Magnesium Deprivation Potentiates Human Mesenchymal Stem Cell Transcriptional Remodeling
Abstract
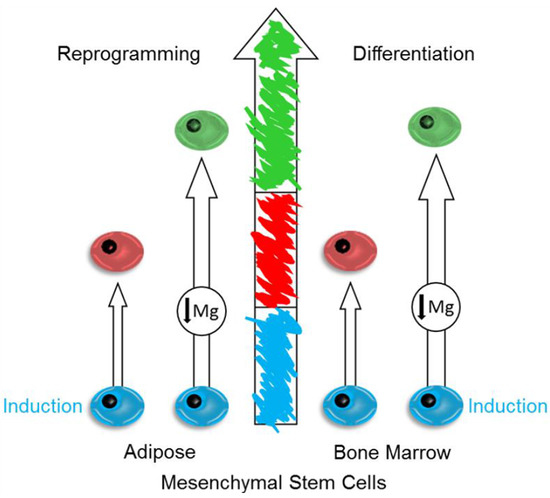
:1. Introduction
2. Results
2.1. Mg and the Transcriptional Remodeling of Adipose-Derived Mesenchymal Stem Cells (AD-MSCs)
2.2. Mg Transcriptional Remodeling and Osteogenic Differentiation of Bone Marrow Mesenchymal Stem Cells (BM-MSCs)
3. Discussion
4. Materials and Methods
4.1. Isolation and Culture of Human Mesenchymal Stem Cells
4.2. Gene Expression Analysis
4.3. Cell Cycle Analysis
4.4. Quantification of Total Cell Mg by Spectrofluorimetric Assay
4.5. Reactive Oxygen Species Evaluation
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| AD-MSCs | Adipose-Derived Mesenchymal Stem Cells |
| BM-MSCs | Bone Marrow Mesenchymal Stem Cells |
| NANOG | Nanog Homeobox |
| GATA-4 | GATA Binding Protein 4 |
| NKX-2.5 | NKX2 Homeobox 5 |
| HGF | Hepatocyte Growth Factor |
| KDR | Kinase Insert Domain Receptor |
| NEUROG | Neurogenin |
| ROS | Reactive Oxygen Species |
| OSX | Osterix |
| NAC | N-acetylcysteine |
| CM | Control Medium |
| RM | Reprogramming Medium |
| OM | Osteogenic Medium |
| GAPDH | Glyceraldehyde-3-Phosphate Dehydrogenase |
| PI | Propidium Iodide |
| DCFH | 2′-7′-Dichlorofluorescein Diacetate |
References
- De Baaij, J.H.F.; Hoenderop, J.G.J.; Bindels, R.J.M. Magnesium in man: Implications for health and disease. Physiol. Rev. 2015, 95, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Li, F.-Y.; Chaigne-Delalande, B.; Kanellopoulou, C.; Davis, J.C.; Matthews, H.F.; Douek, D.C.; Cohen, J.I.; Uzel, G.; Su, H.C.; Lenardo, M.J. Second messenger role for Mg2+ revealed by human T-cell immunodeficiency. Nature 2011, 475, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Long, S.; Romani, A.M. Role of Cellular Magnesium in Human Diseases. Austin J. Nutr. Food Sci. 2014, 2, 1051. [Google Scholar]
- Barbiroli, B.; Iotti, S.; Cortelli, P.; Martinelli, P.; Lodi, R.; Carelli, V.; Montagna, P. Low Brain Intracellular Free Magnesium in Mitochondrial Cytopathies. J. Cereb. Blood Flow Metab. 1999, 19, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Kolisek, M.; Montezano, A.C.; Sponder, G.; Anagnostopoulou, A.; Vormann, J.; Touyz, R.M.; Aschenbach, J.R. PARK7/DJ-1 dysregulation by oxidative stress leads to magnesium deficiency: Implications in degenerative and chronic diseases. Clin. Sci. 2015, 129, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- Barbiroli, B.; Iotti, S.; Lodi, R. Improved brain and muscle mitochondrial respiration with CoQ. An in vivo study by 31P-MR spectroscopy in patients with mitochondrial cytopathies. BioFactors 1999, 9, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Castiglioni, S.; Leidi, M.; Carpanese, E.; Maier, J.A.M. Extracellular magnesium and in vitro cell differentiation: Different behaviour of different cells. Magnes. Res. 2013, 26, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Feyerabend, F.; Schilling, A.F.; Willumeit-Römer, R.; Luthringer, B.J.C. Effects of extracellular magnesium extract on the proliferation and differentiation of human osteoblasts and osteoclasts in coculture. Acta Biomater. 2015, 27, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Tocados, J.M.; Herencia, C.; Martínez-Moreno, J.M.; Montes de Oca, A.; Rodríguez-Ortiz, M.E.; Vergara, N.; Blanco, A.; Steppan, S.; Almadén, Y.; Rodríguez, M.; et al. Magnesium Chloride promotes Osteogenesis through Notch signaling activation and expansion of Mesenchymal Stem Cells. Sci. Rep. 2017, 7, 7839. [Google Scholar] [CrossRef] [PubMed]
