The rs2910164 Genetic Variant of miR-146a-3p Is Associated with Increased Overall Mortality in Patients with Follicular Variant Papillary Thyroid Carcinoma
Abstract
:1. Introduction
2. Results
2.1. The rs2910164 Genetic Variant of miR-146a-3p Is Associated with Increased Mortality in Patients with Follicular Variant PTC
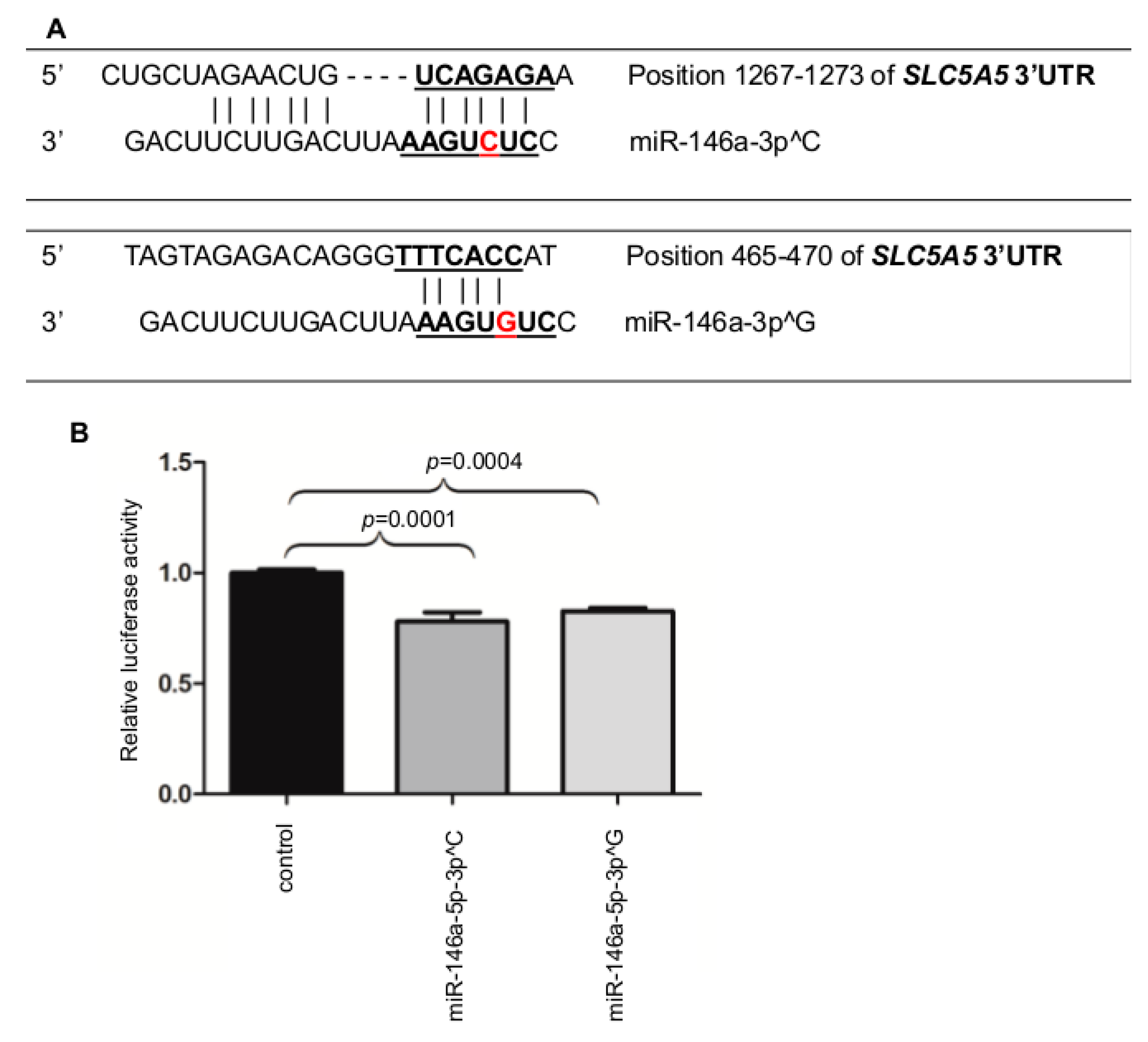
2.2. MiR-146a-3p Directly Binds the 3′UTR of NIS
2.3. Inhibition of the miR-146a-3p Restores the Expression and Function of NIS
2.4. The miR-146a-3p Undergoes Somatic Mutations in Cancer Tissue
3. Discussion
4. Materials and Methods
4.1. Study Patients
4.2. Somatic SNP Genotyping
4.3. In Silico miR Analysis
4.4. Gene Expression Quantification
4.5. Direct miR and NIS Binding Luciferase Assay
4.6. MicroRNA miR-146a Inhibition
4.7. RAIU Assay
4.8. Statistical Analyses
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Swierniak, M.; Wojcicka, A.; Czetwertynska, M.; Dlugosinska, J.; Stachlewska, E.; Gierlikowski, W.; Kot, A.; Gornicka, B.; Koperski, L.; Bogdanska, M.; et al. Association between GWAS-Derived rs966423 Genetic Variant and Overall Mortality in Patients with Differentiated Thyroid Cancer. Clin. Cancer Res. 2016, 22, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Xing, M. BRAF V600E mutation and papillary thyroid cancer. JAMA 2013, 310, 535. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.K. Sodium iodide symporter: Its role in nuclear medicine. J. Nucl. Med. 2002, 43, 1188–1200. [Google Scholar] [PubMed]
- Riesco-Eizaguirre, G.; Wert-Lamas, L.; Perales-Paton, J.; Sastre-Perona, A.; Fernandez, L.P.; Santisteban, P. The miR-146b-3p/PAX8/NIS Regulatory Circuit Modulates the Differentiation Phenotype and Function of Thyroid Cells during Carcinogenesis. Cancer Res. 2015, 75, 4119–4130. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lv, B.; Chen, B.; Guan, M.; Sun, Y.; Li, H.; Zhang, B.; Ding, C.; He, S.; Zeng, Q. Inhibition of miR-146b expression increases radioiodine-sensitivity in poorly differential thyroid carcinoma via positively regulating NIS expression. Mol. Cell Biol. Res. Commun. 