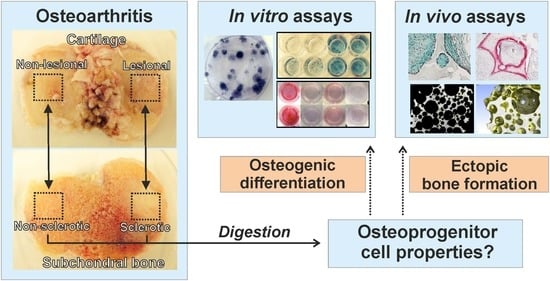
Alterations of Subchondral Bone Progenitor Cells in Human Knee and Hip Osteoarthritis Lead to a Bone Sclerosis Phenotype
Abstract
:1. Introduction
2. Results
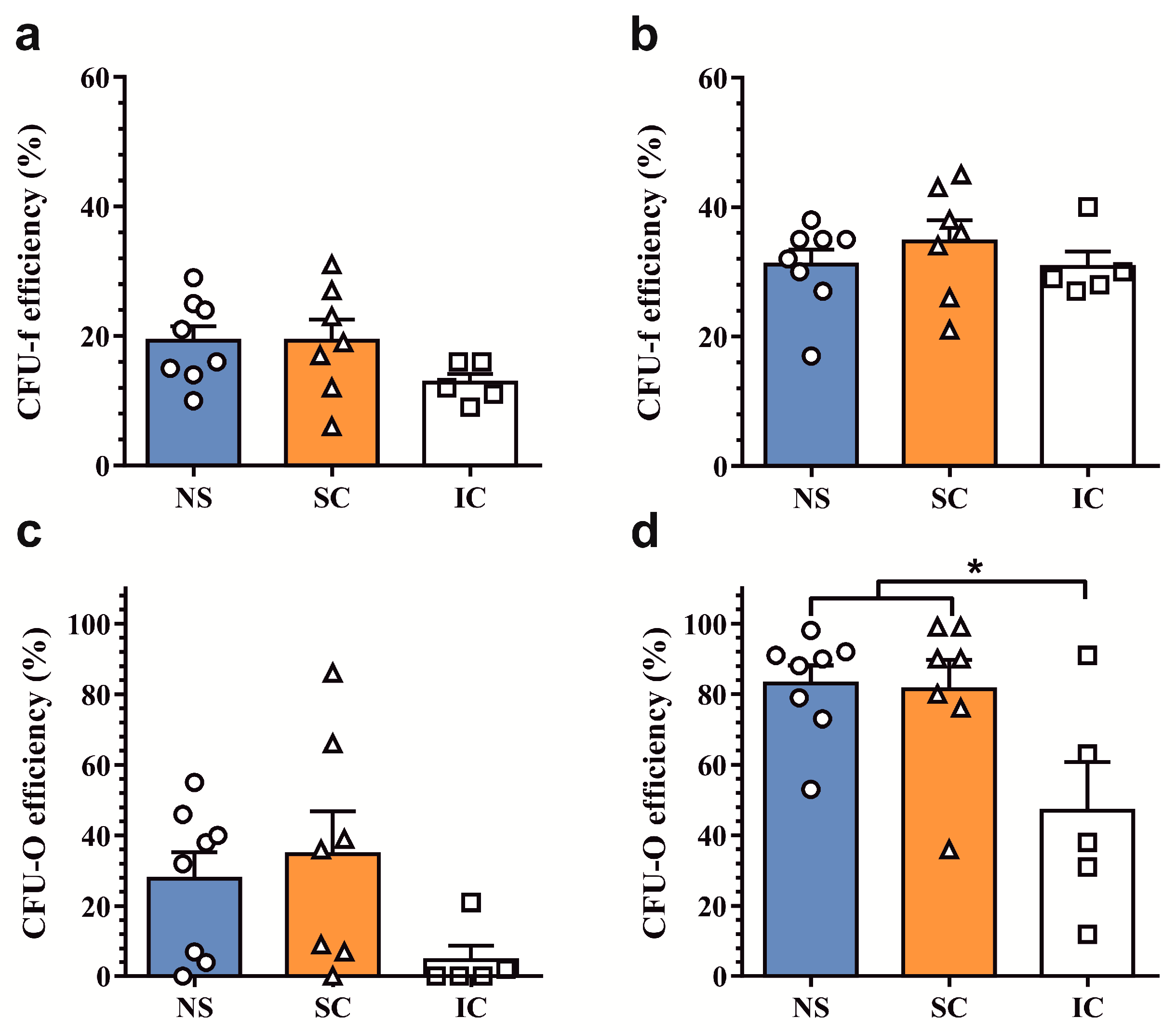
2.1. Assessment of Clonogenic and Osteogenic Potential of Osteoprogenitors from Osteoarthritic Subchondral Bone
2.2. In Vitro Osteogenic Differentiation Properties of Polyclonal Osteoprogenitor Cell Populations
2.3. In Vivo Osteogenic Differentiation Properties of Polyclonal Osteoprogenitor Cell Populations
2.4. Histological Evaluation of De Novo Calcified Tissues
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Enzymatic Release and Culture of Osteoprogenitor Cells
4.3. Colony-Forming Unit Assays
4.4. Osteogenic Differentiation Assays
4.5. Quantitative Alkaline Phosphatase Activity Assay
4.6. Alizarin Red S Staining
4.7. Subcutaneous Implantation Model of Ectopic Bone Formation
4.8. Micro-Computed Tomography Scanning and Analysis
4.9. Histological Analysis
4.10. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| OA | osteoarthritis |
| ALP | alkaline phosphatase |
| BSP | bone sialoprotein |
| CFU | colony forming unit |
References
- Kraus, V.B.; Blanco, F.J.; Englund, M.; Karsdal, M.A.; Lohmander, L.S. Call for standardized definitions of osteoarthritis and risk stratification for clinical trials and clinical use. Osteoarthr. Cartil. 2015, 23, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Hugle, T.; Geurts, J. What drives osteoarthritis?—Synovial versus subchondral bone pathology. Rheumatology 2017, 56, 1461–1471. [Google Scholar] [CrossRef] [PubMed]
- Karsdal, M.A.; Michaelis, M.; Ladel, C.; Siebuhr, A.S.; Bihlet, A.R.; Andersen, J.R.; Guehring, H.; Christiansen, C.; Bay-Jensen, A.C.; Kraus, V.B. Disease-modifying treatments for osteoarthritis (DMOADs) of the knee and hip: Lessons learned from failures and opportunities for the future. Osteoarthr. Cartil. 2016, 24, 2013–2021. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, J.P.; Roubille, C.; Raynauld, J.P.; Abram, F.; Dorais, M.; Delorme, P.; Martel-Pelletier, J. Disease-modifying effect of strontium ranelate in a subset of patients from the Phase III knee osteoarthritis study SEKOIA using quantitative MRI: Reduction in bone marrow lesions protects against cartilage loss. Ann. Rheum. Dis. 2015, 74, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Zhen, G.; Wen, C.; Jia, X.; Li, Y.; Crane, J.L.; Mears, S.C.; Askin, F.B.; Frassica, F.J.; Chang, W.; Yao, J.; et al. Inhibition of TGF-β signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat. Med. 2013, 19, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.L.; Meng, H.Y.; Wang, Y.C.; Peng, J.; Guo, Q.Y.; Wang, A.Y.; Lu, S.B. Bone-cartilage interface crosstalk in osteoarthritis: Potential pathways and future therapeutic strategies. Osteoarthr. Cartil. 