The Sealing Zone in Osteoclasts: A Self-Organized Structure on the Bone
Abstract
:1. Introduction
2. Bone Resorption by Osteoclasts
3. Structure of the Sealing Zone
3.1. Podosomes and the Sealing Zone
3.2. The Sealing Zone on Bone Is Different from the Podosome Belt on Glass
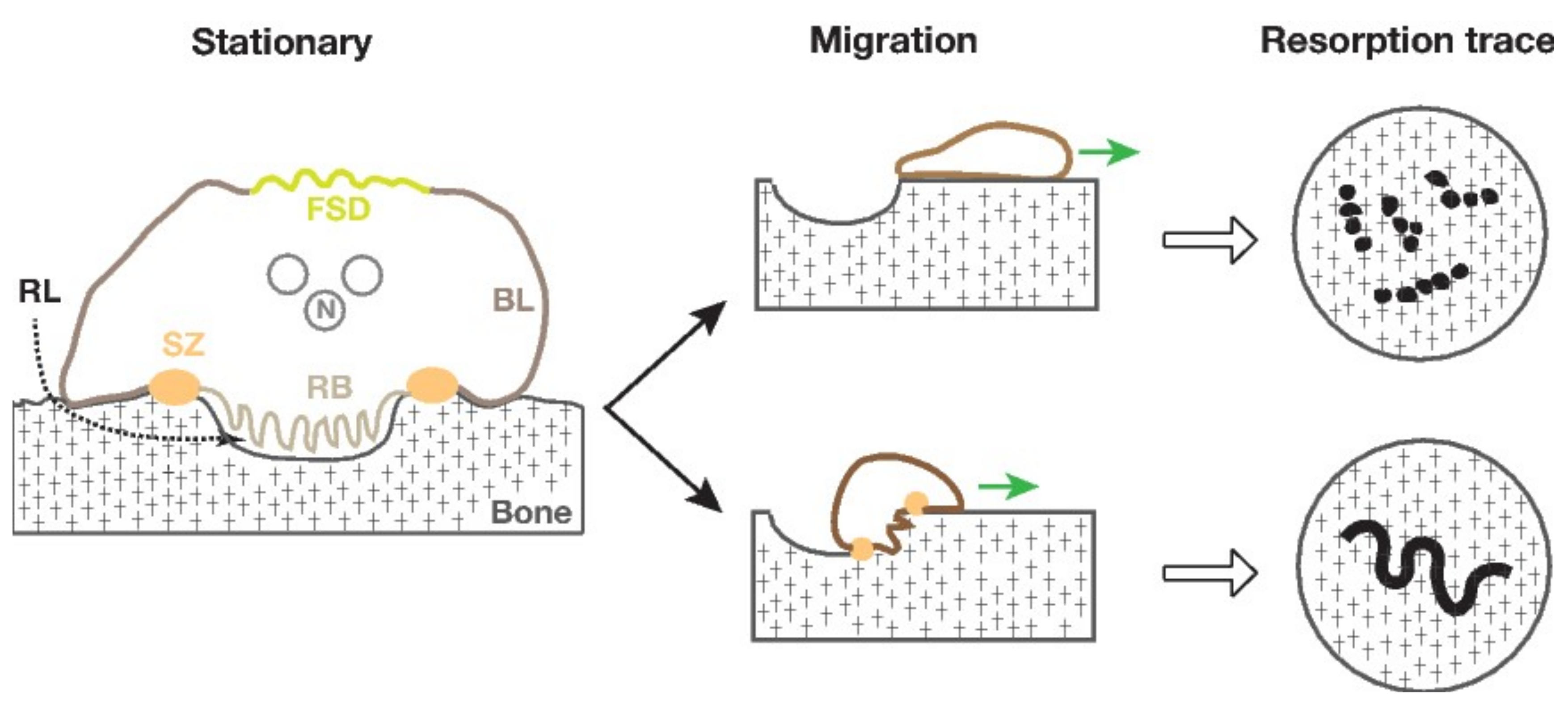
3.3. Size of the Sealing Zone
4. Actin Flow
4.1. Discovery of Self-Organized Actin Flow in Osteoclasts
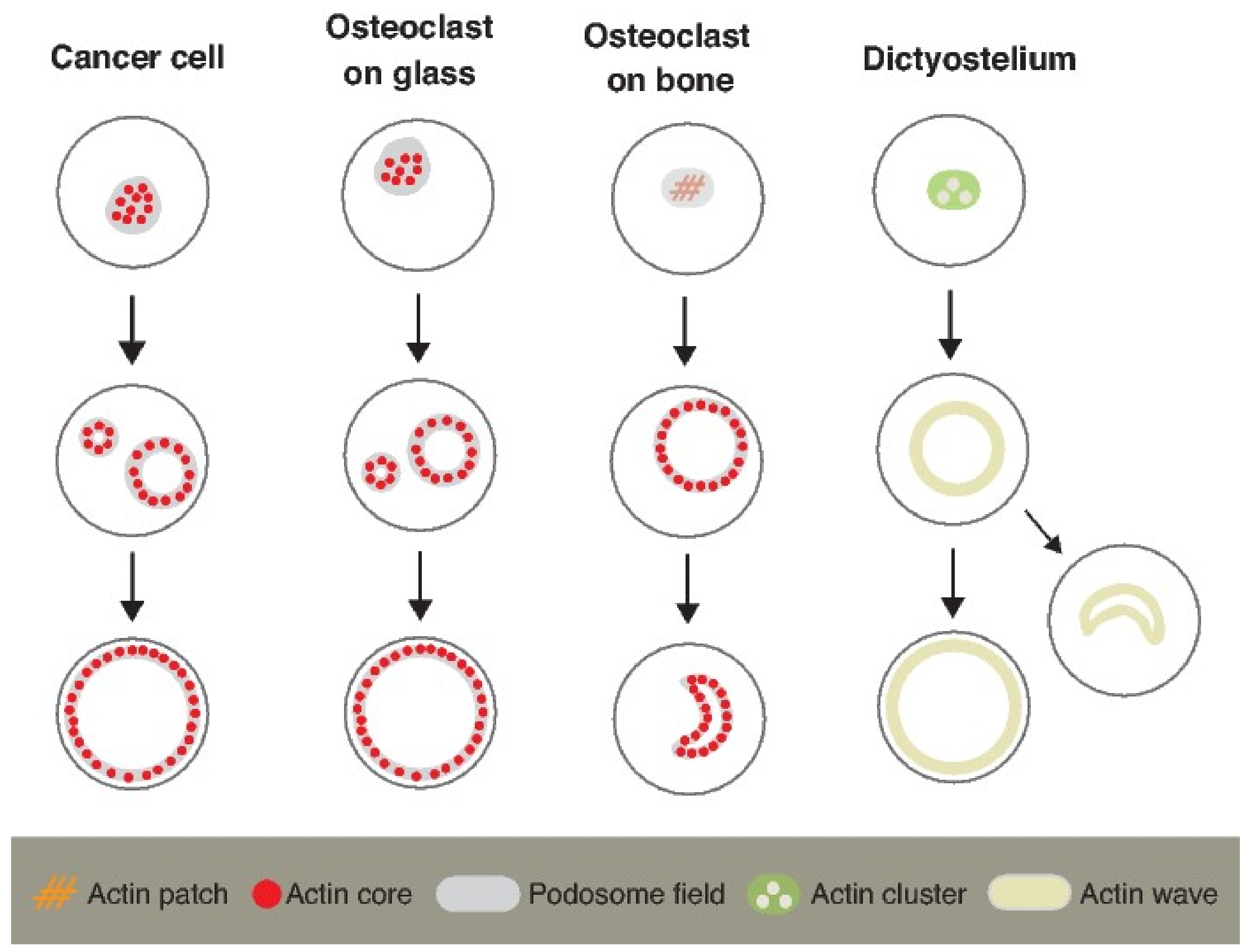
4.2. Actin Waves in Dictyostelium Cells and Actin Flow in Osteoclasts
4.3. The Behavior of the Actin Wavse in a Large Space
5. Organization of the Podosome Field
5.1. Two Substructures of the Podosome Belt
5.2. Formation of Actin Core with the Adhesion Domain
5.3. Formation of the Actin Flow
6. Functions of the Sealing Zone
6.1. Diffusion Barrier
6.2. Matrix Degradation
7. Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Linder, S.; Wiesner, C.; Himmel, M. Degrading devices: Invadosomes in proteolytic cell invasion. Annu. Rev. Cell Dev. Biol. 2011, 27, 185–211. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.A.; Courtneidge, S.A. The “ins” and “outs” of podosomes and invadopodia: Characteristics, formation and function. Nat. Rev. Mol. Cell Biol. 2011, 12, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Albiges-Rizo, C.; Destaing, O.; Fourcade, B.; Planus, E.; Block, M.R. Actin machinery and mechanosensitivity in invadopodia, podosomes and focal adhesions. J. Cell Sci. 2009, 122, 3037–3049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linder, S.; Wiesner, C. Feel the force: Podosomes in mechanosensing. Exp. Cell Res. 2016, 343, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, J.; Henriet, E.; Ezzoukhry, Z.; Goetz, J.G.; Moreau, V.; Saltel, F. The microenvironment controls invadosome plasticity. J. Cell Sci. 2016, 129, 1759–1768. [Google Scholar] [CrossRef] [PubMed]
- Takito, J.; Otsuka, H.; Inoue, S.; Kawashima, T.; Nakamura, M. Symmetrical retrograde actin flow in the actin fusion structure is involved in osteoclast fusion. Biol. Open 2017, 6, 1104–1114. [Google Scholar] [CrossRef] [PubMed]
- Takito, J.; Inoue, S.; Nakamura, M. Emerging role of actin flow in the organization of podosomes in osteoclasts. Macrophage 2017, 4, e1614. [Google Scholar]
- Teti, A.; Marchisio, P.C.; Zallone, A.Z. Clear zone in osteoclast function: Role of podosomes in regulation of bone-resorbing activity. Am. J. Physiol. 1991, 261, C1–C7. [Google Scholar] [CrossRef] [PubMed]
- Väänänen, H.K.; Zhao, H.; Mulari, M.; Halleen, J.M. The cell biology of osteoclast function. J. Cell Sci. 2000, 113, 377–381. [Google Scholar] [PubMed]