2015, 462, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Jazdzewski, K.; Li, W.; Liyanarachchi, S.; Nagy, R.; Volinia, S.; Calin, G.A.; Liu, C.G.; Franssila, K.; Suster, S.; et al. The role of microRNA genes in papillary thyroid carcinoma. Proc. Natl. Acad. Sci. USA 2005, 102, 19075–19080. [Google Scholar] [CrossRef] [PubMed]
- Jazdzewski, K.; Boguslawska, J.; Jendrzejewski, J.; Liyanarachchi, S.; Pachucki, J.; Wardyn, K.A.; Nauman, A.; de la Chapelle, A. Thyroid hormone receptor beta (THRB) is a major target gene for microRNAs deregulated in papillary thyroid carcinoma (PTC). J. Clin. Endocrinol. Metab. 2011, 96, E546–E553. [Google Scholar] [CrossRef] [PubMed]
- Jazdzewski, K.; Murray, E.L.; Franssila, K.; Jarzab, B.; Schoenberg, D.R.; de la Chapelle, A. Common SNP in pre-miR-146a decreases mature miR expression and predisposes to papillary thyroid carcinoma. Proc. Natl. Acad. Sci. USA 2008, 105, 7269–7274. [Google Scholar] [CrossRef] [PubMed]
- Jazdzewski, K.; Liyanarachchi, S.; Swierniak, M.; Pachucki, J.; Ringel, M.D.; Jarzab, B.; de la Chapelle, A. Polymorphic mature microRNAs from passenger strand of pre-miR-146a contribute to thyroid cancer. Proc. Natl. Acad. Sci. USA 2009, 106, 1502–1505. [Google Scholar] [CrossRef] [PubMed]
- Jazdzewski, K.; de la Chapelle, A. Genomic sequence matters: A SNP in microRNA-146a can turn anti-apoptotic. Cell Cycle 2009, 8, 1642–1643. [Google Scholar] [CrossRef] [PubMed]
- Marino, M.; Cirello, V.; Gnarini, V.; Colombo, C.; Pignatti, E.; Casarini, L.; Diazzi, C.; Rochira, V.; Cioni, K.; Madeo, B.; et al. Are pre-miR-146a and PTTG1 associated with papillary thyroid cancer? Endocr. Connect. 2013, 2, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.M.; Howarth, K.M.; Martin, L.; Gorman, M.; Mihai, R.; Moss, L.; Auton, A.; Lemon, C.; Mehanna, H.; Mohan, H.; et al. Thyroid cancer susceptibility polymorphisms: Confirmation of loci on chromosomes 9q22 and 14q13, validation of a recessive 8q24 locus and failure to replicate a locus on 5q24. J. Med. Genet. 