2014, 22, 1077–1089. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Q.; Lu, S.; Du, Z.; Friis, T.; Yao, J.; Crawford, R.; Prasadam, I.; Xiao, Y. Characterization of nano-structural and nano-mechanical properties of osteoarthritic subchondral bone. BMC Musculoskelet. Disord. 2016, 17, 367. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J.; Gerstenfeld, L.; Bishop, G.; Davis, A.D.; Mason, Z.D.; Einhorn, T.A.; Maciewicz, R.A.; Newham, P.; Foster, M.; Jackson, S.; et al. Bone marrow lesions from osteoarthritis knees are characterized by sclerotic bone that is less well mineralized. Arthritis Res. Ther. 2009, 11, R11. [Google Scholar] [CrossRef] [PubMed]
- Burr, D.B.; Gallant, M.A. Bone remodelling in osteoarthritis. Nat. Rev. Rheumatol. 2012, 8, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Cox, L.G.; van Donkelaar, C.C.; van Rietbergen, B.; Emans, P.J.; Ito, K. Decreased bone tissue mineralization can partly explain subchondral sclerosis observed in osteoarthritis. Bone 2012, 50, 1152–1161. [Google Scholar] [CrossRef] [PubMed]
- Geurts, J.; Patel, A.; Hirschmann, M.T.; Pagenstert, G.I.; Muller-Gerbl, M.; Valderrabano, V.; Hugle, T. Elevated marrow inflammatory cells and osteoclasts in subchondral osteosclerosis in human knee osteoarthritis. J. Orthop. Res. 2015. [Google Scholar] [CrossRef] [PubMed]
- Abed, E.; Couchourel, D.; Delalandre, A.; Duval, N.; Pelletier, J.P.; Martel-Pelletier, J.; Lajeunesse, D. Low sirtuin 1 levels in human osteoarthritis subchondral osteoblasts lead to abnormal sclerostin expression which decreases Wnt/beta-catenin activity. Bone 2014, 59, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Couchourel, D.; Aubry, I.; Delalandre, A.; Lavigne, M.; Martel-Pelletier, J.; Pelletier, J.P.; Lajeunesse, D. Altered mineralization of human osteoarthritic osteoblasts is attributable to abnormal type I collagen production. Arthritis Rheumtol. 2009, 60, 1438–1450. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.; Deberg, M.A.; Bellahcene, A.; Castronovo, V.; Msika, P.; Delcour, J.P.; Crielaard, J.M.; Henrotin, Y.E. Phenotypic characterization of osteoblasts from the sclerotic zones of osteoarthritic subchondral bone. Arthritis Rheumtol. 2008, 58, 442–455. [Google Scholar] [CrossRef] [PubMed]
