Thermosensitive Injectable Hydrogel for Simultaneous Intraperitoneal Delivery of Doxorubicin and Prevention of Peritoneal Adhesion
Abstract
:1. Introduction
2. Results
2.1. In Vitro Studies
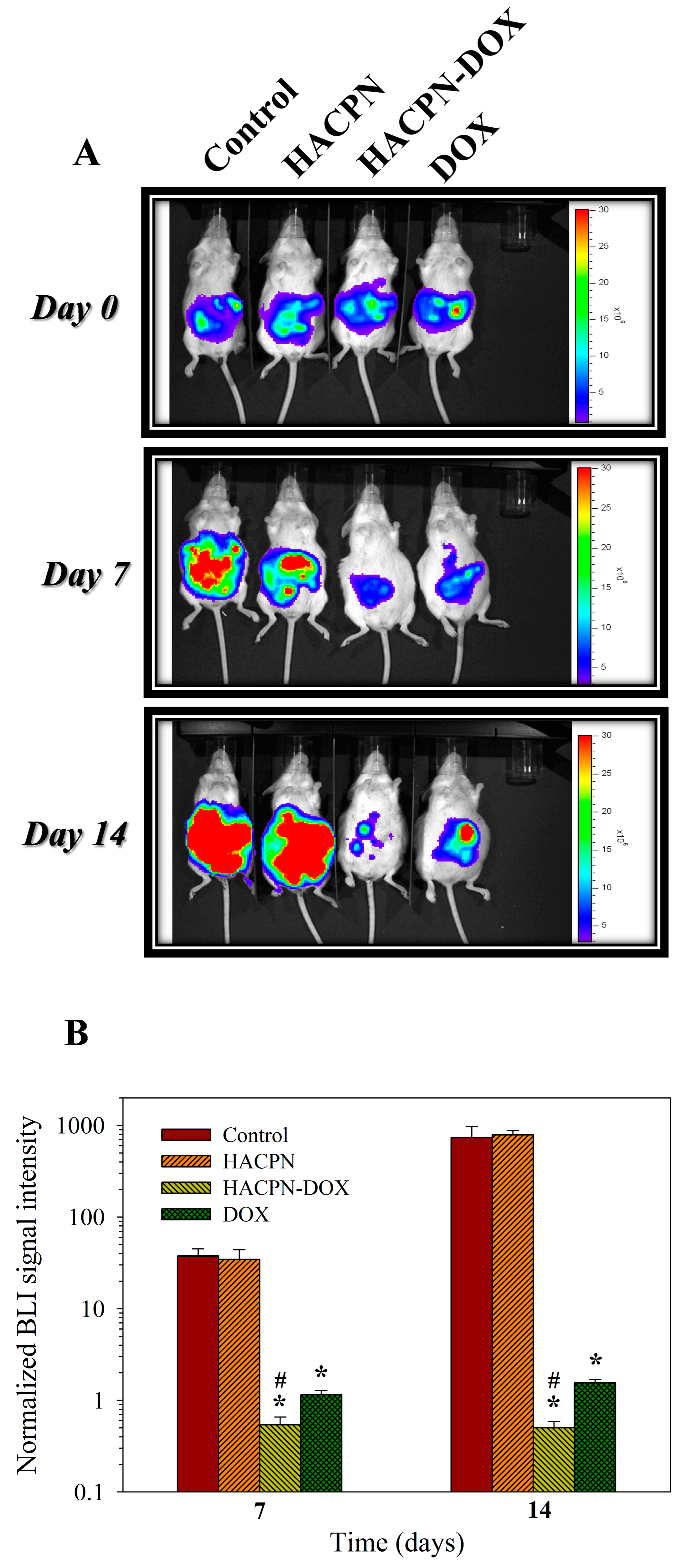
2.2. Antitumor Effects In Vivo Studies
2.3. Anti-Adhesion Effects In Vivo Studies
3. Materials and Methods
3.1. Preparation of and Characterization of HACPN and HACPN-DOX
3.2. In Vitro Degradation and Drug Release Profile of Hydrogel
3.3. In Vitro Cell Culture and Cytotoxicity
3.4. Peritoneal Carcinomatosis Model and Treatment Protocol
3.5. Bioluminescence Imaging (BLI) Using the In Vivo Image System (IVIS)
3.6. The Anti-Adhesion Effects from Sidewall Defect-Cecum Abrasion Model
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| HA | Hyaluronic acid |
| PNIPAM | Poly(N-isoropylacrylamide) |
| HA-CPN | Hyaluronic acid-g-chitosan-g-PNIPAM |
| LCST | Lower critical solution temperature |
| PBS | Phosphate buffered saline |
| DOX | Doxorubicin |
| FBS | Fetal bovine serum |
| IVIS | In vivo imaging system |
| BLI | Bioluminescence imaging |
| UV/VIS | Ultraviolet/visible |
| H&E | Hematoxylin and eosin |
| ROI | Region of interest |
| SEM | Scanning electron microscopy |
References
- Armstrong, D.; Bundy, B.; Wenzel, L.; Ozols, R.; Bundy, B.; Greer, B. Intraperitoneal chemotherapy for ovarian cancer. N. Eng. J. Med. 2006, 2006, 1641–1643. [Google Scholar]
- Elias, D.; Blot, F.; El Otmany, A.; Antoun, S.; Lasser, P.; Boige, V.; Rougier, P.; Ducreux, M. Curative treatment of peritoneal carcinomatosis arising from colorectal cancer by complete resection and intraperitoneal chemotherapy. Cancer 2001, 92, 71–76. [Google Scholar] [CrossRef]
- Heinrich, S.; Lang, H. Neoadjuvant therapy of pancreatic cancer: Definitions and benefits. Int. J. Mol. Sci. 2017, 18, 1622. [Google Scholar] [CrossRef]
- Esquivel, J.; Sticca, R.; Sugarbaker, P.; Levine, E.; Yan, T.; Alexander, R.; Baratti, D.; Bartlett, D.; Barone, R.; Barrios, P. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of peritoneal surface malignancies of colonic origin: A consensus statement. Ann. Surg. Oncol. 2007, 14, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Verwaal, V.J.; Bruin, S.; Boot, H.; van Slooten, G.; van Tinteren, H. 8-year follow-up of randomized trial: Cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann. Surg. Oncol. 2008, 15, 2426–2432. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.D.; Cao, C.Q.; Munkholm-Larsen, S. A pharmacological review on intraperitoneal chemotherapy for peritoneal malignancy. World J. Gastrointest. Oncol. 2010, 2, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Levine, E.A.; Stewart, J.H.t.; Shen, P.; Russell, G.B.; Loggie, B.L.; Votanopoulos, K.I. Intraperitoneal chemotherapy for peritoneal surface malignancy: Experience with 1000 patients. J. Am. Coll. Surg. 2014, 218, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.; Lu, Z.; Wang, J.; Yeh, T.K.; Wientjes, M.G.; Au, J.L. Effects of carrier on disposition and antitumor activity of intraperitoneal paclitaxel. Pharm. Res. 2007, 24, 1691–1701. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Taha, M.S.; Ramsey, B.; Torregrosa-Allen, S.; Elzey, B.D.; Yeo, Y. Intraperitoneal chemotherapy of ovarian cancer by hydrogel depot of paclitaxel nanocrystals. J. Control. Release 2016, 235, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.C.; Ellis, H.; Moran, B.J.; Thompson, J.N.; Wilson, M.S.; Menzies, D.; McGuire, A.; Lower, A.M.; Hawthorn, R.J.; O’brien, F. Postoperative adhesions: Ten-year follow-up of 12,584 patients undergoing lower abdominal surgery. Dis. Colon Rectum 2001, 44, 822–829. [Google Scholar] [CrossRef] [PubMed]
- Coleman, M.; McLain, A.; Moran, B. Impact of previous surgery on time taken for incision and division of adhesions during laparotomy. Dis. Colon Rectum 2000, 43, 1297–1299. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Zhou, C.; Wang, G.; Fan, L.; Wang, K.; Li, X. Keratinocyte growth factor combined with a sodium hyaluronate gel inhibits postoperative intra-abdominal adhesions. Int. J. Mol. Sci. 2016, 17, 1611. [Google Scholar] [CrossRef] [PubMed]
- Kehoe, S.M.; Williams, N.L.; Yakubu, R.; Levine, D.A.; Chi, D.S.; Sabbatini, P.J.; Aghajanian, C.A.; Barakat, R.R.; Abu-Rustum, N.R. Incidence of intestinal obstruction following intraperitoneal chemotherapy for ovarian tubal and peritoneal malignancies. Gynecol. Oncol. 2009, 113, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Shi, J.; Xu, X.; Ding, J.; Zhong, W.; Zhang, L.; Xing, M.; Zhang, L. Study of stiffness effects of poly(amidoamine)–poly(N-isopropyl acrylamide) hydrogel on wound healing. Colloids Surf. B 2016, 140, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Harada, I.; Yanagisawa, S.; Iwasaki, K.; Cho, C.-S.; Akaike, T. Local Mechanical Stimulation of Mardin-Darby Canine Kidney Cell Sheets on Temperature-Responsive Hydrogel. Int. J. Mol. Sci. 2012, 13, 1095–1108. [Google Scholar] [CrossRef] [PubMed]
- Rzaev, Z.M.O.; Dinçer, S.; Pişkin, E. Functional copolymers of N-isopropylacrylamide for bioengineering applications. Prog. Polym. Sci. 2007, 32, 534–595. [Google Scholar] [CrossRef]
- Chen, J.-P.; Cheng, T.-H. Functionalized temperature-sensitive copolymer for tissue engineering of articular cartilage and meniscus. Colloids Surf. A 2008, 313, 254–259. [Google Scholar] [CrossRef]
- Molla, M.R.; Rangadurai, P.; Pavan, G.M.; Thayumanavan, S. Experimental and theoretical investigations in stimuli responsive dendrimer-based assemblies. Nanoscale 2015, 7, 3817–3837. [Google Scholar] [CrossRef] [PubMed]
- Ilmain, F.; Tanaka, T.; Kokufuta, E. Volume transition in a gel driven by hydrogen bonding. Nature 1991, 349, 400–401. [Google Scholar] [CrossRef]