2012, 49, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.J.; Wang, Y.L.; Li, D.S.; Wang, Y.; Wang, X.F.; Zhu, Y.X.; Yang, Y.J.; Wang, Z.Y.; Ma, Y.Y.; Wu, Y.; et al. Association between the rs2910164 polymorphism in pre-Mir-146a sequence and thyroid carcinogenesis. PLoS ONE 2013, 8, e56638. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.J.; Mishra, P.J.; Banerjee, D.; Bertino, J.R. MiRSNPs or MiR-polymorphisms, new players in microRNA mediated regulation of the cell: Introducing microRNA pharmacogenomics. Cell Cycle 2008, 7, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Hagag, P.; Hod, N.; Kummer, E.; Cohenpour, M.; Horne, T.; Weiss, M. Follicular variant of papillary thyroid carcinoma: Clinical-pathological characterization and long-term follow-up. Cancer J. 2006, 12, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Stokowy, T.; Gawel, D.; Wojtas, B. Differences in miRNA and mRNA Profile of Papillary Thyroid Cancer Variants. Int. J. Endocrinol. 2016, 2016, 1427042. [Google Scholar] [CrossRef] [PubMed]
- Kogai, T.; Schultz, J.J.; Johnson, L.S.; Huang, M.; Brent, G.A. Retinoic acid induces sodium/iodide symporter gene expression and radioiodide uptake in the MCF-7 breast cancer cell line. Proc. Natl. Acad. Sci. USA 2000, 97, 8519–8524. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Kim, Y.R.; Kim, K.N.; Choe, J.G.; Chung, J.K.; Kim, M.K. Effect of all-trans retinoic acid on sodium/iodide symporter expression, radioiodine uptake and gene expression profiles in a human anaplastic thyroid carcinoma cell line. Nucl. Med. Biol. 2006, 33, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Hou, Y.L.; Kang, Q.Q.; Chen, X.Y.; Duan, L.Q.; Shu, J.; Li, S.L.; Hu, X.L.; Peng, Z.P. All-trans-retinoic acid promotes iodine uptake via up- regulating the sodium iodide symporter in medullary thyroid cancer stem cells. Asian Pacific journal of cancer prevention. APJCP 2014, 15, 1859–1862. [Google Scholar] [PubMed]