- Campbell, T.M.; Churchman, S.M.; Gomez, A.; McGonagle, D.; Conaghan, P.G.; Ponchel, F.; Jones, E. Mesenchymal Stem Cell Alterations in Bone Marrow Lesions in Patients With Hip Osteoarthritis. Arthritis Rheumatol. 2016, 68, 1648–1659. [Google Scholar] [CrossRef] [PubMed]
- Malaval, L.; Wade-Gueye, N.M.; Boudiffa, M.; Fei, J.; Zirngibl, R.; Chen, F.; Laroche, N.; Roux, J.P.; Burt-Pichat, B.; Duboeuf, F.; et al. Bone sialoprotein plays a functional role in bone formation and osteoclastogenesis. J. Exp. Med. 2008, 205, 1145–1153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, E.L.; Edwards, C.J.; Cooper, C.; Oreffo, R.O. The osteoarthritic niche and modulation of skeletal stem cell function for regenerative medicine. J. Tissue Eng. Regen. Med. 2013, 7, 589–608. [Google Scholar] [CrossRef] [PubMed]
- Karsdal, M.A.; Bay-Jensen, A.C.; Lories, R.J.; Abramson, S.; Spector, T.; Pastoureau, P.; Christiansen, C.; Attur, M.; Henriksen, K.; Goldring, S.R.; et al. The coupling of bone and cartilage turnover in osteoarthritis: Opportunities for bone antiresorptives and anabolics as potential treatments? Ann. Rheum. Dis. 2014, 73, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Akintoye, S.O.; Lam, T.; Shi, S.; Brahim, J.; Collins, M.T.; Robey, P.G. Skeletal site-specific characterization of orofacial and iliac crest human bone marrow stromal cells in same individuals. Bone 2006, 38, 758–768. [Google Scholar] [CrossRef] [PubMed]
- Worthley, D.L.; Churchill, M.; Compton, J.T.; Tailor, Y.; Rao, M.; Si, Y.; Levin, D.; Schwartz, M.G.; Uygur, A.; Hayakawa, Y.; et al. Gremlin 1 identifies a skeletal stem cell with bone, cartilage, and reticular stromal potential. Cell 2015, 160, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Geurts, J.; Ramp, D.; Scharen, S.; Netzer, C. GEORG-SCHMORL-PRIZE OF THE GERMAN SPINE SOCIETY (DWG) 2016: Comparison of in vitro osteogenic potential of iliac crest and degenerative facet joint bone autografts for intervertebral fusion in lumbar spinal stenosis. Eur. Spine J. 2017, 26, 1408–1415. [Google Scholar] [CrossRef] [PubMed]
- Lian, W.S.; Wu, R.W.; Lee, M.S.; Chen, Y.S.; Sun, Y.C.; Wu, S.L.; Ke, H.J.; Ko, J.Y.; Wang, F.S. Subchondral mesenchymal stem cells from osteoarthritic knees display high osteogenic differentiation capacity through microRNA-29a regulation of HDAC4. J. Mol. Med. 2017, 95, 1327–1340. [Google Scholar] [CrossRef] [PubMed]