- Tsao, Y.-T.; Shih, Y.-Y.; Liu, Y.-A.; Liu, Y.-S.; Lee, O.K. Knockdown of SLC41A1 magnesium transporter promotes mineralization and attenuates magnesium inhibition during osteogenesis of mesenchymal stromal cells. Stem Cell Res. Ther. 2017, 8, 39. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Feng, B. Mesendogen, a novel inhibitor of TRPM6, promotes mesoderm and definitive endoderm differentiation of human embryonic stem cells through alteration of magnesium homeostasis. Heliyon 2015, 1, e00046. [Google Scholar] [CrossRef] [PubMed]
- Shoshani, O.; Zipori, D. Mammalian cell dedifferentiation as a possible outcome of stress. Stem Cell Rev. 2011, 7, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zhou, S.; Jiang, Z.; Dai, J.; Puscheck, E.E.; Lee, I.; Parker, G.; Hüttemann, M.; Rappolee, D.A. Hypoxic stress induces, but cannot sustain trophoblast stem cell differentiation to labyrinthine placenta due to mitochondrial insufficiency. Stem Cell Res. 2014, 13, 478–491. [Google Scholar] [CrossRef] [PubMed]
- Berndt, C.; Lillig, C.H. Redox regulation of differentiation and de-differentiation. Biochim. Biophys. Acta 2015, 1850, 1467–1468. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, S.; Hiratsuka, T.; Taniguchi, M.; Shingaki, K.; Kubo, T.; Kiya, K.; Fujiwara, T.; Kanazawa, S.; Kanematsu, R.; Maeda, T.; et al. Physiological ER Stress Mediates the Differentiation of Fibroblasts. PLoS ONE 2015, 10, e0123578. [Google Scholar] [CrossRef] [PubMed]
- Shoshani, O.; Zipori, D. Stress as a fundamental theme in cell plasticity. Biochim. Biophys. Acta 2015, 1849, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Robert, V.; Nechifor, M. Magnesium in the Central Nervous System; University of Adelaide Press: Adelaide, Australia, 2011. [Google Scholar] [CrossRef]
- Zheltova, A.A.; Kharitonova, M.V.; Iezhitsa, I.N.; Spasov, A.A. Magnesium deficiency and oxidative stress: An update. BioMedicine 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.S.; Choi, Y.; Kim, H.-S.; Kim, H.O. Comparison of molecular profiles of human mesenchymal stem cells derived from bone marrow, umbilical cord blood, placenta and adipose tissue. Int. J. Mol. Med. 2016, 37, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Dey, D.; Evans, G.R.D. Generation of Induced Pluripotent Stem (iPS) Cells by Nuclear Reprogramming. Stem Cells Int. 2011, 2011, 619583. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Panetta, N.J.; Gupta, D.M.; Wilson, K.D.; Lee, A.; Jia, F.; Hu, S.; Cherry, A.M.; Robbins, R.C.; Longaker, M.T.; et al. Feeder-free derivation of induced pluripotent stem cells from adult human adipose stem cells. Proc. Natl. Acad. Sci. USA 2009, 106, 15720–15725. [Google Scholar] [CrossRef] [PubMed]
- Strioga, M.; Viswanathan, S.; Darinskas, A.; Slaby, O.; Michalek, J. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev. 2012, 21, 2724–2752. [Google Scholar] [CrossRef] [PubMed]