- Czajka, A.A.; Wojcicka, A.; Kubiak, A.; Kotlarek, M.; Bakula-Zalewska, E.; Koperski, L.; Wiechno, W.; Jazdzewski, K. Family of microRNA-146 Regulates RARbeta in Papillary Thyroid Carcinoma. PLoS ONE 2016, 11, e0151968. [Google Scholar] [CrossRef] [PubMed]
- Wojcicka, A.; Czetwertynska, M.; Swierniak, M.; Dlugosinska, J.; Maciag, M.; Czajka, A.; Dymecka, K.; Kubiak, A.; Kot, A.; Ploski, R.; et al. Variants in the ATM-CHEK2-BRCA1 axis determine genetic predisposition and clinical presentation of papillary thyroid carcinoma. Genes Chromosomes Cancer 2014, 53, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Penston, J. Should we use total mortality rather than cancer specific mortality to judge cancer screening programmes? Yes. BMJ 2011, 343, d6395. [Google Scholar] [CrossRef] [PubMed]
- Ebert, M.S.; Neilson, J.R.; Sharp, P.A. MicroRNA sponges: Competitive inhibitors of small RNAs in mammalian cells. Nat. Methods 2007, 4, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Beyer, S.; Lakshmanan, A.; Liu, Y.Y.; Zhang, X.; Wapnir, I.; Smolenski, A.; Jhiang, S. KT5823 differentially modulates sodium iodide symporter expression, activity, and glycosylation between thyroid and breast cancer cells. Endocrinology 2011, 152, 782–792. [Google Scholar] [CrossRef] [PubMed]
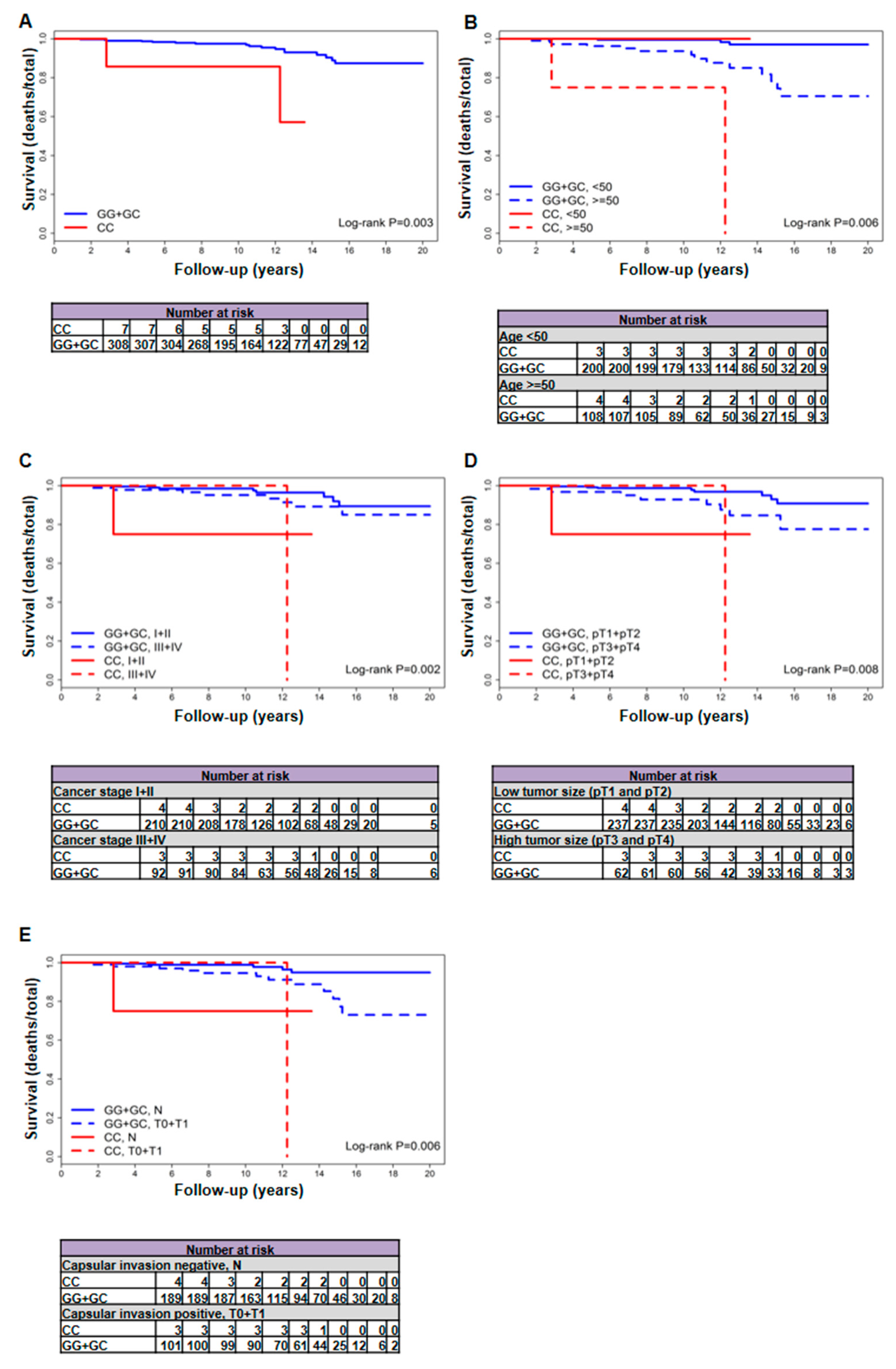

| Mortality, No./Total (%) | Deaths per 1000 Person-Years (95% CI) | Unadjusted | Adjusted | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Category | Overall | miR146a-GG/CG | miR146a-CC | Person-Years Follow-Up | miR146a-GG/CG | miR146a-CC | Log-Rank p-Value | HR (95%CI) | Log-Rank p-Value | HR (95% CI) |
| Female patients | 19/315 (6) | 17/308 (5.5) | 2/7 (28.6) | 3414.58 (3347.25 + 67.33) | 5.08 (2.96–8.13) | 29.7 (3.6–107.3) | 0.003 | 7.03 (1.58–31.19) | 0.006 | 6.21 (1.38–27.93) |
| Age | ||||||||||
| <50 year | 3/203 (1.5) | 3/200 (1.5) | 0/3 (0) | 2277.58 (2240.08 + 37.5) | 1.34 (0.28–3.91) | n/a | n/a | n/a | n/a | n/a |
| ≥50 year | 16/112 (14.3) | 14/108 (13) | 2/4 (50) | 1137 (1107.17 + 29.83) | 12.64 (6.91–21.22) | 67.05 (8.12–242.2) | 0.002 | 7.85 (1.68–36.55) | 0.002 | 7.85 (1.68–36.55) |
| pT | ||||||||||