- Xiao, E.; Li, J.M.; Yan, Y.B.; An, J.G.; Duan, D.H.; Gan, Y.H.; Zhang, Y. Decreased osteogenesis in stromal cells from radiolucent zone of human TMJ ankylosis. J. Dent. Res. 2013, 92, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Klose-Jensen, R.; Hartlev, L.B.; Boel, L.W.; Laursen, M.B.; Stengaard-Pedersen, K.; Keller, K.K.; Hauge, E.M. Subchondral bone turnover, but not bone volume, is increased in early stage osteoarthritic lesions in the human hip joint. Osteoarthr. Cartil. 2015, 23, 2167–2173. [Google Scholar] [CrossRef] [PubMed]
- Bouleftour, W.; Bouet, G.; Granito, R.N.; Thomas, M.; Linossier, M.T.; Vanden-Bossche, A.; Aubin, J.E.; Lafage-Proust, M.H.; Vico, L.; Malaval, L. Blocking the expression of both bone sialoprotein (BSP) and osteopontin (OPN) impairs the anabolic action of PTH in mouse calvaria bone. J. Cell. Physiol. 2015, 230, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Bouleftour, W.; Boudiffa, M.; Wade-Gueye, N.M.; Bouet, G.; Cardelli, M.; Laroche, N.; Vanden-Bossche, A.; Thomas, M.; Bonnelye, E.; Aubin, J.E.; et al. Skeletal development of mice lacking bone sialoprotein (BSP)--impairment of long bone growth and progressive establishment of high trabecular bone mass. PLoS ONE 2014, 9, e95144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Lin, S.; Liu, Y.; Yuan, B.; Harris, S.E.; Feng, J.Q. Dmp1 Null Mice Develop a Unique Osteoarthritis-like Phenotype. Int. J. Biol. Sci. 2016, 12, 1203–1212. [Google Scholar] [CrossRef] [PubMed]
- Jaquiery, C.; Schaeren, S.; Farhadi, J.; Mainil-Varlet, P.; Kunz, C.; Zeilhofer, H.F.; Heberer, M.; Martin, I. In vitro osteogenic differentiation and in vivo bone-forming capacity of human isogenic jaw periosteal cells and bone marrow stromal cells. Ann. Surg. 2005, 242, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Conrozier, T.; Saxne, T.; Fan, C.S.; Mathieu, P.; Tron, A.M.; Heinegard, D.; Vignon, E. Serum concentrations of cartilage oligomeric matrix protein and bone sialoprotein in hip osteoarthritis: A one year prospective study. Ann. Rheum. Dis. 1998, 57, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Waarsing, J.H.; Bierma-Zeinstra, S.M.; Weinans, H. Distinct subtypes of knee osteoarthritis: Data from the Osteoarthritis Initiative. Rheumatology 2015, 54, 1650–1658. [Google Scholar] [CrossRef] [PubMed]
