17β-Hydroxysteroid Dehydrogenase Type 2 Expression Is Induced by Androgen Signaling in Endometrial Cancer
Abstract
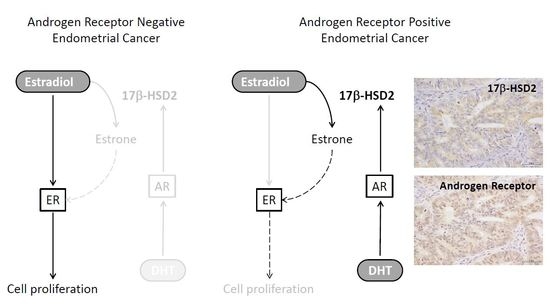
:1. Introduction
2. Results
2.1. Induction of 17β-HSD2 Expression by DHT
2.2. DHT Inhibits E2-Induced Cell Proliferation in HEC-1B Cells
2.3. Immunohistochemistry of 17β-HSD2 and AR in EEA
2.4. 17β-HSD2 Expression Is Correlated with DHT Concentration in Cancer Tissues
2.5. 17β-HSD2 Clinicopathological Parameters in EEA
3. Discussion
4. Materials and Methods
4.1. EEA Patient and Tissue Preparation
4.2. Cell Lines and Chemicals
4.3. Real-Time PCR
4.4. Western Blotting
4.5. Immunohistochemistry
4.6. Evaluation of Immunoreactivity
4.7. LC-MS/MS
4.8. Statistical Analyses
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. 2016 Cancer statistics. CA A Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Bokhman, J.V. Two pathogenetic types of endometrial carcinoma. Gynecol. Oncol. 1983, 15, 10–17. [Google Scholar] [CrossRef]
- Wu, L.; Einstein, M.; Geissler, W.M.; Chan, H.K.; Elliston, K.O.; Andersson, S. Expression cloning and characterization of human 17 beta-hydroxysteroid dehydrogenase type 2, a microsomal enzyme possessing 20 alpha-hydroxysteroid dehydrogenase activity. J. Biol. Chem. 1993, 268, 12964–12969. [Google Scholar] [PubMed]
- Engeli, R.T.; Rohrer, S.R.; Vuorinen, A.; Herdlinger, S.; Kaserer, T.; Leugger, S.; Schuster, D.; Odermatt, A. Interference of Paraben Compounds with Estrogen Metabolism by Inhibition of 17β-Hydroxysteroid Dehydrogenases. Int. J. Mol. Sci. 2017, 18, 2007. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Utsunomiya, H.; Niikura, H.; Yaegashi, N.; Sasano, H. Inhibition of estrogen actions in human gynecological malignancies: New aspects of endocrine therapy for endometrial cancer and ovarian cancer. Mol. Cell Endocrinol. 2011, 340, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Takagi, K.; Miki, Y.; Nagasaki, S.; Hirakawa, H.; Onodera, Y.; Akahira, J.; Ishida, T.; Watanabe, M.; Kimijima, I.; Hayashi, S.; et al. Increased intratumoral androgens in human breast carcinoma following aromatase inhibitor exemestane treatment. Endocr. Relat. Cancer 2010, 17, 415–430. [Google Scholar] [CrossRef] [PubMed]
- Yoda, T.; McNamara, K.M.; Miki, Y.; Takagi, M.; Rai, Y.; Ohi, Y.; Sagara, Y.; Tamaki, K.; Hirakawa, H.; Ishida, T.; et al. Intratumoral androgen metabolism and actions in invasive lobular carcinoma of the breast. Cancer Sci. 2014, 105, 1503–1509. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Miki, Y.; Hashimoto, C.; Takagi, K.; Zhulanqiqige, D.; Li, B.; Yaegashi, N.; Suzuki, T.; Ito, K. The role of 5α-reductase type1 associated with intratumoral dihydrotestosterone concentrations in human endometrial carcinoma. Mol. Cell Endocr. 