- Baron, R.; Neff, L.; Louvard, D.; Courtoy, P.J. Cell-mediated extracellular acidification and bone resorption: Evidence for a low pH in resorbing lacunae and localization of a 100-kD lysosomal membrane protein at the osteoclast ruffled border. J. Cell Biol. 1985, 101, 2210–2222. [Google Scholar] [CrossRef] [PubMed]
- Blair, H.C.; Teitelbaum, S.L.; Ghiselli, R.; Gluck, S. Osteoclastic bone resorption by a polarized vacuolar proton pump. Science 1989, 245, 855–857. [Google Scholar] [CrossRef] [PubMed]
- Nesbitt, S.A.; Horton, M.A. Trafficking of matrix collagens through bone-resorbing osteoclasts. Science 1997, 276, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Salo, J.; Lehenkari, P.; Mulari, M.; Metsikkö, K.; Väänänen, H.K. Removal of osteoclast bone resorption products by transcytosis. Science 1997, 276, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Rumpler, M.; Wurger, T.; Roschger, P.; Zwettler, E.; Sturmlechner, I.; Altmann, P.; Fratzl, P.; Rogers, M.J.; Klaushofer, K. Osteoclasts on bone and dentin in vitro: Mechanism of trail formation and comparison of resorption behavior. Calcif. Tissue Int. 2013, 93, 526–539. [Google Scholar] [CrossRef] [PubMed]
- Søe, K.; Delaissé, J.M. Time-lapse reveals that osteoclasts can move across the bone surface while resorbing. J. Cell Sci. 2017, 130, 2026–2035. [Google Scholar] [CrossRef] [PubMed]
- Soriano, P.; Montgomery, C.; Geske, R.; Bradley, A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell 1991, 64, 693–702. [Google Scholar] [CrossRef]
- Boyce, B.F.; Yoneda, T.; Lowe, C.; Soriano, P.; Mundy, G.R. Requirement of pp60c-src expression for osteoclasts to form ruffled borders and resorb bone in mice. J. Clin. Investig. 1992, 90, 1622–1627. [Google Scholar] [CrossRef] [PubMed]
- Schenk, R.K.; Spiro, D.; Wiener, J. Cartilage resorption in the tibial epiphyseal plate of growing rats. J. Cell Biol. 1967, 34, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Marchisio, P.C.; Cirillo, D.; Naldini, L.; Primavera, M.V.; Teti, A.; Zambonin-Zallone, A. Cell-substratum interaction of cultured avian osteoclasts is mediated by specific adhesion structures. J. Cell Biol. 1984, 99, 1696–1705. [Google Scholar] [CrossRef] [PubMed]
- Destaing, O.; Saltel, F.; Geminard, J.C.; Jurdic, P.; Bard, F. Podosomes display actin turnover and dynamic self-organization in osteoclasts expressing actin-green fluorescent protein. Mol. Biol. Cell 2003, 14, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Jurdic, P.; Saltel, F.; Chabadel, A.; Destaing, O. Podosome and sealing zone: Specificity of the osteoclast model. Eur. J. Cell Biol. 2006, 85, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Luxenburg, C.; Geblinger, D.; Klein, E.; Anderson, K.; Hanein, D.; Geiger, B.; Addadi, L. The architecture of the adhesive apparatus of cultured osteoclasts: From podosome formation to sealing zone assembly. PLoS ONE 2007, 2, e179. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, I.; Takahashi, N.; Sasaki, T.; Jimi, E.; Kurokawa, T.; Suda, T. Chemical and physical properties of the extracellular matrix are required for the actin ring formation in osteoclasts. J. Bone Miner. Res. 1996, 11, 1873–1879. [Google Scholar] [CrossRef] [PubMed]
- Saltel, F.; Destaing, O.; Bard, F.; Eichert, D.; Jurdic, P. Apatite-mediated actin dynamics in resorbing osteoclasts. Mol. Biol. Cell 2004, 15, 5231–5241. [Google Scholar] [CrossRef] [PubMed]
- Collin, O.; Na, S.; Chowdhury, F.; Hong, M.; Shin, M.E.; Wang, F.; Wang, N. Self-organized podosomes are dynamic mechanosensors. Curr. Biol. 2008, 18, 1288–1294. [Google Scholar] [CrossRef] [PubMed]
- Labernadie, A.; Bouissou, A.; Delobelle, P.; Balor, S.; Voituriez, R.; Proag, A.; Fourquaux, I.; Thibault, C.; Vieu, C.; Poincloux, R.; et al. Protrusion force microscopy reveals oscillatory force generation and mechanosensing activity of human macrophage podosomes. Nat. Commun. 2014, 5, 5343. [Google Scholar] [CrossRef] [PubMed]
- Van den Dries, K.; Schwartz, S.L.; Byars, J.; Meddens, M.B.; Bolomini-Vittori, M.; Lidke, D.S.; Figdor, C.G.; Lidke, K.A.; Cambi, A. Dual-color superresolution microscopy reveals nanoscale organization of mechanosensory podosomes. Mol. Biol. Cell 2013, 24, 2112–2123. [Google Scholar] [CrossRef] [PubMed]
- Badowski, C.; Pawlak, G.; Grichine, A.; Chabadel, A.; Oddou, C.; Jurdic, P.; Pfaff, M.; Albiges-Rizo, C.; Block, M.R. Paxillin phosphorylation controls invadopodia/podosomes spatiotemporal organization. Mol. Biol. Cell 2008, 19, 633–645. [Google Scholar] [CrossRef] [PubMed]
- Ory, S.; Brazier, H.; Pawlak, G.; Blangy, A. Rho GTPases in osteoclasts: Orchestrators of podosome arrangement. Eur. J. Cell Biol. 2008, 87, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, J.; Yamanaka, T.; Doi, S.; Turksen, K.; Heersche, J.N.; Aubin, J.E.; Takeuchi, H. A band of F-actin containing podosomes is involved in bone resorption by osteoclasts. Bone 1990, 11, 287–293. [Google Scholar] [CrossRef]
- Takito, J.; Nakamura, M.; Yoda, M.; Tohmonda, T.; Uchikawa, S.; Horiuchi, K.; Toyama, Y.; Chiba, K. The transient appearance of zipper-like actin superstructures during the fusion of osteoclasts. J. Cell Sci. 2012, 125, 662–672. [Google Scholar] [CrossRef] [PubMed]