- Gil, E.S.; Hudson, S.M. Stimuli-reponsive polymers and their bioconjugates. Prog. Polym. Sci. 2004, 29, 1173–1222. [Google Scholar] [CrossRef]
- Jeong, B.; Kim, S.W.; Bae, Y.H. Thermosensitive sol–gel reversible hydrogels. Adv. Drug Deliv. Rev. 2002, 54, 37–51. [Google Scholar] [CrossRef]
- Chen, J.-P.; Cheng, T.-H. Preparation and evaluation of thermo-reversible copolymer hydrogels containing chitosan and hyaluronic acid as injectable cell carriers. Polymer 2009, 50, 107–116. [Google Scholar] [CrossRef]
- Luckanagul, J.A.; Pitakchatwong, C.; Ratnatilaka Na Bhuket, P.; Muangnoi, C.; Rojsitthisak, P.; Chirachanchai, S.; Wang, Q.; Rojsitthisak, P. Chitosan-based polymer hybrids for thermo-responsive nanogel delivery of curcumin. Carbohydr. Polym. 2018, 181, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Delair, T. In situ forming polysaccharide-based 3D-hydrogels for cell delivery in regenerative medicine. Carbohydr. Polym. 2012, 87, 1013–1019. [Google Scholar] [CrossRef]
- Tan, R.; She, Z.; Wang, M.; Fang, Z.; Liu, Y.; Feng, Q. Thermo-sensitive alginate-based injectable hydrogel for tissue engineering. Carbohydr. Polym. 2012, 87, 1515–1521. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, C.; Li, X.; Xu, C.; Zhang, Y.; Sun, Z.; Liu, Y.; Gao, J. Thermosensitive methyl cellulose-based injectable hydrogels for post-operation anti-adhesion. Carbohydr. Polym. 2014, 101, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Peroglio, M.; Grad, S.; Mortisen, D.; Sprecher, C.M.; Illien-Junger, S.; Alini, M.; Eglin, D. Injectable thermoreversible hyaluronan-based hydrogels for nucleus pulposus cell encapsulation. Eur. Spine J. 2012, 21 (Suppl. 6), S839–S849. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Wang, X.; Wu, C. Early stage intercalation of doxorubicin to DNA fragments observed in molecular dynamics binding simulations. J. Mol. Graph. Model. 2012, 38, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Cheewatanakornkool, K.; Niratisai, S.; Manchun, S.; Dass, C.R.; Sriamornsak, P. Thiolated pectin–doxorubicin conjugates: Synthesis, characterization and anticancer activity studies. Carbohy. Polym. 2017, 174, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Lauder, C.I.W.; Garcea, G.; Strickland, A.; Maddern, G.J. Use of a modified chitosan–dextran gel to prevent peritoneal adhesions in a rat model. J. Surg. Res. 2011, 171, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Yeo, Y.; Highley, C.B.; Bellas, E.; Kohane, D.S. Dextran-based in situ cross-linked injectable hydrogels to prevent peritoneal adhesions. Biomaterials 2007, 28, 3418–3426. [Google Scholar] [CrossRef] [PubMed]
- Lauder, C.I.W.; Strickland, A.; Maddern, G.J. Use of a modified chitosan–dextran gel to prevent peritoneal adhesions in a porcine hemicolectomy model. J. Surg. Res. 2012, 176, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Li, L.; He, T.; Wang, N.; Yang, S.; Yang, X.; Zeng, Y.; Zhang, W.; Yang, L.; Wu, Q. Peritoneal adhesion prevention with a biodegradable and injectable N,O-carboxymethyl chitosan-aldehyde hyaluronic acid hydrogel in a rat repeated-injury model. Sci. Rep. 2016, 6, 37600. [Google Scholar] [CrossRef] [PubMed]