- Bruyere, O.; Cooper, C.; Arden, N.; Branco, J.; Brandi, M.L.; Herrero-Beaumont, G.; Berenbaum, F.; Dennison, E.; Devogelaer, J.P.; Hochberg, M.; et al. Can we identify patients with high risk of osteoarthritis progression who will respond to treatment? A focus on epidemiology and phenotype of osteoarthritis. Drugs Aging 2015, 32, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Bianco, P. “Mesenchymal” stem cells. Annu. Rev. Cell Dev. Biol. 2014, 30, 677–704. [Google Scholar] [CrossRef] [PubMed]
- Pippenger, B.E.; Duhr, R.; Muraro, M.G.; Pagenstert, G.I.; Hugle, T.; Geurts, J. Multicolor flow cytometry-based cellular phenotyping identifies osteoprogenitors and inflammatory cells in the osteoarthritic subchondral bone marrow compartment. Osteoarthr. Cartil. 2015, 23, 1865–1869. [Google Scholar] [CrossRef] [PubMed]
- Ismail, T.; Osinga, R.; Todorov, A., Jr.; Haumer, A.; Tchang, L.A.; Epple, C.; Allafi, N.; Menzi, N.; Largo, R.D.; Kaempfen, A.; et al. Engineered, axially-vascularized osteogenic grafts from human adipose-derived cells to treat avascular necrosis of bone in a rat model. Acta Biomater. 2017, 63, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Burger, M.G.; Steinitz, A.; Geurts, J.; Pippenger, B.E.; Schaefer, D.J.; Martin, I.; Barbero, A.; Pelttari, K. Ascorbic Acid Attenuates Senescence of Human Osteoarthritic Osteoblasts. Int. J. Mol. Sci. 2017, 18, 2517. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bianco, D.; Todorov, A.; Čengić, T.; Pagenstert, G.; Schären, S.; Netzer, C.; Hügle, T.; Geurts, J. Alterations of Subchondral Bone Progenitor Cells in Human Knee and Hip Osteoarthritis Lead to a Bone Sclerosis Phenotype. Int. J. Mol. Sci. 2018, 19, 475. https://doi.org/10.3390/ijms19020475
Bianco D, Todorov A, Čengić T, Pagenstert G, Schären S, Netzer C, Hügle T, Geurts J. Alterations of Subchondral Bone Progenitor Cells in Human Knee and Hip Osteoarthritis Lead to a Bone Sclerosis Phenotype. International Journal of Molecular Sciences. 2018; 19(2):475. https://doi.org/10.3390/ijms19020475
Chicago/Turabian StyleBianco, Daniel, Atanas Todorov, Tomislav Čengić, Geert Pagenstert, Stefan Schären, Cordula Netzer, Thomas Hügle, and Jeroen Geurts. 2018. "Alterations of Subchondral Bone Progenitor Cells in Human Knee and Hip Osteoarthritis Lead to a Bone Sclerosis Phenotype" International Journal of Molecular Sciences 19, no. 2: 475. https://doi.org/10.3390/ijms19020475
APA StyleBianco, D., Todorov, A., Čengić, T., Pagenstert, G., Schären, S., Netzer, C., Hügle, T., & Geurts, J. (2018). Alterations of Subchondral Bone Progenitor Cells in Human Knee and Hip Osteoarthritis Lead to a Bone Sclerosis Phenotype. International Journal of Molecular Sciences, 19(2), 475. https://doi.org/10.3390/ijms19020475