Novel Targets for Treating Ischemia-Reperfusion Injury in the Liver
Abstract
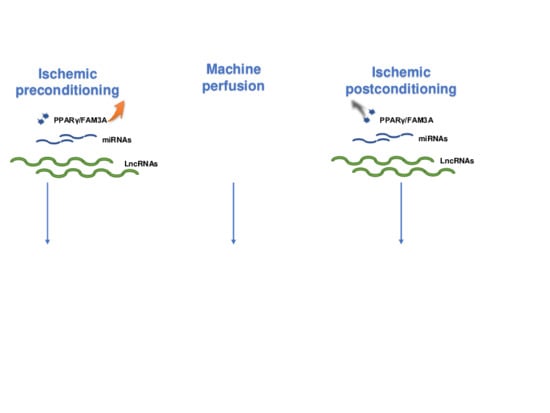
:1. Introduction
2. Current Strategies for Ameliorating Liver Ischemia-Reperfusion Injury (IRI)
3. PPARγ in Liver IRI
4. FAM3A in PPARγ’s Protection in Liver IRI
5. miRNAs and Liver IRI
5.1. miR-122 and Liver IRI
5.2. miR-34a and Liver IRI
5.3. miR-223 and Liver IRI
5.4. miR-370 and Liver IRI
5.5. miR-155 and liver IRI
5.6. Other miRNAs and Liver IRI
6. LncRNAs and Liver IRI
7. Summary and Perspective
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Saidi, R.F.; Kenari, S.K. Liver ischemia/reperfusion injury: An overview. J. Investig. Surg. 2014, 27, 366–379. [Google Scholar] [CrossRef] [PubMed]
- Nastos, C.; Kalimeris, K.; Papoutsidakis, N.; Tasoulis, M.K.; Lykoudis, P.M.; Theodoraki, K.; Nastou, D.; Smyrniotis, V.; Arkadopoulos, N. Global consequences of liver ischemia/reperfusion injury. Oxid. Med. Cell. Longev. 2014, 2014, 906965. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Palanisamy, A.P.; Evans, Z.P.; Sutter, A.G.; Jin, L.; Singh, I.; May, H.; Schmidt, M.G.; Chavin, K.D. Cerulenin blockade of fatty acid synthase reverses hepatic steatosis in ob/ob mice. PLoS ONE 2013, 8, e75980. [Google Scholar] [CrossRef] [PubMed]
- Covington, S.M.; Bauler, L.D.; Toledo-Pereyra, L.H. Akt: A Therapeutic Target in Hepatic Ischemia–Reperfusion Injury. J. Investig. Surg. 2016, 30, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Quillin, R.C., 3rd; Guarrera, J.V. Hypothermic machine perfusion in liver transplantation. Liver Transpl. 2018, 24, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Orci, L.A.; Lacotte, S.; Delaune, V.; Slits, F.; Oldani, G.; Lazarevic, V.; Rossetti, C.; Rubbia-Brandt, L.; Morel, P.; Toso, C. Effects of the gut-liver axis on ischemia-mediated hepatocellular carcinoma recurrence in the mouse liver. J. Hepatol. 2018, 68, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.I.; Liu, Z.; Chen, Y.; Xu, K.; Dong, J. PPARgamma activation reduces ischemia/reperfusion-induced metastasis in a murine model of hepatocellular carcinoma. Exp. Ther. Med. 2016, 11, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Rabb, H.; Womer, K.L. Ischemia-reperfusion and immediate T cell responses. Cell Immunol. 2007, 248, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Gracia-Sancho, J.; Casillas-Ramirez, A.; Peralta, C. Molecular pathways in protecting the liver from ischaemia/reperfusion injury: A 2015 update. Clin. Sci. 2015, 129, 345–362. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Xin, Z.; Di, W.; Yan, X.; Li, X.; Reiter, R.J.; Yang, Y. Melatonin and mitochondrial function during ischemia/reperfusion injury. Cell. Mol. Life Sci. 2017, 74, 3989–3998. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Carroll, M.C. Natural IgM-mediated innate autoimmunity: A new target for early intervention of ischemia-reperfusion injury. Expert Opin. Biol. Ther. 2007, 7, 1575–1582. [Google Scholar] [CrossRef] [PubMed]
- Cannistrà, M.; Ruggiero, M.; Zullo, A.; Gallelli, G.; Serafini, S.; Maria, M.; Naso, A.; Grande, R.; Serra, R.; Nardo, B. Original research: Hepatic ischemia reperfusion injury: A systematic review of literature and the role of current drugs and biomarkers. Int. J. Surg. 2016, 33, S57–S70. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.J.; Li, J.H.; Jiang, L.; Lin, B.Y.; Wang, L.; Su, R.; Zhou, L.; Zheng, S.S. Liver protection strategies in liver transplantation. Hepatobiliary Pancreat. Dis. Int. 2015, 14, 34–42. [Google Scholar] [CrossRef]