- Cavallari, G.; Olivi, E.; Bianchi, F.; Neri, F.; Foroni, L.; Valente, S.; La Manna, G.; Nardo, B.; Stefoni, S.; Ventura, C. Mesenchymal Stem Cells and Islet Cotransplantation in Diabetic Rats: Improved Islet Graft Revascularization and Function by Human Adipose Tissue-Derived Stem Cells Preconditioned with Natural Molecules. Cell Transplant. 2012, 21, 2771–2781. [Google Scholar] [CrossRef] [PubMed]
- Maioli, M.; Contini, G.; Santaniello, S.; Bandiera, P.; Pigliaru, G.; Sanna, R.; Rinaldi, S.; Delitala, A.P.; Montella, A.; Bagella, L.; et al. Amniotic fluid stem cells morph into a cardiovascular lineage: Analysis of a chemically induced cardiac and vascular commitment. Drug Des. Dev. Ther. 2013, 7, 1063–1073. [Google Scholar] [CrossRef]
- Lien, C.L.; Wu, C.; Mercer, B.; Webb, R.; Richardson, J.A.; Olson, E.N. Control of early cardiac-specific transcription of Nkx2-5 by a GATA-dependent enhancer. Development 1999, 126, 75–84. [Google Scholar] [PubMed]
- Duda, D.G.; Fukumura, D.; Jain, R.K. Role of eNOS in neovascularization: NO for endothelial progenitor cells. Trends Mol. Med. 2004, 10, 143–145. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Venema, V.J.; Gu, X.; Venema, R.C.; Marrero, M.B.; Caldwell, R.B. Vascular Endothelial Growth Factor Signals Endothelial Cell Production of Nitric Oxide and Prostacyclin through Flk-1/KDR Activation of c-Src. J. Biol. Chem. 1999, 274, 25130–25135. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.J. Neuronal differentiation: Proneural genes inhibit gliogenesis. Curr. Biol. 2001, 11, R349–R351. [Google Scholar] [CrossRef]
- Mitsui, K.; Tokuzawa, Y.; Itoh, H.; Segawa, K.; Murakami, M.; Takahashi, K.; Maruyama, M.; Maeda, M.; Yamanaka, S. The Homeoprotein Nanog Is Required for Maintenance of Pluripotency in Mouse Epiblast and ES Cells. Cell 2003, 113, 631–642. [Google Scholar] [CrossRef]
- Pitrone, M.; Pizzolanti, G.; Tomasello, L.; Coppola, A.; Morini, L.; Pantuso, G.; Ficarella, R.; Guarnotta, V.; Perrini, S.; Giorgino, F.; et al. NANOG Plays a Hierarchical Role in the Transcription Network Regulating the Pluripotency and Plasticity of Adipose Tissue-Derived Stem Cells. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Cazzaniga, A.; Maier, J.A.M.; Castiglioni, S. Impact of simulated microgravity on human bone stem cells: New hints for space medicine. Biochem. Biophys. Res. Commun. 2016, 473, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.M. Cell Cycle-Driven Heterogeneity: On the Road to Demystifying the Transitions between Poised and Restricted Pluripotent Cell States. Stem Cells Int. 2015, 2015, e219514. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Maier, J.A.M.; Bernardini, D.; Rayssiguier, Y.; Mazur, A. High concentrations of magnesium modulate vascular endothelial cell behaviour in vitro. Biochim. Biophys. Acta 2004, 1689, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Pierantozzi, E.; Gava, B.; Manini, I.; Roviello, F.; Marotta, G.; Chiavarelli, M.; Sorrentino, V. Pluripotency regulators in human mesenchymal stem cells: Expression of NANOG but not of OCT-4 and SOX-2. Stem Cells Dev. 2011, 20, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-C.; Hung, S.-C. Functional roles of pluripotency transcription factors in mesenchymal stem cells. Cell Cycle 2012, 11, 3711–3712. [Google Scholar] [CrossRef] [PubMed]