- Chun, C.; Lee, S.M.; Kim, C.W.; Hong, K.-Y.; Kim, S.Y.; Yang, H.K.; Song, S.-C. Doxorubicin–polyphosphazene conjugate hydrogels for locally controlled delivery of cancer therapeutics. Biomaterials 2009, 30, 4752–4762. [Google Scholar] [CrossRef] [PubMed]
- Lan, K.L.; Yen, S.H.; Liu, R.S.; Shih, H.L.; Tseng, F.W.; Lan, K.H. Mutant bik gene transferred by cationic liposome inhibits peritoneal disseminated murine colon cancer. Clin. Exp. Metastasis 2007, 24, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Ersoy, E.; Ozturk, V.; Yazgan, A.; Ozdogan, M.; Gundogdu, H. Effect of polylactic acid film barrier on intra-abdominal adhesion formation. J. Surg. Res. 2008, 147, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Hooker, G.D.; Taylor, B.M.; Driman, D.K. Prevention of adhesion formation with use of sodium hyaluronate–based bioresorbable membrane in a rat model of ventral hernia repair with polypropylene mesh—A randomized, controlled study. Surgery 1999, 125, 211–216. [Google Scholar] [CrossRef]
- Li, L.; Wang, N.; Jin, X.; Deng, R.; Nie, S.; Sun, L.; Wu, Q.; Wei, Y.; Gong, C. Biodegradable and injectable in situ cross-linking chitosan-hyaluronic acid based hydrogels for postoperative adhesion prevention. Biomaterials 2014, 35, 3903–3917. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-C.; Metters, A.T. Hydrogels in controlled release formulations: Network design and mathematical modeling. Adv. Drug Deliv. Rev. 2006, 58, 1379–1408. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wang, W.; Li, X.; Liu, J.; Dong, A.; Deng, L. Sustained release of PTX-incorporated nanoparticles synergized by burst release of DOX⋅HCl from thermosensitive modified PEG/PCL hydrogel to improve anti-tumor efficiency. Eur. J. Pharm. Sci. 2014, 62, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Chen, S.H.; Mao, S.H.; Tsai, M.J.; Chou, P.Y.; Liao, C.H.; Chen, J.P. Injectable thermosensitive hydrogel containing hyaluronic acid and chitosan as a barrier for prevention of postoperative peritoneal adhesion. Carbohydr. Polym. 2017, 173, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Nowacki, M.; Wisniewski, M.; Werengowska-Ciecwierz, K.; Roszek, K.; Czarnecka, J.; Lakomska, I.; Kloskowski, T.; Tyloch, D.; Debski, R.; Pietkun, K. Nanovehicles as a novel target strategy for hyperthermic intraperitoneal chemotherapy: A multidisciplinary study of peritoneal carcinomatosis. Oncotarget 2015, 6, 22776–22798. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Qian, D.Z.; Rey, S.; Wei, H.; Liu, J.O.; Semenza, G.L. Anthracycline chemotherapy inhibits hif-1 transcriptional activity and tumor-induced mobilization of circulating angiogenic cells. Proc. Natl. Acad. Sci. USA 2009, 106, 2353–2358. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Yang, B.; Qian, Z.; Zhao, X.; Wu, Q.; Qi, X.; Wang, Y.; Guo, G.; Kan, B.; Luo, F. Improving intraperitoneal chemotherapeutic effect and preventing postsurgical adhesions simultaneously with biodegradable micelles. Nanomedicine 2012, 8, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gong, C.; Yang, L.; Wu, Q.; Shi, S.; Shi, H.; Qian, Z.; Wei, Y. 5-FU-hydrogel inhibits colorectal peritoneal carcinomatosis and tumor growth in mice. BMC Cancer 2010, 10, 402. [Google Scholar] [CrossRef] [PubMed]
- Terracina, K.P.; Aoyagi, T.; Huang, W.C.; Nagahashi, M.; Yamada, A.; Aoki, K.; Takabe, K. Development of a metastatic murine colon cancer model. J. Surg. Res. 2015, 199, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Chou, P.Y.; Chen, S.H.; Chen, C.H.; Chen, S.H.; Fong, Y.T.; Chen, J.P. Thermo-responsive in-situ forming hydrogels as barriers to prevent post-operative peritendinous adhesion. Acta Biomater. 2017, 63, 85–95. [Google Scholar] [CrossRef] [PubMed]






| Treatment | Tumor Weight (g) | Tumor Volume (cm3) |
|---|---|---|
| Control | 2.50 ± 0.12 | 2.61 ± 0.16 |
| HACPN | 2.60 ± 0.08 | 2.70 ± 0.10 |
| HACPN-DOX | 0.30 ± 0.03 *,# | 0.46 ± 0.08 *,# |
| DOX | 1.13 ± 0.09 * | 1.46 ± 0.12 * |
| Gross View 1. | Control | HACPN | HACPN-DOX | DOX |
|---|---|---|---|---|
| Score 0 | 0 | 5 (62.5%) | 6 (75.0%) | 0 |
| Score 1 | 0 | 2 (25.0%) | 2 (25.0%) | 0 |
| Score 2 | 1 (12.5%) | 1 (12.5%) | 0 | 3 (37.5%) |
| Score 3 | 7 (87.5%) | 0 | 0 | 5 (62.5%) |
| Histology 2. | Control | HACPN | HACPN-DOX | DOX |
| Score 0 | 0 | 6 (75.0%) | 7 (87.5%) | 0 |
| Score 1 | 0 | 2 (25.0%) | 1 (12.5%) | 1 (12.5%) |
| Score 2 | 1 (12.5%) | 0 | 0 | 2 (25.0%) |
| Score 3 | 7 (87.5%) | 0 | 0 | 5 (62.5%) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-H.; Kuo, C.-Y.; Chen, S.-H.; Mao, S.-H.; Chang, C.-Y.; Shalumon, K.T.; Chen, J.-P. Thermosensitive Injectable Hydrogel for Simultaneous Intraperitoneal Delivery of Doxorubicin and Prevention of Peritoneal Adhesion. Int. J. Mol. Sci. 2018, 19, 1373. https://doi.org/10.3390/ijms19051373
Chen C-H, Kuo C-Y, Chen S-H, Mao S-H, Chang C-Y, Shalumon KT, Chen J-P. Thermosensitive Injectable Hydrogel for Simultaneous Intraperitoneal Delivery of Doxorubicin and Prevention of Peritoneal Adhesion. International Journal of Molecular Sciences. 2018; 19(5):1373. https://doi.org/10.3390/ijms19051373
Chicago/Turabian StyleChen, Chih-Hao, Chang-Yi Kuo, Shih-Hsien Chen, Shih-Hsuan Mao, Chih-Yen Chang, K. T. Shalumon, and Jyh-Ping Chen. 2018. "Thermosensitive Injectable Hydrogel for Simultaneous Intraperitoneal Delivery of Doxorubicin and Prevention of Peritoneal Adhesion" International Journal of Molecular Sciences 19, no. 5: 1373. https://doi.org/10.3390/ijms19051373