- Jeong, J.S.; Kim, D.; Kim, K.Y.; Ryu, S.; Han, S.; Shin, B.S.; Kim, G.S.; Gwak, M.S.; Ko, J.S. Ischemic preconditioning produces comparable protection against hepatic ischemia/reperfusion injury under isoflurane and sevoflurane anesthesia in rats. Transplant. Proc. 2017, 49, 2188–2193. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, T.N.; Victorino, J.P.; Bistafa Liu, J.; Tofoli Queiroz Campos, D.; Graf, C.; Jordani, M.C.; Carneiro D’albuquerque, L.A.; Mendes, K.D.S.; Castro, E.S.O. Effect of hepatic preconditioning with the use of methylene blue on the liver of wistar rats submitted to ischemia and reperfusion. Transplant. Proc. 2018, 50, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Pontes, H.B.D.; Pontes, J.; Azevedo Neto, E.; Vendas, G.; Miranda, J.V.C.; Dias, L.; Oliva, J.; Almeida, M.H.M.; Chaves, I.O.; Sampaio, T.L.; et al. Evaluation of the effects of atorvastatin and ischemic postconditioning preventing on the ischemia and reperfusion injury: experimental study in rats. Braz. J. Cardiovasc. Surg. 2018, 33, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Alva, N.; Bardallo, R.G.; Basanta, D.; Palomeque, J.; Carbonell, T. Preconditioning-like properties of short-term hypothermia in isolated perfused rat liver (IPRL) system. Int. J. Mol. Sci. 2018, 19, 1023. [Google Scholar] [CrossRef] [PubMed]
- Camara-Lemarroy, C.R.; Guzman-de la Garza, F.J.; Alarcon-Galvan, G.; Cordero-Perez, P.; Munoz-Espinosa, L.; Torres-Gonzalez, L.; Fernandez-Garza, N.E. Hepatic ischemia/reperfusion injury is diminished by atorvastatin in Wistar rats. Arch Med. Res. 2014, 45, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Theodoraki, K.; Karmaniolou, I.; Tympa, A.; Tasoulis, M.K.; Nastos, C.; Vassiliou, I.; Arkadopoulos, N.; Smyrniotis, V. Beyond preconditioning: postconditioning as an alternative technique in the prevention of liver ischemia-reperfusion injury. Oxid. Med. Cell. Longev. 2016, 2016, 8235921. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, A.A.; Kalisvaart, M.; Muiesan, P. Machine perfusion in liver transplantation: An essential treatment or just an expensive toy? Minerva Anestesiol. 2018, 84, 236–245. [Google Scholar] [PubMed]
- Donato, M.; Evelson, P.; Gelpi, R.J. Protecting the heart from ischemia/reperfusion injury: An update on remote ischemic preconditioning and postconditioning. Curr. Opin. Cardiol. 2017, 32, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.C.; Liu, S.Y.; Yen, E.Y.; Li, T.K.; Lai, I.R. microRNA-183 mediates protective postconditioning of the liver by repressing Apaf-1. Antioxid. Redox Signal. 2017, 26, 583–597. [Google Scholar] [CrossRef] [PubMed]
- Feyzizadeh, S.; Badalzadeh, R. Application of ischemic postconditioning’s algorithms in tissues protection: Response to methodological gaps in preclinical and clinical studies. J. Cell. Mol. Med. 2017, 21, 2257–2267. [Google Scholar] [CrossRef] [PubMed]
- Lehrke, M.; Lazar, M.A. The many faces of PPARgamma. Cell 2005, 123, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Balakumar, P.; Rose, M.; Singh, M. PPAR Ligands: Are they potential agents for cardiovascular disorders? Pharmacology 2007, 80, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ruan, X.; Zheng, F.; Guan, Y. PPARs and the kidney in metabolic syndrome. Am. J. Physiol. Ren. Physiol. 2008, 294, F1032–F1047. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, D.; Li, J.; Zhang, X.; Fan, F.; Guan, Y. Role of PPARgamma in renoprotection in Type 2 diabetes: Molecular mechanisms and therapeutic potential. Clin. Sci. 2009, 116, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Gross, B.; Pawlak, M.; Lefebvre, P.; Staels, B. PPARs in obesity-induced T2DM, dyslipidaemia and NAFLD. Nat. Rev. Endocrinol. 