- Takito, J.; Nakamura, M. Precursors linked via the zipper-like structure or the filopodium during the secondary fusion of osteoclasts. Commun. Integr. Biol. 2012, 5, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Allard, J.; Mogilner, A. Traveling waves in actin dynamics and cell motility. Curr. Opin. Cell Biol. 2013, 25, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, N.; Katsuno, H. Actin Waves: Origin of Cell Polarization and Migration? Trends Cell Biol. 2017, 27, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Bretschneider, T.; Anderson, K.; Ecke, M.; Muller-Taubenberger, A.; Schroth-Diez, B.; Ishikawa-Ankerhold, H.C.; Gerisch, G. The three-dimensional dynamics of actin waves, a model of cytoskeletal self-organization. Biophys. J. 2009, 96, 2888–2900. [Google Scholar] [CrossRef] [PubMed]
- Schroth-Diez, B.; Gerwig, S.; Ecke, M.; Hegerl, R.; Diez, S.; Gerisch, G. Propagating waves separate two states of actin organization in living cells. HFSP J. 2009, 3, 412–427. [Google Scholar] [CrossRef] [PubMed]
- Gerisch, G.; Ecke, M.; Wischnewski, D.; Schroth-Diez, B. Different modes of state transitions determine pattern in the Phosphatidylinositide-Actin system. BMC Cell Biol. 2011, 12, 42. [Google Scholar] [CrossRef] [PubMed]
- McMichael, B.K.; Cheney, R.E.; Lee, B.S. Myosin X regulates sealing zone patterning in osteoclasts through linkage of podosomes and microtubules. J. Biol. Chem. 2010, 285, 9506–9515. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, M.; Ecke, M.; Walz, M.; Stengl, A.; Beta, C.; Gerisch, G. Actin and PIP3 waves in giant cells reveal the inherent length scale of an excited state. J. Cell Sci. 2014, 127, 4507–4517. [Google Scholar] [CrossRef] [PubMed]
- Yagi, M.; Miyamoto, T.; Sawatani, Y.; Iwamoto, K.; Hosogane, N.; Fujita, N.; Morita, K.; Ninomiya, K.; Suzuki, T.; Miyamoto, K.; et al. DC-STAMP is essential for cell–cell fusion in osteoclasts and foreign body giant cells. J. Exp. Med. 2005, 202, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Palokangas, H.; Mulari, M.; Väänänen, H.K. Endocytic pathway from the basal plasma membrane to the ruffled border membrane in bone-resorbing osteoclasts. J. Cell Sci. 1997, 110, 1767–1780. [Google Scholar] [PubMed]
- Kuroda, H.; Nakamura, M.; Kamiyama, K. Effects of calcitonin and parathyroid hormone on the distribution of F-actin in the clear zone of osteoclasts in vivo. Bone 1996, 19, 115–120. [Google Scholar] [CrossRef]
- Batsir, S.; Geiger, B.; Kam, Z. Dynamics of the sealing zone in cultured osteoclasts. Cytoskeleton 2017, 74, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Pollard, T.D.; Borisy, G.G. Cellular motility driven by assembly and disassembly of actin filaments. Cell 2003, 112, 453–465. [Google Scholar] [CrossRef]
- Chabadel, A.; Banon-Rodriguez, I.; Cluet, D.; Rudkin, B.B.; Wehrle-Haller, B.; Genot, E.; Jurdic, P.; Anton, I.M.; Saltel, F. CD44 and β3 integrin organize two functionally distinct actin-based domains in osteoclasts. Mol. Biol. Cell 2007, 18, 4899–4910. [Google Scholar] [CrossRef] [PubMed]
