St. John’s Wort Regulates Proliferation and Apoptosis in MCF-7 Human Breast Cancer Cells by Inhibiting AMPK/mTOR and Activating the Mitochondrial Pathway
Abstract
:1. Introduction
2. Results
2.1. SJWE Inhibited the Proliferation in MCF-7 Human Breast Cancer Cells
2.2. SJWE Induced Apoptosis in MCF-7 Human Breast Cancer Cells
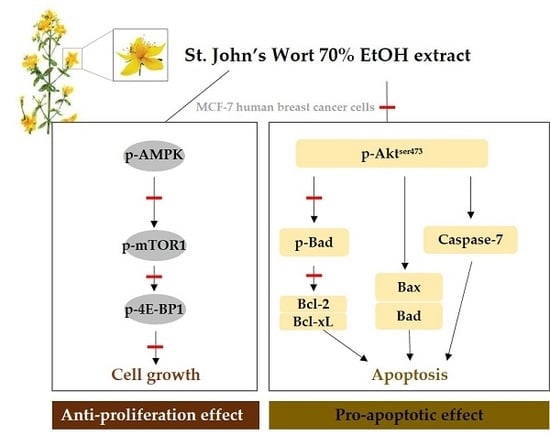
2.3. AMPK/mTOR/4E-BP1 Pathway Was Involved in SJWE Induced Growth Inhibition of MCF-7 Human Breast Cancer Cells
2.4. SJWE Caused Hypophosphorylation of Akt in MCF-7 Human Breast Cancer Cells
2.5. SJWE Induced Apoptosis Was Mitochondrial in Origin and Was Regulated by the Bcl-2 Family in MCF-7 Human Breast Cancer Cells
2.6. SJWE Increased Caspase-7 Activation in MCF-7 Human Breast Cancer Cells
3. Discussion
4. Materials and Methods
4.1. Preparation of St. John’s Wort Extract
4.2. Cell Culture and Treatment
4.3. Cell Proliferation
4.4. Apoptosis
4.5. TUNEL Assay
4.6. Immunoblotting
4.7. Caspase 7 Activation
4.8. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AMPK | AMP-activated protein kinase |
| mTOR | mammalian target of rapamycin |
| S6K1 | kinases p70S6K1 |
| eIF4E | eukaryotic translation initiation factor 4E |
| 4E-BP1 | eIF4E-Binding protein 1 |
| PKC-∝ | protein kinase C-∝ |
| Akt | protein kinase B |
| Bcl-xL | B-cell lymphoma-extra large |
| Bcl-2 | B-cell lymphoma 2 |
| Bad | Bcl-2-associated death promoter |
| Bax | Bcl-2 like protein 4 |
References
- Fehm, T.; Muller, V.; Alix-Panabieres, C.; Pantel, K. Micrometastatic spread in breast cancer: Detection, molecular characterization and clinical relevance. Breast Cancer Res. 2008, 10, S1. [Google Scholar] [CrossRef] [PubMed]
- Harward, J.H.; Bland, K.L. Current management and treatment strategies for breast cancer. Curr. Opin. Obstet. Gynecol. 2012, 24, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Han, S.; Zhang, G.; Sang, Y.; Ji, Z. Antiproliferative activity and apoptosis-inducing mechanism of l-securinine on human breast cancer MCF-7 cells. Pharmazie 2014, 69, 201–223. [Google Scholar]
- Patel, P.B.; Thakkar, V.R. l-carvone induces p53, caspase 3 mediated apoptosis and inhibits the migration of breast cancer cell lines. Nutr. Cancer 2014, 66, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Evan, G.I.; Vousden, K.H. Proliferation, cell cycle and apoptosis in cancer. Nature 2001, 411, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Fogarty, S.; Hardie, D.G. Development of protein kinase activators: AMPK as a target in metabolic disorders and cancer. Biochim. Biophys. Acta 2010, 1804, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G. AMP-activated/SNF1 protein kinases: Conserved guardians of cellular energy. Nat. Rev. Mol. Cell Biol. 2007, 8, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, S.; Bierhoff, H.; Cado, I.; Weber, A.; Tiebe, M.; Grummt, I.; Voit, R. AMP-activated protein kinase adapts rRNA synthesis to cellular energy supply. Proc. Natl. Acad. Sci. USA 2009, 106, 17781–17786. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.Y.; He, Y.Y. Targeting the AMP-activated protein kinase for cancer prevention and therapy. Oncology 2012, 3, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Moschetta, A.; Reale, A.; Marasco, C.; Vacca, A.; Carratu, M.R. Therapeutic targeting of the mTOR-signaling pathway in cancer: Benefits and limitations. Br. J. Phamacol. 2014, 171, 3801–3813. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Kim, S.M.; Bea, H.; Nam, D.; Lee, J.H.; Lee, S.G.; Shim, B.S.; Kim, S.H.; Ahn, K.S.; Choi, S.H.; et al. Embelin inhibits growth and induces apoptosis through the suppression of Akt/mTOR/S6KI signaling cascades. Prostate 2013, 73, 296–305. [Google Scholar] [CrossRef] [PubMed]