2017, 13, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Janani, C.; Ranjitha Kumari, B.D. PPAR gamma gene—A review. Diabetes Metab. Syndr. 2015, 9, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Marion-Letellier, R.; Savoye, G.; Ghosh, S. Fatty acids, eicosanoids and PPAR gamma. Eur. J. Pharmacol. 2016, 785, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Casillas-Ramirez, A.; Amine-Zaouali, M.; Massip-Salcedo, M.; Padrissa-Altes, S.; Bintanel-Morcillo, M.; Ramalho, F.; Serafin, A.; Rimola, A.; Arroyo, V.; Rodes, J.; et al. Inhibition of angiotensin II action protects rat steatotic livers against ischemia-reperfusion injury. Crit. Care Med. 2008, 36, 1256–1266. [Google Scholar] [CrossRef] [PubMed]
- Kuboki, S.; Shin, T.; Huber, N.; Eismann, T.; Galloway, E.; Schuster, R.; Blanchard, J.; Zingarelli, B.; Lentsch, A.B. Peroxisome proliferator-activated receptor-gamma protects against hepatic ischemia/reperfusion injury in mice. Hepatology 2008, 47, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Shin, T.; Kuboki, S.; Huber, N.; Eismann, T.; Galloway, E.; Schuster, R.; Blanchard, J.; Pritts, T.A.; Lentsch, A.B. Activation of peroxisome proliferator-activated receptor-gamma during hepatic ischemia is age-dependent. J. Surg. Res. 2008, 147, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yao, J.; Zou, C.; Zhang, H.; Zhang, S.; Liu, J.; Ma, G.; Jiang, P.; Zhang, W. Asiatic acid protects against hepatic ischemia/reperfusion injury by inactivation of Kupffer cells via PPARgamma/NLRP3 inflammasome signaling pathway. Oncotarget 2017, 8, 86339–86355. [Google Scholar] [PubMed]
- Chen, K.; Li, J.J.; Li, S.N.; Feng, J.; Liu, T.; Wang, F.; Dai, W.Q.; Xia, Y.J.; Lu, J.; Zhou, Y.Q.; et al. 15-Deoxy-Delta(12,14)-prostaglandin J2 alleviates hepatic ischemia-reperfusion injury in mice via inducing antioxidant response and inhibiting apoptosis and autophagy. Acta Pharmacol. Sin. 2017, 38, 672–687. [Google Scholar] [CrossRef] [PubMed]
- Ruan, W.; Liu, Q.; Chen, C.; Li, S.; Xu, J. Limb remote ischemic preconditioning attenuates liver ischemia reperfusion injury by activating autophagy via modulating PPAR-gamma pathway. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2016, 41, 918–928. [Google Scholar] [PubMed]
- Koh, E.J.; Yoon, S.J.; Lee, S.M. Losartan protects liver against ischaemia/reperfusion injury through PPAR-gamma activation and receptor for advanced glycation end-products down-regulation. Br. J. Pharmacol. 2013, 169, 1404–1416. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, A.; Jacob, A.; Wu, R.; Zhou, M.; Aziz, M.; Wang, P. Milk fat globule—EGF factor VIII ameliorates liver injury after hepatic ischemia-reperfusion. J. Surg. Res. 2013, 180, e37–e46. [Google Scholar] [CrossRef] [PubMed]
- Zhai, D.; Zhang, J.; Zheng, Q.; Li, Z.; Zhang, J.; Tian, Y. Significance of rosiglitazone inhibiting TLR4 expression in partial hepatic ischemia/reperfusion of mice. J. Huazhong Univ. Sci. Technol. Med. Sci. 2008, 28, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Casillas-Ramirez, A.; Zaouali, A.; Padrissa-Altes, S.; Ben Mosbah, I.; Pertosa, A.; Alfany-Fernandez, I.; Bintanel-Morcillo, M.; Xaus, C.; Rimola, A.; Rodes, J.; et al. Insulin-like growth factor and epidermal growth factor treatment: New approaches to protecting steatotic livers against ischemia-reperfusion injury. Endocrinology 2009, 150, 3153–3161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casillas-Ramirez, A.; Alfany-Fernandez, I.; Massip-Salcedo, M.; Juan, M.E.; Planas, J.M.; Serafin, A.; Pallas, M.; Rimola, A.; Rodes, J.; Peralta, C. Retinol-binding protein 4 and peroxisome proliferator-activated receptor-gamma in steatotic liver transplantation. J. Pharmacol. Exp. Ther. 2011, 338, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Castro, M.B.; Elias-Miro, M.; Mendes-Braz, M.; Lemoine, A.; Rimola, A.; Rodes, J.; Casillas-Ramirez, A.; Peralta, C. Tauroursodeoxycholic acid affects PPARgamma and TLR4 in Steatotic liver transplantation. Am. J. Transplant. 2012, 12, 3257–3271. [Google Scholar] [CrossRef] [PubMed]