| pT1 + pT2 | 9/241 (3.7) | 8/237 (3.4) | 1/4 (25) | 2513.25 (2479.08 + 34.17) | 3.23 (1.39–6.36) | 29.27 (0.74–163.06) | 0.002 | 13.62 (1.57–118.11) | <0.001 | 25.05 (2.23–280.9) |
| pT3 + pT4 | 9/65 (13.8) | 8/62 (12.9) | 1/3 (33.3) | 737.42 (704.25 + 33.17) | 11.36 (4.9–22.38) | 30.15 (0.76–167.97) | 0.183 | 3.83 (0.46–32.26) | 0.642 | 1.66 (0.19–14.25) |
| pN | ||||||||||
| N0 | 17/245 (6.9) | 16/240 (6.7) | 1/5 (20) | 2607.42 (2562.42 + 45) | 6.24 (3.57–10.14) | 22.22 (0.56–123.81) | 0.143 | 4.09 (0.53–31.52) | 0.143 | 4.09 (0.53–31.52) |
| N1a | 1/30 (3.3) | 0/29 (0) | 1/1 (100) | 381.67 (369.42 + 12.25) | 0 (0–9.99) | 81.63 (2.07–454.83) | n/a | n/a | n/a | n/a |
| N1b | 1/26 (3.8) | 1/25 (4) | 0/1 (0) | 274.83 (264.75 + 10.08) | 3.78 (0.1–21.04) | 0 (0–365.96) | n/a | n/a | n/a | n/a |
| pN | ||||||||||
| N0 + N1a | 18/275 (6.5) | 16/269 (5.9) | 2/6 (33.3) | 2989.08 (2931.83 + 57.25) | 5.46 (3.12–8.86) | 34.93 (4.23–126.2) | 0.002 | 7.38 (1.65–33.11) | 0.002 | 7.84 (1.72–35.69) |
| N1b | 1/26 (3.8) | 1/25 (4) | 0/1 (0) | 274.83 (264.75 + 10.08) | 3.78 (0.1–21.04) | 0 (0–365.96) | n/a | n/a | n/a | n/a |
| capsule | ||||||||||
| N | 6/193 (3.1) | 5/189 (2.6) | 1/4 (25) | 2049.33 (2015.17 + 34.17) | 2.48 (0.81–5.79) | 29.27 (0.74–163.06) | 0.016 | 8.95 (1.03–77.62) | 0.021 | 9.03 (0.94–87.18) |
| T0 + T1 | 13/104 (12.5) | 12/101 (11.9) | 1/3 (33.3) | 1149.92 (1116.75 + 33.17) | 10.75 (5.55–18.77) | 30.15 (0.76–167.97) | 0.11 | 4.73 (0.58–38.59) | 0.282 | 3 (0.37–24.7) |
| angioinvasion | ||||||||||
| N | 12/228 (5.3) | 12/224 (5.4) | 0/4 (0) | 2514.5 (2473.08 + 41.42) | 4.85 (2.51–8.48) | 0 (0–89.06) | n/a | n/a | n/a | n/a |
| T | 5/56 (8.9) | 4/54 (7.4) | 1/2 (50) | 583.17 (569.5 + 13.67) | 7.02 (1.91–17.98) | 73.15 (1.85–407.58) | n/a | n/a | n/a | n/a |
| pM | ||||||||||
| M0 | 18/292 (6.2) | 16/285 (5.6) | 2/7 (28.6) | 3191.25 (3123.92 + 67.33) | 5.12 (2.93–8.32) | 29.7 (3.6–107.3) | 0.003 | 7.04 (1.57–31.54) | 0.005 | 6.5 (1.43–29.52) |
| M1 | 1/10 (10) | 1/10 (10) | 0/0 (NaN) | 89.5 (89.5 + 0) | 11.17 (0.28–62.25) | n/a | n/a | n/a | n/a | n/a |