- McCubrey, J.A.; Steelman, L.S.; Kempf, C.R.; Chappell, W.H.; Abrams, S.L.; Stivala, F.; Malaponte, G.; Nicoletti, F.; Libra, M.; Basecke, J.; et al. Therapeutic resistance resulting from mutations in Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR signaling pathways. J. Cell. Physiol. 2011, 226, 2762–2781. [Google Scholar] [CrossRef] [PubMed]
- Dazert, E.; Hall, M.N. mTOR signaling in disease. Curr. Opin. Cell Biol. 2011, 23, 744–755. [Google Scholar] [CrossRef] [PubMed]
- Mohan, C.D.; Srinivasa, V.; Rangappa, S.; Mervin, L.; Mohan, S.; Paricharak, S.; Baday, S.; Li, F.; Shanmugam, M.K.; Chinnathambi, A.; et al. Trisubstitured-imidazoles induced apoptosis in human breast cancer cells by targeting the oncogenic PI3K/Akt/mTOR signaling pathway. PLoS ONE 2016, 11, e0153155. [Google Scholar] [CrossRef] [PubMed]
- Harrison, M.E.; Power Coombs, M.R.; Melaney, L.M.; Hoskin, D.W. Exposure of breast cancer cells to a subcytotoxic dose of apigenin causes growth inhibition, oxidative stress and hypophosphorylation of Akt. Exp. Mol. Pathol. 2014, 97, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, H.; Zhu, J.; Xu, J.; Ding, K. Mollugin induces tumor cell apoptosis and autophagy via the PI3K/Akt/mTOR/p70S6K and ERK signaling pathways. Biochem. Biophys. Res. Commun. 2014, 450, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Mennini, T.; Gobbi, M. The antidepressant mechanism of Hypericum perforatum. Life Sci. 2004, 5, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Brenner, R.; Azbel, V.; Madhusoodanan, S.; Pawlowska, M. Comparison of an extract of hypericum (LI 160) and sertraline in the treatment of depression: A double-blind, randomized pilot study. Clin. Ther. 2000, 22, 411–419. [Google Scholar] [CrossRef]
- Schrader, E. Equivalence of St. John’s Wort extract (Ze 117) and fluoxetine: A randomized, controlled study in mild-moderate depression. Int. Clin. Psychopharmacol. 2000, 15, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.; Anderson, L.A.; Phillipson, J.D. St John’s Wort (Hypericum perforatum L.): A review of its chemistry, pharmacology and clinical properties. J. Pharm. Pharmacol. 2001, 53, 583–600. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, P.; Vantieghem, A.; Merlevede, W.; de Witte, P.A. Hypericin in cancer treatment: More light on the way. Int. J. Biochem. Cell Biol. 2002, 34, 221–241. [Google Scholar] [CrossRef]
- Fugh-Berman, A.; Ernest, E. Herb-drug interactions: Review and assessment of report reliability. Br. J. Clin. Pharmacol. 2001, 52, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Perloff, M.D.; von Moltke, L.L.; Stromer, E.; Shader, R.I.; Greenblatt, D.J. Saint John’s Wort: An in vitro analysis of P-glycoprotein induction due to extended exposure. Br. J. Pharmacol. 2001, 134, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, D.M.; Arceci, R.J. Clinical relevance of transmembrane drug efflux as a mechanism of multidrug resistance. J. Clin. Oncol. 1998, 16, 3674–3690. [Google Scholar] [CrossRef] [PubMed]
- Waka, A.; Sakaeda, T.; Takara, K.; Hirai, M.; Kinura, T.; Ohmoto, N.; Zhou, J.; Nakamura, T.; Kobayashi, H.; Okamura, N.; et al. Effect of St. John’s Wort and hypericin on cytotoxicity of anticancer drugs. Drug Metab. Pharmacokinet. 2002, 17, 467–474. [Google Scholar]
- Abdali, K.; Khajehei, M.; Tavatavaee, H.R. Effect of St. John’s Wort on severity, frequency, and duration of hot flashes in premenopausal, perimenopausal and postmenopausal women: A randomized, double-blind, placebo-controlled study. Menopause 2010, 17, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Al-Akoum, M.; Maunsell, E.; Verreault, R.; Provencher, L.; Otis, H.; Dodin, S. Effect of Hypericium perforatum (St. John’s Wort) on hot flashes and quality of life in perimenopausal women: A randomized pilot trial. Menopause 2009, 16, 307–314. [Google Scholar] [CrossRef] [PubMed]