- Zaouali, M.A.; Padrissa-Altes, S.; Ben Mosbah, I.; Alfany-Fernandez, I.; Massip-Salcedo, M.; Casillas-Ramirez, A.; Bintanel-Morcillo, M.; Boillot, O.; Serafin, A.; Rimola, A.; et al. Improved rat steatotic and nonsteatotic liver preservation by the addition of epidermal growth factor and insulin-like growth factor-I to University of Wisconsin solution. Liver Transpl. 2010, 16, 1098–1111. [Google Scholar] [CrossRef] [PubMed]
- Somi, M.H.; Hajipour, B.; Asl, N.A.; Estakhri, R.; Azar, A.N.; Zade, M.N.; Haghjou, A.G.; Vatankhah, A.M. Pioglitazone attenuates ischemia/reperfusion-induced liver injury in rats. Transplant. Proc. 2009, 41, 4105–4109. [Google Scholar] [CrossRef] [PubMed]
- Elias-Miro, M.; Jimenez-Castro, M.B.; Mendes-Braz, M.; Casillas-Ramirez, A.; Peralta, C. The Current Knowledge of the Role of PPAR in Hepatic Ischemia-Reperfusion Injury. PPAR Res. 2012, 2012, 802384. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Jia, S.; Wang, C.; Chen, Z.; Chi, Y.; Li, J.; Xu, G.; Guan, Y.; Yang, J. FAM3A is a target gene of peroxisome proliferator-activated receptor gamma. Biochim. Biophys. Acta 2013, 1830, 4160–4170. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chi, Y.; Li, J.; Miao, Y.; Li, S.; Su, W.; Jia, S.; Chen, Z.; Du, S.; Zhang, X.; et al. FAM3A activates PI3K p110alpha/Akt signaling to ameliorate hepatic gluconeogenesis and lipogenesis. Hepatology 2014, 59, 1779–1790. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Wang, J.; Chen, Z.; Chen, J.; Meng, Y.; Chen, L.; Chang, Y.; Geng, B.; Sun, L.; Dou, L.; et al. NFE2 Induces miR-423-5p to Promote Gluconeogenesis and Hyperglycemia by Repressing the Hepatic FAM3A-ATP-Akt Pathway. Diabetes 2017, 66, 1819–1832. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.; Li, J.; Li, N.; Chen, Z.; Ma, L.; Peng, W.; Pan, X.; Li, M.; Yu, W.; He, X.; et al. FAM3A enhances adipogenesis of 3T3-L1 preadipocytes via activation of ATP-P2 receptor-Akt signaling pathway. Oncotarget 2017, 8, 45862–45873. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Chen, Z.; Li, J.; Chi, Y.; Wang, J.; Li, S.; Luo, Y.; Geng, B.; Wang, C.; Cui, Q.; et al. FAM3A promotes vascular smooth muscle cell proliferation and migration and exacerbates neointima formation in rat artery after balloon injury. J. Mol. Cell. Cardiol. 2014, 74, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Carini, R.; Grazia De Cesaris, M.; Splendore, R.; Baldanzi, G.; Nitti, M.P.; Alchera, E.; Filigheddu, N.; Domenicotti, C.; Pronzato, M.A.; Graziani, A.; et al. Role of phosphatidylinositol 3-kinase in the development of hepatocyte preconditioning. Gastroenterology 2004, 127, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Dal Ponte, C.; Alchera, E.; Follenzi, A.; Imarisio, C.; Prat, M.; Albano, E.; Carini, R. Pharmacological postconditioning protects against hepatic ischemia/reperfusion injury. Liver Transpl. 2011, 17, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Ke, B.; Shen, X.D.; Ji, H.; Kamo, N.; Gao, F.; Freitas, M.C.; Busuttil, R.W.; Kupiec-Weglinski, J.W. HO-1-STAT3 axis in mouse liver ischemia/reperfusion injury: Regulation of TLR4 innate responses through PI3K/PTEN signaling. J. Hepatol. 