2015, 401, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.M.; Bulmer, J.N.; DeCruze, S.B.; Stringfellow, H.F.; Martin-Hirsch, P.; Hapangama, D.K. Androgen receptors are acquired by healthy postmenopausal endometrial epithelium and their subsequent loss in endometrial cancer is associated with poor survival. Br. J. Cancer 2016, 114, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Tuohimaa, P. Regulation of 17β-hydroxysteroid dehydrogenase type 2, type 4 and type 5 by calcitriol, LXR agonist and 5alpha-dihydrotestosterone in human prostate cancer cells. J. Steroid Biochem. Mol. Biol. 2007, 107, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Sasano, H.; Suzuki, T.; Miki, Y.; Moriya, T. Intracrinology of estrogens and androgens in breast carcinoma. J. Steroid Biochem. Mol. Biol. 2008, 108, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Miki, Y.; Suzuki, T.; Sasano, H. Intracrinology of sex steroids in ductal carcinoma in situ (DCIS) of human breast: Comparison to invasive ductal carcinoma (IDC) and non-neoplastic breast. J. Steroid Biochem. Mol. Biol. 2009, 114, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Utsunomiya, H.; Suzuki, T.; Saitou, S.; Akahira, J.; Okamura, K.; Yaegashi, N.; Sasano, H. 17β-hydroxysteroid dehydrogenases in human endometrium and its disorders. Mol. Cell Endocrinol. 2006, 248, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Miki, Y.; Fue, M.; Takagi, K.; Hashimoto, C.; Tanaka, S.; Suzuki, T.; Ito, K. Androgen receptor and intracrine androgen signaling in endometrial carcinomas. Recept. Clin. Investig. 2015, 2, e853. [Google Scholar]
- Ito, K.; Miki, Y.; Suzuki, T.; McNamara, K.M.; Sasano, H. In situ androgen and estrogen biosynthesis in endometrial cancer: Focus on androgen actions and intratumoral production. Endocr. Relat. Cancer 2016, 23, R323–R335. [Google Scholar] [CrossRef] [PubMed]
- Gunnarsson, C.; Olsson, B.M.; Stål, O. Southeast Sweden Breast Cancer Group, Abnormal expression of 17beta-hydroxysteroid dehydrogenases in breast cancer predicts late recurrence. Cancer Res. 2001, 61, 8448–8451. [Google Scholar] [PubMed]
- Gunnarsson, C.; Hellqvist, E.; Stål, O. 17beta-Hydroxysteroid dehydrogenases involved in local oestrogen synthesis have prognostic significance in breast cancer. Br. J. Cancer 2005, 92, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Utsunomiya, H.; Suzuki, T.; Kaneko, C.; Takeyama, J.; Nakamura, J.; Kimura, K.; Yoshihama, M.; Harada, N.; Ito, K.; Konno, R.; et al. The analyses of 17beta-hydroxysteroid dehydrogenase isozymes in human endometrial hyperplasia and carcinoma. J. Clin. Endocrinol. Metab. 2001, 86, 3436–3443. [Google Scholar] [PubMed]
- Mustonen, M.V.; Isomaa, V.V.; Vaskivuo, T.; Tapanainen, J.; Poutanen, M.H.; Stenbäck, F.; Vihko, R.K.; Vihko, P.T. Human 17beta-hydroxysteroid dehydrogenase type 2 messenger ribonucleic acid expression and localization in term placenta and in endometrium during the menstrual cycle. J. Clin. Endocrinol. Metab. 1998, 83, 1319–1324. [Google Scholar] [PubMed]
- Lépine, J.; Audet-Walsh, E.; Grégoire, J.; Têtu, B.; Plante, M.; Ménard, V.; Ayotte, P.; Brisson, J.; Caron, P.; Villeneuve, L.; et al. Circulating estrogens in endometrial cancer cases and their relationship with tissular expression of key estrogen biosynthesis and metabolic pathways. J. Clin. Endocrinol. Metab. 2010, 95, 2689–2698. [Google Scholar] [CrossRef] [PubMed]