- You, M.K.; Kim, D.W.; Jeong, K.S.; Bang, M.A.; Kim, H.S.; Rhuy, J.; Kim, H.A. St. John’s Wort (Hypericum perforatum) stimulates human osteoblastic MG-63 cell proliferation and attenuates trabecular bone loss induced by ovariectomy. Nutr. Res. Pract. 2015, 9, 459–465. [Google Scholar] [CrossRef] [PubMed]
- You, M.K.; Rhuy, J.; Jeong, K.S.; Bang, M.A.; Kim, M.S.; Kim, H.A. Effect of St. John’s Wort (Hypericum perforatum) on obesity, lipid metabolism and uterine epithelial proliferation in ovariectomized rats. Nutr. Res. Pract. 2014, 8, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Gallo, D.; Zannoni, G.F.; Apollpnio, P.; Martinelli, E.; Ferlini, C.; Pasetti, G.; Riva, A.; Morazzoni, P.; Bombardelli, E.; Scambia, G. Characterization of the pharmacologic profile of a standardized soy extract in the ovariectomized rat model of menopause: Effects on bone, uterus and lipid profile. Menopause 2005, 12, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Kwan, D.; Saxton, R.E.; McFadden, D.W. Hypericin and photodynamic therapy decreases human pancreatic cancer in vitro and in vivo. J. Surg. Res. 2000, 93, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Vanderwerf, Q.M.; Saxton, R.E.; Chang, A.; Horton, D.; Paiva, M.B.; Anderson, J.; Foote, C.; Soudant, J.; Mathey, A.; Castro, D.J. Hypericin: A new lase phototargeting agent for human cancer cells. Laryngoscope 1996, 106, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Ferenc, P.; Solar, P.; Kleban, J.; Mikes, J.; Fedorocko, P. Down-regulation of Bcl-2 and Akt induced by combination of photoactivated hypericin and genistein in human breast cancer cells. J. Photochem. Photobiol. 2010, 98, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Mirmalek, S.A.; Azizi, M.A.; Jangholi, E.; Yadollah-Damavandi, S.; Javidi, M.A.; Parsa, T.; Salimi-Tabatabaee, S.A.; Ghasemzadeh Kolagar, H.; Alizadeh-Navaei, R. Cytotoxic and apoptogenic effect of hypericin, the bioactive component of Hypericum perforatum on the MCF-7 human breast cancer cell line. Cancer Cell Int. 2016, 13, 3. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G. AMP-activated protein kinase-an energy sensor that regulates all aspects of cell function. Genes Dev. 2011, 25, 1895–1908. [Google Scholar] [CrossRef] [PubMed]
- Dowling, R.J.; Topisirovic, I.; Alain, T.; Bidinosti, M.; Fonseca, B.D.; Petroulakin, E.; Wang, X.; Larsson, O.; Selvarai, A.; Liu, Y.; et al. mTORC1-mediated cell proliferation, but not cell growth, controlled by the 4E-BPs. Science 2010, 328, 1172–1176. [Google Scholar] [CrossRef] [PubMed]
- Marone, R.; Cmiljanovic, V.; Giese, B.; Wymann, M.P. Targeting phosphoinositide 3-kinase-moving towards therapy. Biochim. Biophys. Acta 2008, 1784, 159–185. [Google Scholar] [CrossRef] [PubMed]
- Pause, A.; Methot, N.; Svitkin, Y.; Merrick, W.C.; Sonenberg, N. Dominant negative mutants of mammalian translation initiation factor eIF-4A define a critical role for eIF-4F in cap-dependent and cap-independent initiation of translation. EMBO J. 1994, 13, 1205–1215. [Google Scholar] [PubMed]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Igney, F.H.; Krammer, P.H. Death and anti-death: Tumour resistance to apoptosis. Nature 2002, 2, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Nunez, G.; Benedict, M.A.; Hu, Y.; Inihara, N. Caspases: The proteases of the apoptosis. Cardiovasc. Res. 1998, 45, 528–537. [Google Scholar]