2012, 56, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Kamo, N.; Ke, B.; Busuttil, R.W.; Kupiec-Weglinski, J.W. PTEN-mediated Akt/beta-catenin/Foxo1 signaling regulates innate immune responses in mouse liver ischemia/reperfusion injury. Hepatology 2013, 57, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, J.; Yang, W.; Chen, J.; Meng, Y.; Geng, B.; Cui, Q.; Yang, J. FAM3A mediates PPARγ’s protection in liver ischemia-reperfusion injury by activating Akt survival pathway and repressing inflammation and oxidative stress. Oncotarget 2017, 8, 49882–49896. [Google Scholar] [PubMed]
- Lentsch, A.B.; Kato, A.; Yoshidome, H.; McMasters, K.M.; Edwards, M.J. Inflammatory mechanisms and therapeutic strategies for warm hepatic ischemia/reperfusion injury. Hepatology 2000, 32, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Gou, W.L.; Zhang, R. FAM3A Protects HT22 Cells Against Hydrogen Peroxide-Induced Oxidative Stress Through Activation of PI3K/Akt but not MEK/ERK Pathway. Cell. Physiol. Biochem. 2015, 37, 1431–1441. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Gou, W.L.; Zhang, R. FAM3A attenuates ER stress-induced mitochondrial dysfunction and apoptosis via CHOP-Wnt pathway. Neurochem. Int. 2016, 94, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Kapranov, P.; Cheng, J.; Dike, S.; Nix, D.A.; Duttagupta, R.; Willingham, A.T.; Stadler, P.F.; Hertel, J.; Hackermuller, J.; Hofacker, I.L.; et al. RNA maps reveal new RNA classes and a possible function for pervasive transcription. Science 2007, 316, 1484–1488. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Garcia, I.; Miska, E.A. MicroRNA functions in animal development and human disease. Development 2005, 132, 4653–4662. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Farwell, M.A. microRNAs: A new emerging class of players for disease diagnostics and gene therapy. J. Cell. Mol. Med. 2008, 12, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Schickel, R.; Boyerinas, B.; Park, S.M.; Peter, M.E. MicroRNAs: Key players in the immune system, differentiation, tumorigenesis and cell death. Oncogene 2008, 27, 5959–5974. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.T.; Lo, C.M.; Wong, N.; Li, C.X.; Qi, X.; Liu, X.B.; Geng, W.; Yeung, O.W.; Ma, Y.Y.; Chan, S.C.; et al. Early-phase circulating miRNAs predict tumor recurrence and survival of hepatocellular carcinoma patients after liver transplantation. Oncotarget 2016, 7, 19824–19839. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Men, H.; Li, J.; Xing, Y.; Wu, B.; Wang, Z.; Li, J.; Teng, D.; Shi, Y.; Li, J.; et al. Global MicroRNA Expression Profiling of Mouse Livers following Ischemia-Reperfusion Injury at Different Stages. PLoS ONE 2016, 11, e0148677. [Google Scholar] [CrossRef] [PubMed]
- Morita, T.; Ishikawa, M.; Sakamoto, A. Identical MicroRNAs Regulate Liver Protection during Anaesthetic and Ischemic Preconditioning in Rats: An animal study. PLoS ONE 2015, 10, e0125866. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.F.; Yu, C.H.; Li, Y.M. Regulation of hepatic microRNA expression in response to ischemic preconditioning following ischemia/reperfusion injury in mice. OMICS J. Integr. Biol. 2009, 13, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Gehrau, R.C.; Mas, V.R.; Dumur, C.I.; Ladie, D.E.; Suh, J.L.; Luebbert, S.; Maluf, D.G. Regulation of molecular pathways in ischemia-reperfusion injury after liver transplantation. Transplantation 2013, 96, 926–934. [Google Scholar] [CrossRef] [PubMed]