- Pauklin, S.; Vallier, L. The cell-cycle state of stem cells determines cell fate propensity. Cell 2013, 155, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Sato, F.; Matsukawa, Y.; Matsumoto, K.; Nishino, H.; Sakai, T. Apigenin Induces Morphological Differentiation and G2-M Arrest in Rat Neuronal Cells. Biochem. Biophys. Res. Commun. 1994, 204, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Zarrilli, R.; Pignata, S.; Apicella, A.; Di Popolo, A.; Memoli, A.; Ricchi, P.; Salzano, S.; Acquaviva, A.M. Cell cycle block at G1-S or G2-M phase correlates with differentiation of Caco-2 cells: Effect of constitutive insulin-like growth factor II expression. Gastroenterology 1999, 116, 1358–1366. [Google Scholar] [CrossRef]
- Van Oudenhove, J.J.; Grandy, R.A.; Ghule, P.N.; Del Rio, R.; Lian, J.B.; Stein, J.L.; Zaidi, S.K.; Stein, G.S. Lineage-Specific Early Differentiation of Human Embryonic Stem Cells Requires a G2 Cell Cycle Pause. Stem Cells 2016, 34, 1765–1775. [Google Scholar] [CrossRef] [PubMed]
- Maier, J.A.M. Endothelial cells and magnesium: Implications in atherosclerosis. Clin. Sci. 2012, 122, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xu, Y.; Gao, C.Y.; Zhai, Y.P. Adaptive protection against damage of preconditioning human umbilical cord-derived mesenchymal stem cells with hydrogen peroxide. Genet. Mol. Res. 2014, 13, 7304–7317. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.-S.; Park, B.-S.; Sung, J.-H. The wound-healing and antioxidant effects of adipose-derived stem cells. Expert Opin. Biol. Ther. 2009, 9, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Atashi, F.; Modarressi, A.; Pepper, M.S. The role of reactive oxygen species in mesenchymal stem cell adipogenic and osteogenic differentiation: A review. Stem Cells Dev. 2015, 24, 1150–1163. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, C.I.; Suda, T. Regulation of reactive oxygen species in stem cells and cancer stem cells. J. Cell. Physiol. 2012, 227, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, D.; Barroca, V.; Ducongé, F.; Bayer, J.; Van Nhieu, J.T.; Pestourie, C.; Fouchet, P.; Tavitian, B.; Roméo, P.-H. In vivo cellular imaging pinpoints the role of reactive oxygen species in the early steps of adult hematopoietic reconstitution. Blood 2010, 115, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Le Belle, J.E.; Orozco, N.M.; Paucar, A.A.; Saxe, J.P.; Mottahedeh, J.; Pyle, A.D.; Wu, H.; Kornblum, H.I. Proliferative neural stem cells have high endogenous ROS levels that regulate self-renewal and neurogenesis in a PI3K/Akt-dependant manner. Cell Stem Cell 2011, 8, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Tkach, M.; Théry, C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell 2016, 164, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-F.; Yang, G.-H.; Pan, X.-H.; Zhang, S.-J.; Zhao, C.; Qiu, B.-S.; Gu, H.-F.; Hong, J.-F.; Cao, L.; Chen, Y.; et al. Altered microRNA expression profile in exosomes during osteogenic differentiation of human bone marrow-derived mesenchymal stem cells. PLoS ONE 2014, 9, e114627. [Google Scholar] [CrossRef] [PubMed]