- Janicke, R.U.; Sprengart, M.L.; Wati, M.R.; Prtter, A.G. Caspase-3 is required for DNA fragmentation and morphological changers associated with apoptosis. J. Biol. Chem. 1998, 273, 9357–9360. [Google Scholar] [CrossRef] [PubMed]
- Strasser, A.; O’Connor, L.; Dixit, V.M. Apoptosis signaling. Annu. Rev. Biochem. 2000, 69, 217–245. [Google Scholar] [CrossRef] [PubMed]
- Bortner, C.D.; Oldenburg, N.B.; Cidlowski, J.A. The role of DNA fragmentation in apoptosis. Trends Cell Biol. 1995, 5, 21–26. [Google Scholar] [CrossRef]
- Borders, E.B.; Bivona, C.; Medina, P.J. Mammalian target of rapamycin: Biological function and target for novel anticancer agents. Am. J. Health Syst. Pharm. 2010, 67, 2095–2106. [Google Scholar] [CrossRef] [PubMed]
- Yap, T.A.; Garrett, M.D.; Walton, M.I.; Raynaud, F.; de Bono, J.S.; Workman, P. Targeting the PI3K-AKT-mTOR pathway: Progress, pitfalls and promises. Curr. Opin. Pharmacol. 2008, 8, 393–412. [Google Scholar] [CrossRef] [PubMed]
- Giannelli, G.; Sgarra, C.; Porcelli, L.; Azzariti, A.; Salvotore, A.; Paradiso, A. EGFR and VEGFR as potential target for biological therapies in HCC cells. Cancer Lett. 2008, 261, 527–564. [Google Scholar] [CrossRef] [PubMed]
- Willis, S.N.; Chen, L.; Dewson, G.; Wei, A.; Naik, E.; Fletcher, J.I.; Adams, J.M.; Huang, D.C. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005, 19, 1294–1305. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.R.; Ranger, A.M.; Lin, M.Z.; Sturgill, J.F.; Ma, Y.C.; Cowan, C.W.; Dikkes, P.; Korsmeyer, S.J.; Greenberg, M.E. Survival factor-mediated BAD phosphorylation raises the mitochondrial threshold for apoptosis. Dev. Cell 2002, 3, 631–643. [Google Scholar] [CrossRef]
- Nachshon-Kedmi, M.; Yannai, S.; Fares, F.A. Induction of apoptosis in human prostate cancer cell line, PC3, by 3,3′-diindolylmethane through the mitochondrial pathway. Br. J. Cancer 2004, 91, 1358–1363. [Google Scholar] [CrossRef] [PubMed]
- Cardone, M.H.; Roy, N.; Stennicke, H.R.; Salvesen, G.S.; Franke, T.F.; Stanbridge, E.; Frisch, S.; Reed, J.C. Regulation of cell death protease caspase-9 by phosphorylation. Science 1998, 282, 1318–1321. [Google Scholar] [CrossRef] [PubMed]
- Schempp, C.M.; Kirkin, V.; Simon-Haarhaus, B.; Kersten, A.; Kiss, J.; Termeer, C.C.; Cilb, B.; Kaufmann, T.; Borner, C.; Sleeman, J.P.; et al. Inhibition of tumour cell growth by hypericin, a novel anticancer drug from St. John’s Wort that acts by induction of apoptosis. Oncogene 2002, 21, 1242–1250. [Google Scholar] [CrossRef] [PubMed]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, M.-K.; Kim, H.-J.; Kook, J.H.; Kim, H.-A. St. John’s Wort Regulates Proliferation and Apoptosis in MCF-7 Human Breast Cancer Cells by Inhibiting AMPK/mTOR and Activating the Mitochondrial Pathway. Int. J. Mol. Sci. 2018, 19, 966. https://doi.org/10.3390/ijms19040966
You M-K, Kim H-J, Kook JH, Kim H-A. St. John’s Wort Regulates Proliferation and Apoptosis in MCF-7 Human Breast Cancer Cells by Inhibiting AMPK/mTOR and Activating the Mitochondrial Pathway. International Journal of Molecular Sciences. 2018; 19(4):966. https://doi.org/10.3390/ijms19040966
Chicago/Turabian StyleYou, Mi-Kyoung, Hwa-Jin Kim, Ji Hyun Kook, and Hyeon-A Kim. 2018. "St. John’s Wort Regulates Proliferation and Apoptosis in MCF-7 Human Breast Cancer Cells by Inhibiting AMPK/mTOR and Activating the Mitochondrial Pathway" International Journal of Molecular Sciences 19, no. 4: 966. https://doi.org/10.3390/ijms19040966
APA StyleYou, M.-K., Kim, H.-J., Kook, J. H., & Kim, H.-A. (2018). St. John’s Wort Regulates Proliferation and Apoptosis in MCF-7 Human Breast Cancer Cells by Inhibiting AMPK/mTOR and Activating the Mitochondrial Pathway. International Journal of Molecular Sciences, 19(4), 966. https://doi.org/10.3390/ijms19040966