- Lagos-Quintana, M.; Rauhut, R.; Yalcin, A.; Meyer, J.; Lendeckel, W.; Tuschl, T. Identification of tissue-specific microRNAs from mouse. Curr. Biol. 2002, 12, 735–739. [Google Scholar] [CrossRef]
- Chang, J.; Nicolas, E.; Marks, D.; Sander, C.; Lerro, A.; Buendia, M.A.; Xu, C.; Mason, W.S.; Moloshok, T.; Bort, R.; et al. miR-122, a mammalian liver-specific microRNA, is processed from hcr mRNA and may downregulate the high affinity cationic amino acid transporter CAT-1. RNA Biol. 2004, 1, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Xu, Y.; Hao, J.; Wang, S.; Li, C.; Meng, S. MiR-122 in hepatic function and liver diseases. Protein Cell 2012, 3, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.C.; Hsu, S.D.; Hsu, C.S.; Lai, T.C.; Chen, S.J.; Shen, R.; Huang, Y.; Chen, H.C.; Lee, C.H.; Tsai, T.F.; et al. MicroRNA-122 plays a critical role in liver homeostasis and hepatocarcinogenesis. J. Clin. Investig. 2012, 122, 2884–2897. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Friedman, J.R. miR-122 regulates hepatic lipid metabolism and tumor suppression. J. Clin. Investig. 2012, 122, 2773–2776. [Google Scholar] [CrossRef] [PubMed]
- Andersson, P.; Gidlof, O.; Braun, O.O.; Gotberg, M.; van der Pals, J.; Olde, B.; Erlinge, D. Plasma levels of liver-specific miR-122 is massively increased in a porcine cardiogenic shock model and attenuated by hypothermia. Shock 2012, 37, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Roderburg, C.; Benz, F.; Vargas Cardenas, D.; Koch, A.; Janssen, J.; Vucur, M.; Gautheron, J.; Schneider, A.T.; Koppe, C.; Kreggenwinkel, K.; et al. Elevated miR-122 serum levels are an independent marker of liver injury in inflammatory diseases. Liver Int. 2015, 35, 1172–1184. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Antoine, D.J.; Weemhoff, J.L.; Jenkins, R.E.; Farhood, A.; Park, B.K.; Jaeschke, H. Biomarkers distinguish apoptotic and necrotic cell death during hepatic ischemia/reperfusion injury in mice. Liver Transpl. 2014, 20, 1372–1382. [Google Scholar] [CrossRef] [PubMed]
- Van Caster, P.; Brandenburger, T.; Strahl, T.; Metzger, S.; Bauer, I.; Pannen, B.; Braun, S. Circulating microRNA-122, -21 and -223 as potential markers of liver injury following warm ischaemia and reperfusion in rats. Mol. Med. Rep. 2015, 12, 3146–3150. [Google Scholar] [CrossRef] [PubMed]
- Selten, J.W.; Verhoeven, C.J.; Heedfeld, V.; Roest, H.P.; de Jonge, J.; Pirenne, J.; van Pelt, J.; Ijzermans, J.N.M.; Monbaliu, D.; van der Laan, L.J.W. The release of microRNA-122 during liver preservation is associated with early allograft dysfunction and graft survival after transplantation. Liver Transpl. 2017, 23, 946–956. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Ye, Q.F.; Wang, W.; Fu, B.Q.; Xia, Z.P.; Liu, Z.Z.; Zhang, X.J.; Wang, Y.F. Mild hypothermia pretreatment protects hepatocytes against ischemia reperfusion injury via down-regulating miR-122 and IGF-1R/AKT pathway. Cryobiology 2017, 75, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Akbari, G.; Mard, S.A.; Dianat, M.; Mansouri, E. The hepatoprotective and microRNAs downregulatory effects of crocin following hepatic ischemia-reperfusion injury in rats. Oxid. Med. Cell. Longev. 2017, 2017, 1702967. [Google Scholar] [CrossRef] [PubMed]