- Saltel, F.; Chabadel, A.; Bonnelye, E.; Jurdic, P. Actin cytoskeletal organisation in osteoclasts: A model to decipher transmigration and matrix degradation. Eur. J. Cell Biol. 2008, 87, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Chellaiah, M.; Kizer, N.; Silva, M.; Alvarez, U.; Kwiatkowski, D.; Hruska, K.A. Gelsolin deficiency blocks podosome assembly and produces increased bone mass and strength. J. Cell Biol. 2000, 148, 665–678. [Google Scholar] [CrossRef] [PubMed]
- Calle, Y.; Jones, G.E.; Jagger, C.; Fuller, K.; Blundell, M.P.; Chow, J.; Chambers, T.; Thrasher, A.J. WASp deficiency in mice results in failure to form osteoclast sealing zones and defects in bone resorption. Blood 2004, 103, 3552–3561. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Nakchbandi, I.; Ruppert, R.; Kawelke, N.; Hess, M.W.; Pfaller, K.; Jurdic, P.; Fässler, R.; Moser, M. Kindlin-3-mediated signaling from multiple integrin classes is required for osteoclast-mediated bone resorption. J. Cell Biol. 2011, 192, 883–897. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.H.; Rafiq, N.B.; Krishnasamy, A.; Hartman, K.L.; Jones, G.E.; Bershadsky, A.D.; Sheetz, M.P. Integrin-matrix clusters form podosome-like adhesions in the absence of traction forces. Cell Rep. 2013, 5, 1456–1468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alberti, S. The wisdom of crowds: Regulating cell function through condensed states of living matter. J. Cell Sci. 2017, 130, 2789–2796. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.; Brangwynne, C.P. Liquid phase condensation in cell physiology and disease. Science 2017, 357, eaaf4382. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, A.E. Dendritic actin filament nucleation causes traveling waves and patches. Phys. Rev. Lett. 2010, 104, 228102. [Google Scholar] [CrossRef] [PubMed]
- Kawska, A.; Carvalho, K.; Manzi, J.; Boujemaa-Paterski, R.; Blanchoin, L.; Martiel, J.L.; Sykes, C. How actin network dynamics control the onset of actin-based motility. Proc. Natl. Acad. Sci. USA 2012, 109, 14440–14445. [Google Scholar] [CrossRef] [PubMed]
- Loisel, T.P.; Boujemaa, R.; Pantaloni, D.; Carlier, M.F. Reconstitution of actin-based motility of Listeria and Shigella using pure proteins. Nature 1999, 401, 613–616. [Google Scholar] [CrossRef] [PubMed]
- Bernheim-Groswasser, A.; Wiesner, S.; Golsteyn, R.M.; Carlier, M.F.; Sykes, C. The dynamics of actin-based motility depend on surface parameters. Nature 2002, 417, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Glansdorff, P.; Prigogine, I. Thermodynamic Theory of Structure, Stability and Fluctuations; Wiley-Interscience: London, UK, 1978; pp. 247–271. ISBN 0471302805. [Google Scholar]