- Cornel, K.M.; Kruitwagen, R.F.; Delvoux, B.; Visconti, L.; Van de Vijver, K.K.; Day, J.M.; Van Gorp, T.; Hermans, R.J.; Dunselman, G.A.; Romano, A. Overexpression of 17β-hydroxysteroid dehydrogenase type 1 increases the exposure of endometrial cancer to 17β-estradiol. J. Clin. Endocrinol. Metab. 2012, 97, E591–E601. [Google Scholar] [CrossRef] [PubMed]
- Sinreih, M.; Hevir, N.; Rižner, T.L. Altered expression of genes involved in progesterone biosynthesis, metabolism and action in endometrial cancer. Chem. Biol. Interact. 2013, 202, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Bao, W.; Wang, J.; Yang, T.; He, X.; Liao, Y.; Wan, X. FOXA1 promotes tumor cell proliferation through AR involving the Notch pathway in endometrial cancer. BMC Cancer 2014, 14, 78. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Sharma, C.G.; Jordan, V.C. Estrogen regulation of X-box binding protein-1 and its role in estrogen induced growth of breast and endometrial cancer cells. Horm. Mol. Biol. Clin. Investig. 2010, 2, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Wang, H.; Liu, D.; Zhang, C.; Deng, Y.; Yang, F.; Zhang, C. Methylation of tumor suppressor gene CDH13 and SHP1 promoters and their epigenetic regulation by the UHRF1/PRMT5 complex in endometrial carcinoma. Gynecol. Oncol. 2016, 140, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, X.; Yu, Q.; Eng, C. Androgen receptor-induced tumor suppressor, KLLN, inhibits breast cancer growth and transcriptionally activates p53/p73-mediated apoptosis in breast carcinomas. Hum. Mol. Genet. 2013, 22, 2263–2272. [Google Scholar] [CrossRef] [PubMed]
- Geng, C.; Rajapakshe, K.; Shah, S.S.; Shou, J.; Eedunuri, V.K.; Foley, C.; Bond, R. Androgen receptor is the key transcriptional mediator of the tumor suppressor SPOP in prostate cancer. Cancer Res. 2014, 74, 5631–5643. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.L.; Macarthur, S.; Ross-Innes, C.S.; Tilley, W.D.; Neal, D.E.; Mills, I.G.; Carroll, J.S. Androgen receptor driven transcription in molecular apocrine breast cancer is mediated by FoxA1. EMBO J. 2011, 30, 3019–3027. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, D.R.; Bernales, S.; Jacobsen, B.M.; Cittelly, D.M.; Howe, E.N.; D’Amato, N.C.; Spoelstra, N.S.; Edgerton, S.M.; Jean, A.; Guerrero, J.; et al. Role of the androgen receptor in breast cancer and preclinical analysis of enzalutamide. Breast Cancer Res. 2014, 16, R7. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.; Li, Y.; Song, W.; Xu, Y.; Yang, F.; Zhang, W.; Yin, Y.; Guan, X. Antiproliferative Effect of Androgen Receptor Inhibition in Mesenchymal Stem-Like Triple-Negative Breast Cancer. Cell Physiol. Biochem. 2016, 38, 1003–1014. [Google Scholar] [CrossRef] [PubMed]
- Boostanfar, R.; Amezcua, C.A.; Tourgeman, D.E.; Roy, S.; Felix, J.C.; Stanczyk, F.Z. Growth effects of raloxifene, estradiol, medroxy-progesterone acetate, and progesterone on human endometrial adenocarcinoma cells. Fertil. Steril. 2003, 79, 223–225. [Google Scholar] [CrossRef]