- Mard, S.A.; Akbari, G.; Dianat, M.; Mansouri, E. Protective effects of crocin and zinc sulfate on hepatic ischemia-reperfusion injury in rats: A comparative experimental model study. Biomed. Pharmacother. 2017, 96, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Gao, Y.; Qin, J.; Lu, S. The role of miR-34a in the hepatoprotective effect of hydrogen sulfide on ischemia/reperfusion injury in young and old rats. PLoS ONE 2014, 9, e113305. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Joe, Y.; Yu, J.K.; Chen, Y.; Jeong, S.O.; Mani, N.; Cho, G.J.; Pae, H.O.; Ryter, S.W.; Chung, H.T. Carbon monoxide protects against hepatic ischemia/reperfusion injury by modulating the miR-34a/SIRT1 pathway. Biochim. Biophys. Acta 2015, 1852, 1550–1559. [Google Scholar] [CrossRef] [PubMed]
- Shan, W.; Gao, L.; Zeng, W.; Hu, Y.; Wang, G.; Li, M.; Zhou, J.; Ma, X.; Tian, X.; Yao, J. Activation of the SIRT1/p66shc antiapoptosis pathway via carnosic acid-induced inhibition of miR-34a protects rats against nonalcoholic fatty liver disease. Cell Death Dis. 2015, 6, e1833. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.H.; Xu, C.F.; Li, Y.M. Association of MicroRNA-223 expression with hepatic ischemia/reperfusion injury in mice. Dig. Dis. Sci. 2009, 54, 2362–2366. [Google Scholar] [CrossRef] [PubMed]
- Schueller, F.; Roy, S.; Loosen, S.H.; Alder, J.; Koppe, C.; Schneider, A.T.; Wandrer, F.; Bantel, H.; Vucur, M.; Mi, Q.S.; et al. miR-223 represents a biomarker in acute and chronic liver injury. Clin. Sci. 2017, 131, 1971–1987. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, G.; Yu, C.; Shen, Z.; Xu, C.; Feng, Z.; Zhang, X.; Li, Y. A role of microRNA-370 in hepatic ischaemia-reperfusion injury by targeting transforming growth factor-beta receptor II. Liver Int. 2015, 35, 1124–1132. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhu, F.; Song, W.; Zhang, B.; Zhang, X.; Jin, X.; Li, H. Altered miR-370 expression in hepatic ischemia-reperfusion injury correlates with the level of nuclear kappa B (NF-kappaB) related factors. Gene 2017, 607, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Wang, Z.; Qi, G.; Yuan, S.; Yu, S.; Li, B.; Wei, Y.; Huang, Q.; Zhai, R.; He, S. MicroRNA-155 deficiency attenuates ischemia-reperfusion injury after liver transplantation in mice. Transpl. Int. 2015, 28, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ma, D.; Wang, Z.; Yang, J. MicroRNA-155 deficiency in Kupffer cells ameliorates liver ischemia-reperfusion injury in mice. Transplantation 2017, 101, 1600–1608. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; He, D.; Lu, X.; Sun, C.; Luo, Q.; Wu, Z. The miR-148a alleviates hepatic ischemia/reperfusion injury in mice via targeting CaMKIIalpha. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2016, 32, 1202–1206. [Google Scholar] [PubMed]
- Chen, Q.; Kong, L.; Xu, X.; Geng, Q.; Tang, W.; Jiang, W. Down-regulation of microRNA-146a in the early stage of liver ischemia-reperfusion injury. Transplant. Proc. 2013, 45, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yi, S.; Deng, Y.; Cheng, J.; Wu, X.; Liu, W.; Tai, Y.; Chen, G.; Zhang, Q.; Yang, Y. MiR-124 protects human hepatic L02 cells from H2O2-induced apoptosis by targeting Rab38 gene. Biochem. Biophys. Res. Commun. 2014, 450, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Li, S.P.; He, J.D.; Wang, Z.; Yu, Y.; Fu, S.Y.; Zhang, H.M.; Zhang, J.J.; Shen, Z.Y. miR-30b inhibits autophagy to alleviate hepatic ischemia-reperfusion injury via decreasing the Atg12-Atg5 conjugate. World J. Gastroenterol. 2016, 22, 4501–4514. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, J.; Wang, Z.; Wang, T.; Yu, Y.; He, J.; Zhang, H.; Yang, T.; Shen, Z. MicroRNA-17 regulates autophagy to promote hepatic ischemia/reperfusion injury via suppression of signal transductions and activation of transcription-3 expression. Liver Transpl. 2016, 22, 1697–1709. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Gu, C.; Huang, X. Sevoflurane protects against hepatic ischemia/reperfusion injury by modulating microRNA-200c regulation in mice. Biomed. Pharmacother. 2016, 84, 1126–1136. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Liu, G.; Tang, W. MicroRNA-182-5p Ameliorates Liver Ischemia-Reperfusion Injury by Suppressing Toll-Like Receptor 4. Transplant. Proc. 2016, 48, 2809–2814. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Zhao, Z.H.; Meng, Q.T.; Tie, M.E.; Lei, S.Q.; Xia, Z.Y. Propofol protects against hepatic ischemia/reperfusion injury via miR-133a-5p regulating the expression of MAPK6. Cell Biol. Int. 2017, 41, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Bantel, H.; Wandrer, F.; Schneider, A.T.; Gautheron, J.; Vucur, M.; Tacke, F.; Trautwein, C.; Luedde, T.; Roderburg, C. miR-1224 inhibits cell proliferation in acute liver failure by targeting the antiapoptotic gene Nfib. J. Hepatol. 2017, 67, 966–978. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Huang, J.; An, W. Hepatic stimulator substance resists hepatic ischemia/reperfusion injury by regulating Drp1 translocation and activation. Hepatology 2017, 66, 1989–2001. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Luo, Y.; Yang, W.; Ding, L.; Wang, J.; Tu, J.; Geng, B.; Cui, Q.; Yang, J. Comparison Analysis of Dysregulated LncRNA Profile in Mouse Plasma and Liver after Hepatic Ischemia/Reperfusion Injury. PLoS ONE 2015, 10, e0133462. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Liu, J.; He, K.; Zhang, M.; Feng, C.; Peng, F.; Li, B.; Xia, X. Overexpression of the long noncoding RNA TUG1 protects against cold-induced injury of mouse livers by inhibiting apoptosis and inflammation. FEBS J. 2016, 283, 1261–1274. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Jia, S.; Li, D.; Cai, J.; Tu, J.; Geng, B.; Guan, Y.; Cui, Q.; Yang, J. Silencing of long noncoding RNA AK139328 attenuates ischemia/reperfusion injury in mouse livers. PLoS ONE 2013, 8, e80817. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, W.; Chen, Z.; Chen, J.; Meng, Y.; Feng, B.; Sun, L.; Dou, L.; Li, J.; Cui, Q.; et al. Long Non-coding RNA LncSHGL recruits hnRNPA1 to suppress hepatic gluconeogenesis and lipogenesis. Diabetes 2018. [Google Scholar] [CrossRef] [PubMed]


| miRNAs | Change in IRI | Effects | Proposed Mechanism |
|---|---|---|---|
| miR-34a | Increased in liver and circulation | Exaggerating liver IRI | Suppressing Nrf2 and SIRT1 pathways |
| miR-122 | Increased in liver and circulation | Exaggerating liver IRI | Inhibiting IGF-1R/Akt pathway |
| miR-155 | Increased in liver and circulation | Exaggerating liver IRI | Activating Kupffer cells and promoting inflammation |
| miR-223 | Increased in liver and circulation | Exaggerating liver IR | Unclear |
| miR-370 | Increased in liver and circulation | Exaggerating liver IRI | Inhibiting TβRII pathway and activating NF-κB pathway |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.; Chen, J.; Meng, Y.; Chen, Z.; Yang, J. Novel Targets for Treating Ischemia-Reperfusion Injury in the Liver. Int. J. Mol. Sci. 2018, 19, 1302. https://doi.org/10.3390/ijms19051302
Yang W, Chen J, Meng Y, Chen Z, Yang J. Novel Targets for Treating Ischemia-Reperfusion Injury in the Liver. International Journal of Molecular Sciences. 2018; 19(5):1302. https://doi.org/10.3390/ijms19051302
Chicago/Turabian StyleYang, Weili, Ji Chen, Yuhong Meng, Zhenzhen Chen, and Jichun Yang. 2018. "Novel Targets for Treating Ischemia-Reperfusion Injury in the Liver" International Journal of Molecular Sciences 19, no. 5: 1302. https://doi.org/10.3390/ijms19051302