- Gerisch, G.; Ecke, M.; Schroth-Diez, B.; Gerwig, S.; Engel, U.; Maddera, L.; Clarke, M. Self-organizing actin waves as planar phagocytic cup structures. Cell Adhes. Migr. 2009, 3, 373–382. [Google Scholar] [CrossRef]
- Silver, I.A.; Murrills, R.J.; Etherington, D.J. Microelectrode studies on the acid microenvironment beneath adherent macrophages and osteoclasts. Exp. Cell Res. 1988, 175, 266–276. [Google Scholar] [CrossRef]
- Väänänen, H.K.; Horton, M. The osteoclast clear zone is a specialized cell-extracellular matrix adhesion structure. J. Cell Sci. 1995, 108, 2729–2732. [Google Scholar] [PubMed]
- Stenbeck, G.; Horton, M.A. A new specialized cell-matrix interaction in actively resorbing osteoclasts. J. Cell Sci. 2000, 113, 1577–1587. [Google Scholar] [PubMed]
- Takagi, M.; Yagasaki, H.; Baba, T.; Baba, H. Ultrastructural visualization of selective peanut agglutinin binding sites in rat osteoclasts. J. Histochem. Cytochem. 1988, 36, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Salo, J.; Metsikkö, K.; Palokangas, H.; Lehenkari, P.; Väänänen, H.K. Bone-resorbing osteoclasts reveal a dynamic division of basal plasma membrane into two different domains. J. Cell Sci. 1996, 109, 301–307. [Google Scholar] [PubMed]
- Golebiewska, U.; Kay, J.G.; Masters, T.; Grinstein, S.; Im, W.; Pastor, R.W.; Scarlata, S.; McLaughlin, S. Evidence for a fence that impedes the diffusion of phosphatidylinositol 4,5-bisphosphate out of the forming phagosomes of macrophages. Mol. Biol. Cell 2011, 22, 3498–3507. [Google Scholar] [CrossRef] [PubMed]
- Freeman, S.A.; Goyette, J.; Furuya, W.; Woods, E.C.; Bertozzi, C.R.; Bergmeier, W.; Hinz, B.; van der Merwe, P.A.; Das, R.; Grinstein, S. Integrins Form an Expanding Diffusional Barrier that Coordinates Phagocytosis. Cell 2016, 164, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, I.; Pilkington, M.F.; Lakkakorpi, P.T.; Lipfert, L.; Sims, S.M.; Dixon, S.J.; Rodan, G.A.; Duong, L.T. Role of α(v)β(3) integrin in osteoclast migration and formation of the sealing zone. J. Cell Sci. 1999, 112, 3985–3993. [Google Scholar] [PubMed]
- Mulari, M.T.; Zhao, H.; Lakkakorpi, P.T.; Väänänen, H.K. Osteoclast ruffled border has distinct subdomains for secretion and degraded matrix uptake. Traffic 2003, 4, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Edwards, J.C.; Hruska, K.A. Cellular distribution and regulation of NHE-1 isoform of the NA-H exchanger in the avian osteoclast. Bone 1996, 18, 87–95. [Google Scholar] [CrossRef]