- Ray, S.; Johnston, R.; Campbell, D.C.; Nugent, S.; McDade, S.S.; Waugh, D.; Panov, K.I. Androgens and estrogens stimulate ribosome biogenesis in prostate and breast cancer cells in receptor dependent manner. Gene 2013, 526, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Hamada, T.; Souda, M.; Yoshimura, T.; Sasaguri, S.; Hatanaka, K.; Tasaki, T.; Yoshioka, T.; Ohi, Y.; Yamada, S.; Tsutsui, M.; et al. Anti-apoptotic effects of PCP4/PEP19 in human breast cancer cell lines: A novel oncotarget. Oncotarget 2014, 5, 6076–6086. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Ito, K.; Nagase, S.; Suzuki, T.; Akahira, J.; Okamura, K.; Yaegashi, N.; Sasano, H. Progesterone receptor isoforms as a prognostic marker in human endometrial carcinoma. Cancer Sci. 2006, 97, 1308–1314. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Miki, Y.; Moriya, T.; Akahira, J.; Ishida, T.; Hirakawa, H.; Yamaguchi, Y.; Hayashi, S.; Sasano, H. 5alpha-Reductase type 1 and aromatase in breast carcinoma as regulators of in situ androgen production. Int. J. Cancer 2007, 120, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Miki, Y.; Suzuki, T.; Tazawa, C.; Yamaguchi, Y.; Kitada, K.; Honma, S.; Moriya, T.; Hirakawa, H.; Evans, D.B.; Hayashi, S.; et al. Aromatase localization in human breast cancer tissues: Possible interactions between intratumoral stromal and parenchymal cells. Cancer Res. 2007, 67, 3945–3954. [Google Scholar] [CrossRef] [PubMed]






| Parameter | Total (n = 53) | 17β-HSD2 | ||
|---|---|---|---|---|
| + (n = 19) | − (n = 34) | p-Value | ||
| Grade 1 (G1) | 22 | 11 | 11 | |
| 2 (G2) | 20 | 7 | 13 | |
| 3 (G3) | 11 | 1 | 10 | 0.0480 |
| Stage | ||||
| I, II | 43 | 19 | 24 | |
| III, IV | 10 | 0 | 10 | 0.0218 |
| LVI | ||||
| No | 37 | 15 | 22 | |
| Yes | 16 | 4 | 12 | 0.2704 |
| MI | ||||
| None or less than half | 31 | 14 | 17 | |
| More than half | 22 | 5 | 17 | 0.0884 |
| Androgen receptor | ||||
| Positive | 40 | 17 | 23 | |
| Negative | 13 | 2 | 11 | 0.0629 |
| Estrogen receptor | ||||
| Positive | 37 | 15 | 22 | |
| Negative | 16 | 4 | 12 | 0.2704 |
| Progesterone receptor | ||||
| Positive | 33 | 17 | 16 | |
| Negative | 20 | 2 | 18 | 0.0023 |
| Ki-67 LI median (min-max) (%) | 20.4 (3–90) | 30.6 (0–96) | 0.0212 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashimoto, C.; Miki, Y.; Tanaka, S.; Takagi, K.; Fue, M.; Doe, Z.; Li, B.; Yaegashi, N.; Suzuki, T.; Ito, K. 17β-Hydroxysteroid Dehydrogenase Type 2 Expression Is Induced by Androgen Signaling in Endometrial Cancer. Int. J. Mol. Sci. 2018, 19, 1139. https://doi.org/10.3390/ijms19041139
Hashimoto C, Miki Y, Tanaka S, Takagi K, Fue M, Doe Z, Li B, Yaegashi N, Suzuki T, Ito K. 17β-Hydroxysteroid Dehydrogenase Type 2 Expression Is Induced by Androgen Signaling in Endometrial Cancer. International Journal of Molecular Sciences. 2018; 19(4):1139. https://doi.org/10.3390/ijms19041139
Chicago/Turabian StyleHashimoto, Chiaki, Yasuhiro Miki, Sota Tanaka, Kiyoshi Takagi, Misaki Fue, Zhulanqiqige Doe, Bin Li, Nobuo Yaegashi, Takashi Suzuki, and Kiyoshi Ito. 2018. "17β-Hydroxysteroid Dehydrogenase Type 2 Expression Is Induced by Androgen Signaling in Endometrial Cancer" International Journal of Molecular Sciences 19, no. 4: 1139. https://doi.org/10.3390/ijms19041139