- Chiarello, D.I.; Pardo, F.; Salsoso, R.; Fuenzalida, B.; Gutiérrez, J.; Leiva, A.; Sobrevia, L. Effect of MgSO4 on protein expression and exosomes release from human placental microvascular endothelial cells from late-onset preeclampsia. Placenta 2017, 51, 127–128. [Google Scholar] [CrossRef]
- Bianchi, F.; Maioli, M.; Leonardi, E.; Olivi, E.; Pasquinelli, G.; Valente, S.; Mendez, A.J.; Ricordi, C.; Raffaini, M.; Tremolada, C.; et al. A new nonenzymatic method and device to obtain a fat tissue derivative highly enriched in pericyte-like elements by mild mechanical forces from human lipoaspirates. Cell Transplant. 2013, 22, 2063–2077. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, F.; Baldassarre, M.; Giannone, F.A.; Laggetta, M.; Valente, S.; Cavallini, C.; Tassinari, R.; Canaider, S.; Pasquinelli, G.; Tremolada, C.; et al. Lipogems, a New Modality of Fat Tissue Handling to Enhance Tissue Repair in Chronic Hind Limb Ischemia. CellR4 2014, 2, e1289. [Google Scholar]
- Quirici, N.; Soligo, D.; Bossolasco, P.; Servida, F.; Lumini, C.; Deliliers, G.L. Isolation of bone marrow mesenchymal stem cells by anti-nerve growth factor receptor antibodies. Exp. Hematol. 2002, 30, 783–791. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Sargenti, A.; Farruggia, G.; Malucelli, E.; Cappadone, C.; Merolle, L.; Marraccini, C.; Andreani, G.; Prodi, L.; Zaccheroni, N.; Sgarzi, M.; et al. A novel fluorescent chemosensor allows the assessment of intracellular total magnesium in small samples. Analyst 2014, 139, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Sargenti, A.; Farruggia, G.; Zaccheroni, N.; Marraccini, C.; Sgarzi, M.; Cappadone, C.; Malucelli, E.; Procopio, A.; Prodi, L.; Lombardo, M.; et al. Synthesis of a highly Mg2+-selective fluorescent probe and its application to quantifying and imaging total intracellular magnesium. Nat. Protoc. 2017, 12, 461–471. [Google Scholar] [CrossRef] [PubMed]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sargenti, A.; Castiglioni, S.; Olivi, E.; Bianchi, F.; Cazzaniga, A.; Farruggia, G.; Cappadone, C.; Merolle, L.; Malucelli, E.; Ventura, C.; et al. Magnesium Deprivation Potentiates Human Mesenchymal Stem Cell Transcriptional Remodeling. Int. J. Mol. Sci. 2018, 19, 1410. https://doi.org/10.3390/ijms19051410
Sargenti A, Castiglioni S, Olivi E, Bianchi F, Cazzaniga A, Farruggia G, Cappadone C, Merolle L, Malucelli E, Ventura C, et al. Magnesium Deprivation Potentiates Human Mesenchymal Stem Cell Transcriptional Remodeling. International Journal of Molecular Sciences. 2018; 19(5):1410. https://doi.org/10.3390/ijms19051410
Chicago/Turabian StyleSargenti, Azzurra, Sara Castiglioni, Elena Olivi, Francesca Bianchi, Alessandra Cazzaniga, Giovanna Farruggia, Concettina Cappadone, Lucia Merolle, Emil Malucelli, Carlo Ventura, and et al. 2018. "Magnesium Deprivation Potentiates Human Mesenchymal Stem Cell Transcriptional Remodeling" International Journal of Molecular Sciences 19, no. 5: 1410. https://doi.org/10.3390/ijms19051410
APA StyleSargenti, A., Castiglioni, S., Olivi, E., Bianchi, F., Cazzaniga, A., Farruggia, G., Cappadone, C., Merolle, L., Malucelli, E., Ventura, C., Maier, J. A. M., & Iotti, S. (2018). Magnesium Deprivation Potentiates Human Mesenchymal Stem Cell Transcriptional Remodeling. International Journal of Molecular Sciences, 19(5), 1410. https://doi.org/10.3390/ijms19051410