- Henriksen, K.; Sørensen, M.G.; Jensen, V.K.; Dziegiel, M.H.; Nosjean, O.; Karsdal, M.A. Ion transporters involved in acidification of the resorption lacuna in osteoclasts. Calcif. Tissue Int. 2008, 83, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Merrild, D.M.; Pirapaharan, D.C.; Andreasen, C.M.; Kjærsgaard-Andersen, P.; Møller, A.M.; Ding, M.; Delaissé, J.M.; Søe, K. Pit- and trench-forming osteoclasts: A distinction that matters. Bone Rep. 2015, 3, 15032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.T. Proteolytic activity of specialized surface protrusions formed at rosette contact sites of transformed cells. J. Exp. Zool 1989, 251, 167–185. [Google Scholar] [CrossRef] [PubMed]
- Bourguignon, L.Y.; Singleton, P.A.; Diedrich, F.; Stern, R.; Gilad, E. CD44 interaction with Na+–H+ exchanger (NHE1) creates acidic microenvironments leading to hyaluronidase-2 and cathepsin B activation and breast tumor cell invasion. J. Biol. Chem. 2004, 279, 26991–27007. [Google Scholar] [CrossRef] [PubMed]
- Busco, G.; Cardone, R.A.; Greco, M.R.; Bellizzi, A.; Colella, M.; Antelmi, E.; Mancini, M.T.; Dell’Aquila, M.E.; Casavola, V.; Paradiso, A.; et al. NHE1 promotes invadopodial ECM proteolysis through acidification of the peri-invadopodial space. FASEB J. 2010, 24, 3903–3915. [Google Scholar] [CrossRef] [PubMed]
- Greco, M.R.; Antelmi, E.; Busco, G.; Guerra, L.; Rubino, R.; Casavola, V.; Reshkin, S.J.; Cardone, R.A. Protease activity at invadopodial focal digestive areas is dependent on NHE1-driven acidic pHe. Oncol. Rep. 2014, 31, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Astro, V.; de Curtis, I. Plasma membrane-associated platforms: Dynamic scaffolds that organize membrane-associated events. Sci. Signal. 2015, 8, re1. [Google Scholar] [CrossRef] [PubMed]
- Sala, K.; Raimondi, A.; Tonoli, D.; Tacchetti, C.; de Curtis, I. Identification of a membrane-less compartment regulating invadosome function and motility. Sci. Rep. 2018, 8, 1164. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takito, J.; Inoue, S.; Nakamura, M. The Sealing Zone in Osteoclasts: A Self-Organized Structure on the Bone. Int. J. Mol. Sci. 2018, 19, 984. https://doi.org/10.3390/ijms19040984
Takito J, Inoue S, Nakamura M. The Sealing Zone in Osteoclasts: A Self-Organized Structure on the Bone. International Journal of Molecular Sciences. 2018; 19(4):984. https://doi.org/10.3390/ijms19040984
Chicago/Turabian StyleTakito, Jiro, Satoshi Inoue, and Masanori Nakamura. 2018. "The Sealing Zone in Osteoclasts: A Self-Organized Structure on the Bone" International Journal of Molecular Sciences 19, no. 4: 984. https://doi.org/10.3390/ijms19040984
APA StyleTakito, J., Inoue, S., & Nakamura, M. (2018). The Sealing Zone in Osteoclasts: A Self-Organized Structure on the Bone. International Journal of Molecular Sciences, 19(4), 984. https://doi.org/10.3390/ijms19040984