| multifocality | ||||||||||
| single | 14/207 (6.8) | 13/203 (6.4) | 1/4 (25) | 2254.33 (2207.58 + 46.75) | 5.89 (3.14–10.07) | 21.39 (0.54–119.18) | 0.169 | 3.79 (0.49–29.25) | 0.232 | 3.26 (0.42–25.41) |
| multifocality | 5/95 (5.3) | 4/92 (4.3) | 1/3 (33.3) | 990.42 (969.83 + 20.58) | 4.12 (1.12–10.56) | 48.59 (1.23–270.73) | n/a | n/a | n/a | n/a |
| Stage | ||||||||||
| I + II | 9/214 (4.2) | 8/210 (3.8) | 1/4 (25) | 2218.83 (2184.67 + 34.17) | 3.66 (1.58–7.22) | 29.27 (0.74–163.06) | 0.004 | 12.01 (1.38–104.19) | <0.001 | 22.72 (2.04–253.66) |
| III + IV | 9/95 (9.5) | 8/92 (8.7) | 1/3 (33.3) | 1100.33 (1067.17 + 33.17) | 7.5 (3.24–14.77) | 30.15 (0.76–167.97) | 0.073 | 5.63 (0.67–47.38) | 0.522 | 1.99 (0.23–17.16) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotlarek, M.; Kubiak, A.; Czetwertyńska, M.; Świerniak, M.; Gierlikowski, W.; Kolanowska, M.; Bakuła-Zalewska, E.; Jhiang, S.M.; Jażdżewski, K.; Wójcicka, A. The rs2910164 Genetic Variant of miR-146a-3p Is Associated with Increased Overall Mortality in Patients with Follicular Variant Papillary Thyroid Carcinoma. Int. J. Mol. Sci. 2018, 19, 655. https://doi.org/10.3390/ijms19030655
Kotlarek M, Kubiak A, Czetwertyńska M, Świerniak M, Gierlikowski W, Kolanowska M, Bakuła-Zalewska E, Jhiang SM, Jażdżewski K, Wójcicka A. The rs2910164 Genetic Variant of miR-146a-3p Is Associated with Increased Overall Mortality in Patients with Follicular Variant Papillary Thyroid Carcinoma. International Journal of Molecular Sciences. 2018; 19(3):655. https://doi.org/10.3390/ijms19030655
Chicago/Turabian StyleKotlarek, Marta, Anna Kubiak, Małgorzata Czetwertyńska, Michał Świerniak, Wojciech Gierlikowski, Monika Kolanowska, Elwira Bakuła-Zalewska, Sissy M. Jhiang, Krystian Jażdżewski, and Anna Wójcicka. 2018. "The rs2910164 Genetic Variant of miR-146a-3p Is Associated with Increased Overall Mortality in Patients with Follicular Variant Papillary Thyroid Carcinoma" International Journal of Molecular Sciences 19, no. 3: 655. https://doi.org/10.3390/ijms19030655





